Abstract
Context: Malva parviflora L. (Malvaceae) is widely distributed throughout Africa. It has several uses in traditional medicinal practice. Leaves of this plant are used in the treatment of some inflammatory disorders.
Objective: The anti-inflammatory and the antioxidant activities of the methanol extract (Met. E) and aqueous extract (Aq. E) of M. parviflora leaves were investigated.
Materials and methods: Croton oil-induced ear edema and acetic acid-induced vascular permeability were applied as acute inflammatory models to evaluate the anti-inflammatory activity of the extracts. The antioxidant effects were evaluated using the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical assay and the measurement of the metal-chelating activity.
Results: Results demonstrated that Met. E inhibited the croton oil-induced ear edema by 57%. In contrast, the Aq. E did not show any activity. Furthermore, Met. E and Aq. E inhibited significantly the acetic acid-induced vascular permeability by 36 and 40%, respectively. However, Met. E and Aq. E exerted a strong scavenging activity with IC50 values of 89.03 ± 2.65 and 76.67 ± 0.29 µg/mL, respectively. Moreover, Met. E and Aq. E were able to chelate ferrous ions in a concentration-dependent manner.
Discussion and conclusion: These findings demonstrate that M. parviflora leaf extracts possess anti-inflammatory and antioxidant activities and thus have great potential as an interesting source for natural health products.
Introduction
During the past two decades, a growing number of studies investigated the diverse health benefits and protective effects of natural substances present in the plants. The role of medicinal plants and traditional medicine for developing new drugs is incontestable. Herbs have been used for flavoring foods and beverages and for medicinal purposes (CitationDraughon, 2004). Medicinal plants are considered to be an important source of therapeutic compounds and the therapeutic benefit of many medicinal plants is often attributed to their anti-inflammatory and antioxidant properties (CitationRice-Evans, 2004; CitationShale et al., 2005; CitationZhang et al., 2009). The preservative effect of many plant species and herbs suggests the presence of antioxidative and antimicrobial constituents such as flavonoids, phenolic acids, and phenolic diterpenes (CitationShahidi & Wanasundara, 1992; CitationHammer et al., 1999; CitationDutra et al., 2008). Malva parviflora L. (Malvaceae) is widely distributed throughout Africa. Traditional healers and herbalists use M. parviflora leaves and roots to clean wounds and sores. A hot poultice made from leaves is used to treat wounds and swelling, and incorporated into a lotion to treat bruised and broken limbs (CitationShale et al., 1999). Leaves of this plant are used in the treatment of boils (CitationGrierson & Afolayan, 1999). Despite the use of M. parviflora in traditional medicine, very few pharmacological and phytochemical studies are reported assessing its therapeutic properties. The methanol extract (Met. E) of M. parviflora has been reported to possess high COX-1-inhibiting activity as well as antibacterial activity against Gram-negative and Gram-positive bacteria (CitationShale et al., 1999). CitationGrierson and Afolayan (1999) showed that M. parviflora possessed an inhibitory effect against some fungi but was ineffective against some species of bacteria. In contrast, CitationShale et al. (1999) reported the antibacterial activity of the hexane and Met. E of the roots but noted the poor activity of the methanol leaf extract.
In order to better understand the differences in effects of Met. E and aqueous extracts (Aq. E) of M. parviflora leaves, the present investigation compared the anti-inflammatory and the antioxidant properties of both extracts in several in vivo and in vitro models. These data provide a rational basis for its traditional use to treat inflammations.
Materials and methods
Plant materials
M. parviflora leaves were collected in April 2008 from Bordj Bou Arréridj, Algeria. The plant material was identified by Dr. Houssine Laouar, University of Sétif. The voucher specimen (No. 062459) was deposited at the laboratory of botany in the University of Sétif, Algeria. The leaves were cleaned, shadow-dried, and pulverized to dry powder.
Preparation of M. parviflora leaf extracts
Met. E was prepared by maceration of 100 g of powdered plant material with 80% methanol at room temperature for 48 h with frequent agitation. After filtration, the filtrate was concentrated under reduced pressure at 40°C. The residue was lyophilized using a lyophilizator (PHYWE chrisa) to give a brown powder (yield: 17%). Aq. E was prepared according to the traditional method by boiling 50 g of powdered plant material in 500 mL of distilled water for 20 min. After filtration, the filtrate was lyophilized to give a brown powder (yield: 19% w/w).
Animals
Swiss albino mice weighing 20–25 g were purchased from the Pasteur Institute of Algiers, Algeria. All animals were divided into different groups each consisting of 6–9 animals, and were allowed to acclimatize to the animal room conditions for 1 week and had free access to food and water ad libitum. Animals were fasted overnight prior the experiments, and the test substances were given orally with access to water. All procedures were performed in accordance with the European Union Guidelines for Animals Experimentation (2007/526/EC).
Croton oil-induced ear edema in mice
To evaluate the effect of M. parviflora leaf extracts on acute inflammation, croton oil-induced ear edema was performed according to the method of CitationManga et al. (2004). Cutaneous inflammation was induced to the inner surface of the right ear of mice (7 mice/group) by application of 15 µL of acetone containing 80 µg of croton oil as irritant. Treated animals received topically 2 mg/ear of Met. E or Aq. E of M. parviflora leaves. Indomethacin as reference drug was applied topically (0.5 mg/ear). The thickness of ears was measured before and 6 h after induction of inflammation using a dial caliper (CitationDelaporte et al., 2004). The edema was expressed as an increase in the ear thickness due to croton oil application.
Acetic acid-induced vascular permeability in mice
The effect of M. parviflora leaf extracts on vascular permeability was evaluated according to the method of CitationKou et al. (2006) with slight modifications. Mice were divided into four groups with seven mice each. They obtained orally 0.2 mL of either 500 mg/kg of the Met. E or 360 mg/kg of the Aq. E or 50 mg/kg indomethacin. The control group received the same volume (0.2 mL) of normal saline solution. One hour later, 10 mL/kg of 1% solution of Evans Blue dissolved in normal saline solution was intravenously administrated. Then 10 mL/kg of 0.7% acetic acid was intraperitoneally injected. Thirty minutes later, mice peritoneal exudates were collected after being washed with 3 mL of normal saline, and centrifuged at 2000 rpm for 10 min. The absorbance of the supernatant was read at 610 nm. The dye content of the exudates, which refers to the rate of vascular permeability, was calculated according to the standard curve of Evans Blue.
DPPH radical-scavenging assay
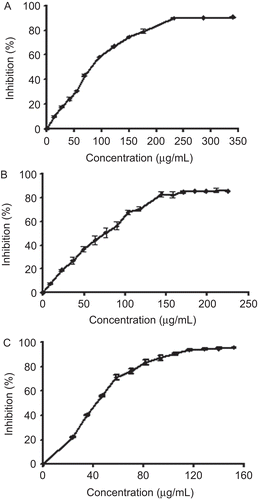
The potential antioxidant activity of M. parviflora leaf extracts was assessed using the stable 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical according to CitationBrand-Williams et al. (1995). DPPH (2.35 mg) was dissolved in 100 mL of methanol. Different dilutions (10–340 µg/mL) of the extracts or butylated hydroxytoluene (BHT), as a positive control, were prepared and 0.1 mL of these solutions was mixed with 3.9 mL of DPPH solution in test tubes to complete the final reaction media (4 mL). The mixture was shaken vigorously and incubated for 30 min in the dark at room temperature and the decreases in the absorbance values were measured at 517 nm. The decrease in absorbance is a measure of the scavenging of the DPPH radical by extracts. All experiments were performed at least in triplicate. The percentage of DPPH-scavenging activity was calculated using the following equation:
where Acontrol is the absorbance of the control reaction mixture without the test compounds, and Asample is the absorbance of the test compounds. IC50 values, which represented the concentration of the extract that caused 50% neutralization of DPPH radicals, were calculated from the plot of inhibition percentages against concentration.
Ferrous ion chelating activity
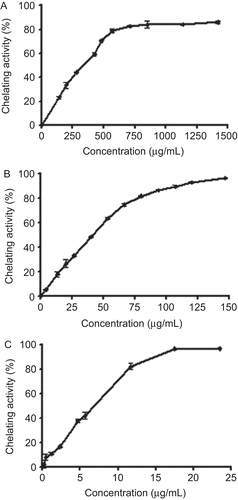
Ferrous ion chelating activity was measured by inhibition of formation of the iron (II)-ferrozine complex after the treatment of test material with Fe2+ according to CitationLe et al. (2007). The reaction mixture contained 500 µL of different concentrations (5–1500 µg/mL) of M. parviflora leaf extracts or the standard chelator ethylenediaminetetraacetic acid (EDTA), 100 µL FeCl2 (0.6 mM), and 900 µL methanol. The control contained all the reaction reagents except the extracts and EDTA. The mixture was thoroughly shaken and allowed to react at room temperature for 5 min. Ferrozine (5 mM) (100 μL) was then added, the mixture shaken again, followed by further reaction at room temperature for 10 min to complex the residual Fe2+ ion. The absorbance of the Fe2+-ferrozine complex was measured at 562 nm. All experiments were performed at least in triplicate. The chelating effect was calculated as a percentage using the following equation:
where Acontrol is the absorbance of the control reaction mixture without the test compounds, and Asample is the absorbance of the test compounds. IC50 values, which represented the concentration of the extract that caused 50% of Fe2+ ion chelation, were calculated from the plot of chelating percentage against concentration.
Statistical analysis
Data obtained in vivo are expressed as mean values ± SEM. Differences between the control and the treatments in these experiments were tested for significance using analysis of variance followed by Dunnett’s test. The data obtained in vitro are expressed as mean ± SD. A probability of P < 0.05 was considered significant.
Results
Anti-inflammatory activity
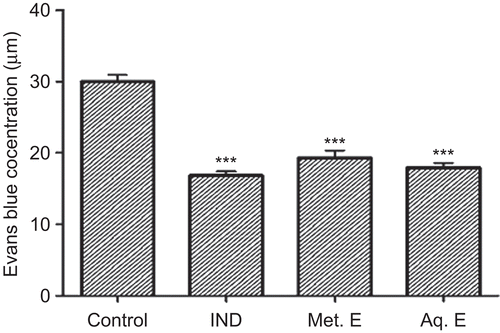
The topical application of 2 mg/ear of the Met. E of M. parviflora inhibited significantly (P ≤ 0.001) the croton oil-induced ear edema (). The inhibition was 57%. This inhibition was higher than that obtained with indomethacin (46%), which was used as positive control. In contrast, the Aq. E did not show any activity. Furthermore, oral administration of 500 and 360 mg/kg of Met. E and Aq. E, respectively, inhibited significantly (P ≤ 0.001) the acetic acid-induced vascular permeability with inhibition rates of 36 and 40%, respectively. These inhibition values were close to that obtained with 50 mg/kg of indomethacin ().
Figure 1. Effect of M. parviflora leaf extracts on ear edema induced by croton oil. Mice were treated with 0.5 mg/ear of indomethacin (IND), 2 mg/ear of methanol extract (Met. E), and 2 mg/ear of aqueous extract (Aq. E). Control group received croton oil solution only. Edema is expressed as mean thickness increase of ears before and 6 h after croton oil application. Values are expressed as means ± SEM (n = 7). ***P < 0.001. NS: not significant versus the control.
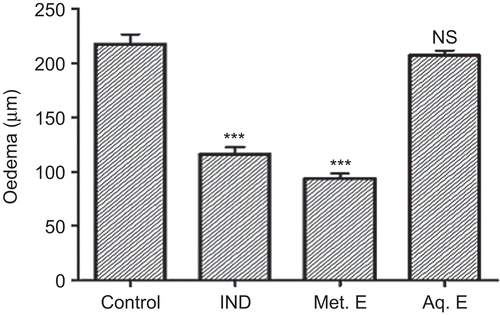
Figure 2. Effect of M. parviflora leaf extracts on acetic acid-induced vascular permeability. Mice were treated orally with 50 mg/kg of indomethacin (IND), 500 mg/kg of methanol extract (Met. E), and 360 mg/kg of aqueous extract (Aq. E). Control group received 0.2 mL of normal saline solution. Values are expressed as means ± SEM (n = 7). ***P < 0.001. NS: not significant versus the control.
Antioxidant activity
Met. E and Aq. E of M. parviflora exhibited a strong scavenging activity against the DPPH radical in a concentration-dependent manner ( and ). The values of IC50 were 89.03 ± 2.65 and 76.67 ± 0.29 µg/mL for Met. E and Aq. E, respectively. As shown in , the scavenging activity of both extracts was comparable with that obtained with the standard antioxidant BHT. Moreover, Met. E and Aq. E were able to chelate ferrous ions in a concentration-dependent manner ( and ). Nevertheless, the Met. E showed a lower chelating activity than Aq. E. The IC50 values were 346.71 ± 7.25 µg/mL and 42.26 ± 0.61 µg/mL for Met. E and Aq. E, respectively. However, chelating activities of both extracts were lower than the activity of the standard chelator EDTA ().
Discussion
Extracts from M. parviflora leaves contain different constituents that possess anti-inflammatory and antioxidant activities. Effects of extracts on croton oil-induced ear edema are probably attributed to lipophilic, methanol- but not water-soluble substances that are able to penetrate through the skin barrier (CitationOkoli et al., 2007). Likely candidates for these anti-inflammatory substances are flavonoids, polyphenols, and proanthocyanidins that were isolated from Met. E of M. parviflora (CitationAfolayan et al., 2008, Citation2010). Phenolic compounds are known to interact with and penetrate through lipid bilayers (CitationRice-Evans, 2004).
In contrast, acetic acid-induced vascular permeability was efficiently inhibited by both extracts of M. parviflora indicating that either other less lipophilic components that are distributed in both extracts are responsible for the observed effects or, alternatively, a hydrophilic constituent in the Aq. E may also inhibit the increased vascular permeability. May be this later component acts via scavenging of free metal ions depressing, thus, transition metal ion-depending oxidative processes. Both extracts possessed marked differences in their ability to scavenge free metal ions. This property was more expressed by the Aq. E. On the other hand, data on DPPH radical scavenging support that both extracts have a similar ability to scavenge free radicals. This indicates the presence of antioxidants in both extracts.
In general, our data give a good rational for the broad application of M. parviflora extracts in traditional medicine to combat inflammations. Extracts of M. parviflora have a high inhibiting activity against cyclooxygenase-1 (COX-1) that is caused by at least two components acting synergistically (CitationShale et al., 2005). Indomethacin, a nonselective COX inhibitor, inhibited the impact of inflammation in both inflammatory models. It is likely that the activity of the examined extracts is due to the presence of flavonoids and phenolic components. The total flavonoid content of M. parviflora was high compared with the phenolic and proanthocyanidin contents (CitationAfolayan et al., 2008). These compounds have been shown to have an anti-inflammatory activity (CitationKüpeli et al., 2007).
The oxidative damage to cellular components is believed to be associated with the development of degenerative diseases including cardiovascular disorders, cancer, arthritis, immune system suppression, brain dysfunction, cataract, and others (CitationAruoma, 1998, CitationLee et al., 2004). The therapeutic effect of many plants and herbs is caused by the presence of antioxidant constituents such as flavonoids (CitationRice-Evans, 2004), phenolic acids, and phenolic diterpenes (CitationShahidi & Wanasundara, 1992). Our data suggest that both extracts of M. parviflora contained constituents that are good radical scavengers. Flavonoids, phenolic acids, tannins, and volatile oils have been isolated from Malva silvestris (CitationProestos et al., 2005; CitationCutillo et al., 2006). Moreover, oxidative damage also depends on the availability of free metal ions. Although these ions are largely sequestered in vivo, an enhanced level of ferrous ions might result from release of these ions from internal sources under inflammatory conditions (CitationCao et al., 1997). Leaves of M. parviflora contained considerable amount of water-soluble ligands that efficiently compete with ferrozine for chelation of ferrous ions. Iron chelators mobilize tissue iron by forming soluble, stable complexes that are then excreted in the feces and/or urine (CitationShinar and Rachmilewitz, 1990). Antioxidants capable of chelating Fe2+ will minimize the ion’s concentration and inhibit its capacity to catalyze free radical formation, resulting in protection against oxidative damage and related diseases (CitationWu et al., 2006). Chelation therapy reduces iron-related inflammatory diseases such as arteriosclerosis (CitationLamar, 1964) and Alzheimer’s disease (CitationReznichenko et al., 2006).
Conclusions
Our results suggest that the ability of M. parviflora leaf extracts to scavenge free radicals and chelate ions may be a mechanism underlying the anti-inflammatory activity of this plant. The health beneficial effect of M. parviflora leaf extracts is likely caused by a cocktail of substances contained in both Met. E and Aq. E. These substances exhibit both anti-inflammatory and antioxidant activities in various in vivo and in vitro models. Flavonoids and phenolic acids could be responsible for these activities. Further experiments are necessary to verify the relationship between chemical composition and these activities.
Acknowledgement
The authors are grateful to the Algerian Ministry of High Education for providing a research grant and fellowships (F01220100036).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Afolayan AJ, Aboyade OM, Adedapo AA, Sofidiya MO. (2010). Anti-inflammatory and analgesic activity of the methanol extract of Malva parviflora Linn (Malvaceae) in rats. Afr J Biotechnol, 9, 1225–1229.
- Afolayan AJ, Aboyade OM, Sofidiya MO. (2008). Total phenolic content and free radical scavenging activity of Malva parviflora L. (Malvaceae). J Biol Sci, 8, 945–949.
- Aruoma OI. (1998). Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc, 75, 199–212.
- Brand-Williams W, Cuvelier ME, Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol, 28, 25–30.
- Cao G, Prior RL, Cutler RG, Yu BP. (1997). Effect of dietary restriction on serum antioxidant capacity in rats. Arch Gerontol Geriatr, 25, 245–253.
- Cutillo F, D’Abrosca B, Dellagreca M, Fiorentino A, Zarrelli A. (2006). Terpenoids and phenol derivatives from Malva silvestris. Phytochemistry, 67, 481–485.
- Delaporte RH, Sarragiotto MH, Takemura OS, Sánchez GM, Filho BP, Nakamura CV. (2004). Evaluation of the antioedematogenic, free radical scavenging and antimicrobial activities of aerial parts of Tillandsia streptocarpa Baker-Bromeliaceae. J Ethnopharmacol, 95, 229–233.
- Draughon FA. (2004). Use of botanicals as biopreservatives in foods. Food Technol, 58, 20–28.
- Dutra RC, Leite MN, Barbosa NR. (2008). Quantification of phenolic constituents and antioxidant activity of Pterodon emarginatus vogel seeds. Int J Mol Sci, 9, 606–614.
- Grierson DS, Afolayan AJ. (1999). Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. J Ethnopharmacol, 66, 103–106.
- Hammer KA, Carson CF, Riley TV. (1999). Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol, 86, 985–990.
- Kou J, Si M, Dai G, Lin Y, Zhu D. (2006). Antiinflammatory activity of Polygala japonica extract. Fitoterapia, 77, 411–415.
- Küpeli E, Sahin FP, Calis I, Yesilada E, Ezer N. (2007). Phenolic compounds of Sideritis ozturkii and their in vivo anti-inflammatory and antinociceptive activities. J Ethnopharmacol, 112, 356–360.
- Lamar CP. (1964). Chelation therapy of occlusive arteriosclerosis in diabetic patients. Angiology, 15, 379–395.
- Le K, Chiu F, Ng K. (2007). Identification and quantification of antioxidants in Fructus lycii. Food Chem, 105, 353–363
- Lee J, Koo N, Min DB. (2004). Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp Rev Food Sci Food Safety, 3, 21–33.
- Manga HM, Brkic D, Marie DE, Quetin-Leclercq J. (2004). In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. (Euphorbiaceae). J Ethnopharmacol, 92, 209–214.
- Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O. (2007). Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol, 109, 219–225.
- Proestos C, Chorianopoulos N, Nychas GJ, Komaitis M. (2005). RP-HPLC analysis of the phenolic compounds of plant extracts. investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem, 53, 1190–1195.
- Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MB, Weinreb O, Mandel S. (2006). Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J Neurochem, 97, 527–536.
- Rice-Evans C. (2004). Flavonoids and isoflavones: Absorption, metabolism, and bioactivity. Free Radic Biol Med, 36, 827–828.
- Shahidi F, Wanasundara PK. (1992). Phenolic antioxidants. Crit Rev Food Sci Nutr, 32, 67–103.
- Shale TL, Stirk WA, van Staden J. (1999). Screening of medicinal plants used in Lesotho for anti-bacterial and anti-inflammatory activity. J Ethnopharmacol, 67, 347–354.
- Shale TL, Stirk WA, van Staden J. (2005). Variation in antibacterial and anti-inflammatory activity of different growth forms of Malva parviflora and evidence for synergism of the anti-inflammatory compounds. J Ethnopharmacol, 96, 325–330.
- Shinar E, Rachmilewitz EA. (1990). Oxidative denaturation of red blood cells in thalassemia. Semin Hematol, 27, 70–82.
- Wu C, Chen F, Wang X, Kim H, He G, Haley-Zitlin V, Huang G. (2006). Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem, 96, 220–227.
- Zhang L, Hu JJ, Lin JW, Fang WS, Du GH. (2009). Anti-inflammatory and analgesic effects of ethanol and aqueous extracts of Pterocephalus hookeri (C.B. Clarke) Höeck. J Ethnopharmacol, 123, 510–514.