Abstract
Context: Oxidative stress is believed to increase delayed neuronal death in the brain following ischemia. As a consequence, many attempts to reduce the damage resulting from cerebral ischemia under more highly oxidized conditions have focused on treatments aimed at maintaining the redox equilibrium of the local environment. Many antioxidants were shown to be neuroprotective in experimental models of cerebral ischemia and reperfusion.
Objective: The present study was designed to investigate the potential protective effects of ethanol extract of Ocimum gratissimum Linn. (Lamiaceae) (EEOg) against focal ischemia and reperfusion (I/R) insult in rat brain.
Materials and methods: The animal model of focal I/R was established by occluding the middle cerebral artery (MCA) of male Wistar rats for 2 h, followed by 24 h reperfusion. The thiobarbituric acid reactive substances concentration, superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity were determined by colorimetric assays. The characterization and quantitative analysis of phenolic content was determined using HPLC.
Results: MCA occlusion led to significant rise in cerebral infarct volume and lipid peroxidation, and depletion in SOD and GPx in brain. The neurological deficits were also significantly elevated by MCA occlusion. All the brain oxidative stress, damage and neurological deficits were significantly attenuated by pre-treatment with EEOg (150 or 300 mg/kg, p.o.).
Conclusion: The overall finding suggests the neuroprotective potential of O. gratissimum in cerebral ischemia, and is mediated through its antioxidant activity. Therefore, O. gratissimum should be investigated further as a possible strategy against cerebral stroke.
Introduction
Stroke is a major public health problem in the world. Stroke results in neurological deficits (CitationE-Jian et al., 2009). It is well documented that oxidative stress is one of the primary factors that exacerbate damage by cerebral ischemia and reperfusion (CitationChan, 2001; CitationJanardhan & Qureshi, 2004). Several components of reactive oxygen species (superoxide, hydroxyl radical, hydrogen peroxide, peroxynitrite radical, etc.) that are generated after ischemia and reperfusion injury play an important role in neuronal injury (CitationImaizumi et al., 1984; CitationOliver et al., 1990). Superoxide and hydroxyl radical cause destruction of the cell membrane by inducing lipid peroxidation (CitationBromont et al., 1989). Inducible nitric oxide synthase is up-regulated after ischemia and reperfusion injury. This results in excessive nitric oxide production. This excess nitric oxide reacts with superoxide to form peroxynitrite, a powerful radical that produces neuronal death after cerebral ischemia. The brain is particularly vulnerable to oxidative stress injury because of its high rate of oxidative metabolic activity, depletion of endogenous antioxidant systems, intense production of reactive oxygen species metabolites, high content of polyunsaturated fatty acids, relatively low antioxidant capacity, low repair mechanism activity and non-replicating nature of its neuronal cells (CitationEvans, 1993; CitationGupta et al., 2003). Several natural and synthetic antioxidants have been shown to produce neuroprotective effect in ischemia and reperfusion-induced cerebral injury (CitationEaston et al., 1998; CitationYousuf et al., 2009).
Ocimum gratissimum (Linn.) (Lamiaceae), an erect small shrub, is native from India to West Africa and has become naturalized in Europe and America (CitationOffiah & Chikwendu, 1999). Though a lot of scientific work has been carried out on some members of this genus, sporadic information is available in the literature about the medicinal uses of O. gratissimum. Traditionally, the plant has been used as a stomachic, expectorant, anthelmintic, diaphoretic, laxative, febrifuge, for curing headache, angina and madness (CitationJanssen et al., 1989; CitationNdounga & Ouamba, 1997), and in central nervous system diseases (CitationCristiana et al., 2006).
Phytochemically, O. gratissimum contains a high quantity of essential oil (3.2-4.1%), ocimol, gratissimin, β-sitosterol (CitationList & Horhammer, 1977), flavonoids (CitationRoberto et al., 2001), linolenic acid and polyphenolic compounds (CitationLukmanul et al., 2008).
Pharmacological reports revealed that O. gratissimum possesses antioxidant, anti-inflammatory (CitationLukmanul et al., 2008), antimicrobial, antifungal, antimutagenic, immunomodulating, smooth muscle contraction effects of lipid-soluble principles, hepatoprotective (CitationLin et al., 1995; CitationVeronica & Unoma, 1999), antimycobacterial and antidiarrheal effects in experimental animals (CitationOffiah & Chikwendu, 1999). The ethanol extract of leaves of O. gratissimum has been reported to exhibit significant hypoglycemic effect in normal and alloxan-induced diabetes in rats (CitationAguiyi et al., 2000).
Recently, it has been reported that O. gratissimum possesses antioxidant and anti-inflammatory properties, (CitationLukmanul et al., 2008) but so far no work has been carried out to evaluate neuroprotective effect of O. gratissimum on ischemia and reperfusion-induced cerebral injury. Therefore, the present study has been designed to investigate the effect of O. gratissimum on focal cerebral ischemia and reperfusion-induced cerebral injury in rats.
Materials and methods
Drugs and chemicals
Thiobarbituric acid (TBA), triphenyltetrazolium chloride (TTC), trichloroacetic acid (TCA), nitroblue tetrazolium (NBT), phenazine methosulfate (PMS), xanthine oxidase, gallic acid (GA), glutathione and nicotinamide adenine dinucleotide phosphate (NADPH) were obtained from Sigma, St. Louis, MO. Chloral hydrate was obtained from Reidel-de Haen, Seelze, Germany. All chemicals were of the highest purity commercially available. All drug solutions were freshly prepared before use.
Plant materials and preparation of the extract
Aerial parts of the plant O. gratissimum were obtained in August 2009 from the medicinal garden, Panjab University, Chandigarh, India. Identity of the plant material was authenticated by S. Negi, Director, Forest Research Institute, Dehradoon, India. Voucher specimen of the plant was deposited in Punjabi University Herbarium. Dried plant material was pulverized using a mechanical grinder. Powdered material (100 g) was extracted with ethanol (95%) continuously for 15 h using a Soxhlet apparatus. Thereafter, the resulting ethanol extract was dried under reduced pressure using a Buchi 461 rotary vacuum evaporator (Postfach, Switzerland) and was preserved in a vacuum desiccator containing anhydrous silica gel blue. Phytochemical screening (CitationFarnsworth, 1966; CitationEvans, 2002) of the extract revealed the presence of polyphenolic compounds, terpenes, flavonoids and carbohydrates.
Standardization of extract
Determination of total phenolic content
Total phenol content was estimated as gallic acid equivalents (CitationSingleton et al., 1999). Briefly, a 100 μL aliquot of dissolved extract was transferred to a 10 mL flask, containing 6 mL double distilled water, to which was subsequently added 500 μL undiluted Folin-Ciocalteu reagent. After 1 min, 1.5 mL Na2CO3 (20%, w/v) was added and the volume was made up to 10 mL with ultra-pure water. After 30 min incubation at 25°C, the absorbance was measured spectrophotometrically (Beckman DU 640B, Nyon, Switzerland) at 760 nm.
High-performance liquid chromatography - Phenolic compounds analysis
A high-performance liquid chromatography (HPLC) system comprising a vacuum degasser, quaternary pump, autosampler, thermostat column compartment, and photodiode array detector (Analytical Technologies, Vadodara, India) was used for the quantification of individual phenolic compounds. The column, Phenomenex C18 5 μm (250 × 4.6 mm), was maintained at 26°C. Different proportions of solvents such as acetonitrile/water/acetic acid (15:84:0.85) as eluant B and methanol as eluant A were used for the separation. The multiple gradient used for chromatographic separation consisted of different proportions of eluant A/B (50:50 for 5 min, 40:60 for 5–10 min, 30:70 for 10–15 min, 15:85 for 15–20 min). The flow rate was 1 mL/min, the sample injection volume was 50 μL and the chromatogram monitored at 330 nm. The peak purity of the tested sample was determined by comparing its ultraviolet spectra to that of the reference standards. Quantification was made on the basis of the corresponding peak area recorded by chromatopac c-R6A. Reference standards were used for the preparation of standard curves.
Animals
Male Wistar rats (250–300g) were maintained on standard environmental conditions and fed with standard rodent diet and tap water ad libitum. They were housed in the institutional animal house and were exposed to natural photoperiod. The experimental protocol was approved by Punjabi University Animal Ethics Committee and care of the animals was carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. no. 107/1999/CPCSEA).
Experimental protocol
A total of four groups of fourteen rats each were employed in the present study. Half the animals from each group were subjected to the elevated plus maze test, inclined beam test and rota-rod test before focal cerebral ischemia, and after 24 h reperfusion. The remaining half of the animals from each group were used to estimate infarct volume, lipid peroxidation, superoxide dismutase, and glutathione peroxidase activity.
The first group (n = 7) were sham operated (animals were subjected to surgical procedure, but did not suffer middle cerebral artery occlusion (MCAO), except for exposure of the right internal carotid artery (ICA) and right external carotid artery (ECA)). A second group (n = 7) served as control [the rats were orally administered vehicle (distilled water + 5% Tween 80, 10 mL/kg) 60 min before subjecting to 2 h focal ischemia by MCAO followed by 24 h reperfusion]. The third and fourth groups of rats (n = 7 each) received 100 mg kg−1 and 200 mg kg−1 doses of ethanol extract of O. gratissimum, respectively. The vehicle and drugs were administered orally, 60 min before subjecting them to focal cerebral ischemia. A pilot study using infarct volume as the end point was performed to select the doses.
Middle cerebral artery occlusion
The rats were subjected to 2 h right MCAO using the intraluminal filament technique (CitationLonga et al., 1989). The rats were anesthetized with chloral hydrate (400 mg/kg, i.p.). The right common carotid artery was exposed at the level of the ECA and ICA bifurcation. A 4-0 monofilament nylon suture was inserted into the ECA and advanced into the ICA for 17–20 mm until a slight resistance was felt, to block the origin of the middle cerebral artery. Two hours after MCAO, the suture was slowly withdrawn. The animals were then returned to their cages for 24 h.
Cerebral infarct volume assessment
After 24 h reperfusion, the animals were sacrificed by spinal dislocation and the brains removed. The brains were kept overnight at −4°C. Frozen brain was sliced into uniform sections of about 1 mm thickness. The slices were incubated in 1% triphenyl tetrazolium chloride (TTC) at 37°C in 0.2 M tris buffer (pH 7.4) for 20 min (CitationBochelen et al., 1999). TTC is converted to red formazone pigment by NAD and dehydrogenase present in living cells. Hence viable cells were stained deep red. The infarcted cells had lost the enzyme and thus remained unstained dull yellow. The area of infarction was measured in coronal brain sections using image analysis software Leica Qwin (Leica Microsystems, Cambridge, UK.). Infarct areas of all sections were cumulated to give the total infarct area which was multiplied by the thickness of the brain sections to get the volume of infarction.
Estimation of mitochondrial thiobarbituric acid reactive substances (TBARS)
The brain was weighed, minced and suspended in a buffer containing 30 mM Tris-HCl and 2.5 mM CaCl2 (pH 7.6). The above mixture was homogenized and the homogenate was centrifuged at 750 rpm to separate cellular debris. The supernatant was accurately divided into two parts. Both portions were centrifuged at 8200 rpm to obtain the mitochondrial fraction. One was utilized for determination of TBARS (CitationYagi, 1982), and the other one was employed for protein estimation (CitationLowry et al., 1951).
Superoxide dismutase
Superoxide dismutase (SOD) activity was measured by the method explained by CitationBeauchamp and Fridovich (1971). The reaction mixture of total volume 1 mL consisted of 0.6 mL phosphate buffer (0.5 M, pH 7.4), 0.1 mL PMS (10%, w/v), 0.1 mL of xanthine (1 mM), 0.1 mL of NBT (57 µM) was incubated for 15 min at room temperature and reaction was initiated by the addition of xanthine oxidase (50 mU). The rate of reaction was measured by recording change in the absorbance at 550 nm due to formation of formazan, a reduction product of NBT.
Glutathione peroxidase (GPx)
Glutathione peroxidase (GPx) activity was measured according to the procedure described by CitationMohandas et al. (1984). The reaction mixture consisted of 1.44 mL phosphate buffer (0.05 M, pH 7.0), 0.1 mL EDTA (1 mM), 0.1 mL sodium azide (1 mM), 0.05 mL of glutathione reductase (1 EU/mL), 0.1 mL of glutathione (1 mM), 0.1 mL NADPH (0.2 mM), 0.01 mL hydrogen peroxide (0.25 mM) and 0.1 mL PMS (10%, w/v) in a final volume of 2 mL. The disappearance of NADPH at 340 nm was recorded at room temperature. The enzyme activity was calculated as nM NADPH oxidized/min/mg protein.
Short-term memory evaluation
The elevated plus maze was employed to evaluate the short-term memory, which consisted of two enclosed (500 × 100 × 400 mm) and two open (500 × 100 mm) arms. It was elevated to a height of 500 mm above the floor (CitationKulkarni, 2007). All the animals were given a single trial on the plus maze. Individually, each rat was placed at the end of the open arm facing away from central platform of the maze. The time taken by the rat to enter from the open arm with all four legs within the enclosed arm was taken as transfer latency time (TLT). If the animal did not enter the enclosed arm within 90 s, it was gently pushed into the enclosed arm and a TLT of 90 s was assigned to it. The animal was allowed to explore the maze for an additional 10 s after the measurement of TLT (CitationItoh et al., 1990). With an aim to attain a low level of TLT the exposure to the elevated plus maze was repeated on days 2 and 3 (CitationZdenek & Ivan, 1998). After the third training trial the animals were subjected to focal cerebral ischemia for 90 min followed by reperfusion for 24 h. Then they were put on the elevated plus maze. TLT measured on the plus maze on the third training trial served as an index of learning or acquisition, whereas TLT recorded 24 h after reperfusion (day 4) served as an index of retrieval or memory.
Inclined beam walking test
To evaluate fore and hind limb motor coordination, the inclined beam walking test was employed (CitationFeeney et al., 1981). The inclined beam walking test was performed before ischemia and 24 h after reperfusion.
Rota-rod test
The CitationDunham and Miya (1957) method was used to evaluate the rota-rod performance. It is used to evaluate motor coordination by testing the ability of rats to remain on a revolving rod. The apparatus consisted of a horizontal rough metal rod of 30 mm diameter attached to a motor with a variable speed. The rod was 700 mm in length and was divided into four sections by wooden partitions, thereby allowing the simultaneous testing of four rats. The rod was at a height of about 500 mm above the table top in order to discourage the animals from jumping off the roller. The rate of rotation of the rod was adjusted so that the normal rat was able to stay on the rotating rod for a period of 5 min. The difference in the fall-off time from the rotating rod between the control and treated animals is taken as an index of motor uncoordination. Each rat was given four or five trials before the actual reading was taken. The rats that were able to stay on the rotating rod for a period of 5 min before focal cerebral ischemia were selected, and the test was again performed 24 h after focal cerebral ischemia and reperfusion.
Statistical analyses
All the data are presented as mean ± SD. The data of infarct volume and biochemical parameters were statistically analyzed using one-way ANOVA followed by Tukey’s post hoc multiple range test for multiple comparisons among various groups. However, the data of percentage change in TLT was statistically analyzed using repeated measure ANOVA followed by Tukey’s post hoc multiple range test. The inclined beam walking test and Rota-rod test were statistically analyzed using one-way ANOVA followed by post hoc Kruskal-Wallis test and Bonferroni’s correction test respectively. A p value of <0.05 was considered statistically significant.
Results
Extract yield, total phenolic content and HPLC analysis
The yield of extract was 8.45% (w/w). The total phenolic content was estimated by the Folin-Ciocalteu reagent method. The total phenolic content was estimated to be 166.2 ± 4.1 mg GA equivalents/g dry extract of O. gratissimum from triplicate measurements.
In the HPLC analysis, phenolic compounds of O. gratissimum were quantified at 330 nm as phenolic acids, flavonoids and hydroxycinnamates. The components rosmarinic acid, p-coumaric acid, hydroxybenzoic acid, ferulic acid, cinnamic acid and lithospermic acid () were identified by comparing their retention time with those of standards, and quantitatively determined by their respective peak areas.
Table 1. Quantitative analyses of phenolic content of O. gratissimum ethanol extract (EEOg).
Protective effect of O. gratissimum
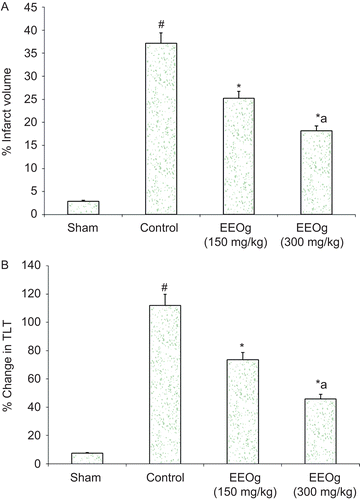
Focal cerebral ischemia of 2 h followed by reperfusion for 24 h produced significant increase in cerebral infarct volume (37.13%) (). Administrations of ethanol extract of O. gratissimum markedly attenuated (about 51%, p < 0.05) ischemia and reperfusion-induced increase in cerebral infarct volume.
Figure 1. A) Neuroprotective effect of ethanol extract of O. gratissimum (EEOg) on brain infarction in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Tukey’s post hoc multiple range test; *p <0.05 versus control; #p < 0.05 versus sham; ap <0.05 versus 150 mg/kg dose of EEOg. B) Effect of EEOg on impairment of short-term memory (plus maze test) in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Tukey’s post hoc multiple range test; *p <0.05 versus control; #p < 0.05 versus sham; ap <0.05 versus 150 mg/kg dose of EEOg.
Biochemical observations
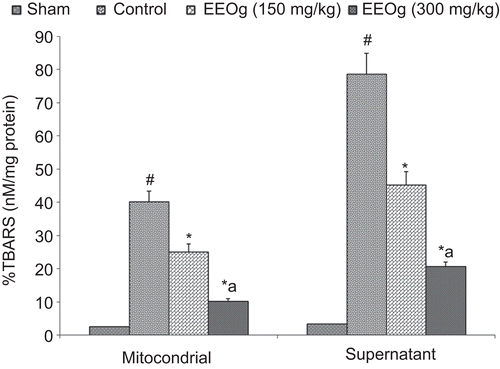
TBARS concentration in brain mitochondrial and supernatant fractions was significantly increased in rat ischemic brain due to ischemia and reperfusion injury (). Administrations of ethanol extract of O. gratissimum markedly decreased ischemia and reperfusion-induced increase in TBARS concentration in brain mitochondrial and supernatant fractions.
Figure 2. Effect of ethanol extract of O. gratissimum (EEOg) on thiobarbituric acid reactive substances (TBARS) formation in brain of rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Tukey’s post hoc multiple range test; #p <0.05 versus sham; *p <0.05 versus control; ap <0.05 versus 150 mg/kg dose of EEOg.
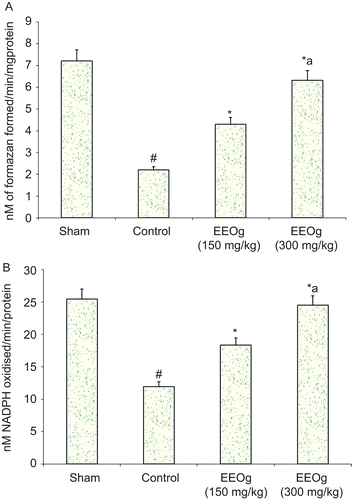
SOD and GPx showed a significant decrease in the MCAO induced control group (). Pre-treatment with ethanol extract of O. gratissimum significantly reversed the alterations in biochemical parameters brought by ischemia and reperfusion. SOD and GPx were significantly elevated.
Figure 3. A) Effect of ethanol extract of O. gratissimum (EEOg) on superoxide dismutase activity in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Tukey’s post hoc multiple range test; *p <0.05 versus control; #p <0.05 versus sham; ap <0.05 versus 150 mg/kg dose of EEOg. B) Effect of EEOg on glutathione peroxidase (GPx) activity in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Tukey’s post hoc multiple range test; *p <0.05 versus control; #p <0.05 versus sham; ap <0.05 versus 150 mg/kg dose of EEOg.
Behavioral observations
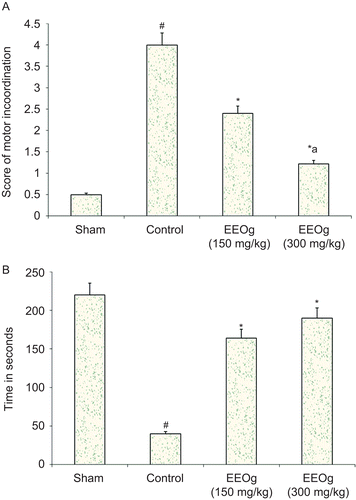
Focal cerebral ischemia and reperfusion produced a marked impairment of short-term memory by decreasing the TLT using the elevated plus maze. Pre-treatment with ethanol extract of O. gratissimum significantly prevented ischemia and reperfusion-induced increase in the percentage change in TLT (). In the same way, focal cerebral ischemia and reperfusion produced a marked impairment of motor performance. Administration of ethanol extract of O. gratissimum significantly prevented ischemia- and reperfusion-induced impairment of motor performance ().
Figure 4. A) Effect of ethanol extract of O. gratissimum (EEOg) on impairment of motor performance (inclined beam test) in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± S.D.; n = 7; ANOVA followed by post hoc Kruskal-Wallis test; #p <0.05 versus sham; *p <0.05 versus control; ap <0.05 versus 150 mg/kg dose of EEOg. B) Effect of EEOg on impairment of motor performance (rota-rod test) in rats subjected to focal cerebral ischemia and 24 h reperfusion injury. The data are expressed as mean ± SD; n = 7; ANOVA followed by Bonferroni’s post hoc correction test; *p <0.05 versus control; #p <0.05 versus sham.
Discussion
Experimental models of stroke have been developed in animals in an attempt to mimic the events of human cerebral ischemia. The focal ischemic animal model involves the transient or permanent occlusion of the middle cerebral artery to be used as a model of cerebral ischemia (CitationCrack et al., 2001). In the present study we used the temporary MCAO rat model to determine the actions of in vivo administration of O. gratissimum ethanol extract. The extract was standardized on the basis of total phenolic and flavonoid content. The results demonstrated that ethanol extract of O. gratissimum exhibited neuroprotection as was evident from the significant reduction in neuronal cell death (decreased infarct volume).
It is well documented that free radicals are involved in ischemia- and reperfusion-induced cerebral injury, among which, superoxide and hydroxyl radicals are potent inducers of lipid peroxidation (CitationBromont et al., 1989). Reactive oxygen species (ROS) produce malondialdehyde (MDA), an end product of lipid peroxidation. MDA reacts with TBA and is, thus, estimated as TBARS (CitationDib et al., 2002). Therefore, in the present study, MDA was estimated using TBARS assay to estimate extent of ROS. Administration of ethanol extract of O. gratissimum significantly decreased the extent of lipid peroxidation (decreased level of TBARS).
Mitochondria produce superoxide anion radicals and hydrogen peroxide under normal physiological conditions (CitationTaku et al., 2004). These constantly produced ROS are scavenged by SOD and GPx. SOD specifically processes superoxide anion radicals and produces hydrogen peroxide, which is then detoxified by GPx, and finally changed to water and superoxide. In MCAO groups, SOD and GPx activity were significantly reduced. Pre-treatment with ethanol extract of O. gratissimum significantly prevented MCAO-induced decline in SOD and GPx activity.
It is well documented that hippocampal neurons are highly susceptible to ischemia- and reperfusion-induced injury (CitationJenkin et al., 1981). Therefore, in the present study, the elevated plus maze model has been employed to evaluate impairment of short-term memory as a result of MCAO. Moreover, neuronal ischemia is reported to impair sensory motor ability. The inclined beam and rota-rod test have been employed to investigate the effect of cerebral ischemia and reperfusion on motor performance. Oral administration of ethanol extract of O. gratissimum, before focal cerebral ischemia has prevented ischemia- and reperfusion-induced impairment of short-term memory and motor uncoordination.
Ocimum species have been extensively reported to contain essential oil; however, the antioxidant capacity of the plant extracts is mainly dependent on phenolic compounds. Phenolic compounds are an important group of secondary metabolites, which are synthesized by plants due to plant adaptation in response to biotic and abiotic stresses (infection, water stress, cold stress, high visible light) (CitationPitchersky & Gang, 2000). In the present study, we quantified our extract for total phenolic content. The phenolic components rosmarinic acid, p-coumaric acid, hydroxybenzoic acid, ferulic acid, lithospermic acid and cinnamic acid were identified and quantified at 330 nm. In our study, rosmarinic acid was the most abundant component identified in O. gratissimum extract (0.025% w/w) as compared to other compounds.
In conclusion, O. gratissimum exhibited neuroprotection by reducing brain infarction, restoring endogenous antioxidant system, and attenuating impairment in short-term memory and motor uncoordination. The protection may be due to the reduction of oxidative stress by phenolic compounds which are identified and quantified in the present study. These observations suggest that O. gratissimum may be a clinically viable protective agent against a variety of conditions where cellular damage is a consequence of oxidative stress. In addition, O. gratissimum may have the potential to be used in the prevention of neurodegenerative diseases such as cerebral stroke.
Declaration of interest
Authors are grateful to the L.L.R. Educational Trust, which runs the L.R. Institute of Pharmacy, Solan, Himachal Pradesh, India, for providing funds to carry out the present investigation. The authors alone are responsible for the content and writing of the paper.
References
- Aguiyi JC, Obi CI, Gang SS, Igweh AC. (2000). Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia, 71, 444–446.
- Beauchamp C, Fridovich I. (1971). Superoxide dismutase, improved assays and an assay applicable to acrylamide gels. Anal Biochem, 44, 276–287.
- Bochelen D, Rudin M, Sauter A. (1999). Calcineurin inhibitor FK 506 and SDZASM 981 alleviate the outcome of focal ischemia/reperfusion injury. J Pharmacol Exp Ther, 288, 653–659.
- Bromont C, Marie C, Bralet J. (1989). Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke, 20, 918–924.
- Chan PH. (2001). Reactive oxygen radicals in signalling and damage in the ischemic brain. J Cereb Blood Metab, 21, 2–14.
- Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC. (2001). Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem, 78, 1389–1399.
- Cristiana M, Murbach FM, Ortiz M, Marques MC. (2006). Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J Ethnopharmacol, 105, 161–166.
- Dib M, Garrel C, Favier A, Robin V, Desnuelle C. (2002). Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol, 249, 367–374.
- Dunham NW, Miya TS. (1957). A note on simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc Am Pharm Assoc, 46, 208–211.
- Easton JD, Hansen SL, Martin JB. (1998). Cerebrovascular diseases. In: Fanci AS, Bravnwald E, Isselbacher KJ,Wilson JD, Martin JB, Kasper DL, eds. Principles of Internal Medicine. New York: McGraw-Hill, 2325.
- E-Jian L, Hung-Yi C, Yu-Chang H, Tsung-Ying C, Ming-Yang L, Shu-Ching Y. (2009). Therapeutic window for cinnamophilin following oxygen-glucose deprivation and transient focal cerebral ischemia. Exp Neurol, 217, 74–83.
- Evans PH. (1993). Free radicals in brain metabolism and pathology. Br Med Bull, 49, 577–587.
- Evans WC. (2002). Carbohydrates. In: Evans WC, Evans D, eds. Trease and Evan’s Pharmacognosy, fifteenth edition. Edinburgh, London: WB Saunders, 93–213.
- Farnsworth NR. (1966). Biological and phytochemical screening of plants. J Pharm Sci, 55, 225–276.
- Feeney DM, Beyeson MG, Linn RT, Murray HM, Dail WG. (1981). Responses to cortical injury: Methodology and local effects of contusions in the rat. Brain Research, 211, 67–77.
- Gupta R, Singh M, Sharma A. (2003). Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol Res, 48, 209–215.
- Imaizumi S, Kayama T, Suzuki J. (1984). Chemiluminescence in hypoxic brain-the first report. Correlation between energy metabolism and free radical reaction. Stroke, 15, 1061–1065.
- Itoh J, Nabeshima T, Kammeyama T. (1990). Utility of an elevated plus maze for the evaluation of memory in mice. Effect of nootropic scopolamine and electroconvulsive shock. Psychopharmacology, 101, 27–32.
- Janardhan V, Qureshi AI. (2004). Mechanisms of ischemic brain injury. Curr Cardiol Rep, 6, 117–123.
- Janssen AM, Scheffer JJC, Ntezurubanza L, Baerheim SA. (1989). Antimicrobial activities of some Ocimum species grown in Rwanda. J Ethnopharmacol, 26, 57–63.
- Jenkin LW, Povlishock JT, Lewett W, Miller JD, Becker DP. (1981). The role of post-ischemic recirculation in the development of ischemic neuronal injury following complete cerebral ischemia. Acta Neuropathologica, 5, 205–220.
- Kulkarni SK. (2007). Hand Book of Experimental Pharmacology, third edition. Delhi: Vallabh Prakashan, 36–38.
- Lin CC, Lin JK, Chang CH. (1995). Evaluation of hepatoprotective effects of “Chhit-Chan-Than” from Taiwan. Int J Pharmacog, 33, 139–143.
- List PH, Horhammer L. (1977). Hagers Handbuch der Pharmazeutischen Praxis, fourth edition. Band VI A, Berlin-Heidelberg, Germany: Springer.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20, 84–91.
- Lowry OH, Rosebrough NJ, Far AL, Randall RJ. (1951). Protein measurement with folin-phenol reagent. J Biol Chem, 93, 265–275.
- Lukmanul HF, Girija A, Boopathy R. (2008). Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plants Res, 2, 250–257.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller D. (1984). Differential distribution of glutathione and glutathione related enzymes in rabbit kidney, possible implications in analgesic neuropathy. Cancer Res, 44, 5086–5091.
- Ndounga M, Ouamba JM. (1997). Antibacterial and antifungal activities of essential oils of Ocimum gratissimum and O. basilicum from Congo. Fitoterapia, 68, 190–191.
- Offiah VN, Chikwendu UA. (1999). Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol, 68, 327–330.
- Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. (1990). Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA, 87, 5144–5147.
- Pitchersky E, Gang DR. (2000). Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci, 5, 459–445.
- Roberto FV, Renee JG, Alan P, James ES. (2001). Genetic diversity of Ocimum gratissimum L. based on volatile oil constituents, flavonoids and RAPD markers. Biochem Syst Ecol, 29, 287–304.
- Singleton VL, Orthofer R, Lamuela-Ravento’s RM. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Packer L, ed. Methods in Enzymology. San Diego CA: Academic Press, 152–178.
- Taku SM, Fujimura NN, Gyung WK, Atsushi ST, Hayashi P, Narasimhan C. (2004). Neuronal death/survival signaling pathways in cerebral ischemia. J Am Soc Exp Neuro Ther, 1, 17–25.
- Veronica NO, Unoma AC. (1999). Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol, 68, 327–330.
- Yagi K. (1982). Assay for serum lipid peroxide level and its clinical significance. In: Yagi K, ed. Lipid Peroxides in Biology and Medicine. New York: Academic Press, 232.
- Yousuf S, Atif F, Ahmad M, Hoda N, Ishrat T, Khan B. (2009). Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res, 1250, 242–253.
- Zdenek H, Ivan K. (1998). Concurrent administration of sub effective doses of scopolamine and MK-801 produces a short-term amnesia for elevated plus-maze in mice. Behav Brain Res, 91, 83–89.
