Abstract
Context: Citronellal is a monoterpene present in the oil of many species, including Cymbopogon winterianus Jowitt (Poaceae).
Objective: The present study investigated the effect of citronellal on inflammatory nociception induced by different stimuli and examined the involvement of the NO–cGMP–ATP-sensitive K+ channel pathway.
Materials and methods: We used male Swiss mice (n = 6 per group) that were treated intraperitoneally with citronellal (25, 50 or 100 mg/kg) 0.5 h after the subplantar injection of 20 μl of carrageenan (CG; 300 µg/paw), tumor necrosis factor-α (TNF-α; 100 pg/paw), prostaglandin E2 (PGE2; 100 ng/paw) or dopamine (DA; 30 μg/paw). The mechanical nociception was evaluated at 0.5, 1, 2 and 3 h after the injection of the agents, using a digital analgesimeter (von Frey). The effects of citronellal were also evaluated in the presence of L-NAME (30 mg/kg) or glibenclamide (5 mg/kg).
Results: At all times, citronellal in all doses inhibited the development of mechanical nociception induced by CG (p < 0.001 and p < 0.01) and TNF-α (p < 0.001, p < 0.01, and p < 0.05). The citronellal was able to increase the pain threshold in the DA test (p < 0.001, p < 0.01, and p < 0.05) and in the PGE2 test at all times (p < 0.001 and p < 0.05). L-NAME and glibenclamide reversed the antinociceptive effects of the citronellal at higher doses in the PGE2 test.
Discussion and conclusion: These data suggest that citronellal attenuated mechanical nociception, mediated in part by the NO-cGMP--ATP-sensitive K+ channel pathway.
Introduction
The inflammatory response is an important cause of painful conditions resulting from tissue injury. This response is established with the participation of several mediators, such as neurotrophic factors, neuropeptides, prostanoids and kinins that are capable of carrying out and strengthening the nociceptive responses (Coutaux et al., Citation2005). Besides these mediators, it is well known that cytokines, produced by either immune or central nervous system (CNS) cells, can directly sensitize the peripheral nociceptors (Obreja et al., Citation2002).
Inflammatory hypernociception is characterized by the activation of a cytokine cascade that acts in a distinct sequence, but the final effect is indirect and mediated through the release of prostanoids and sympathomimetic amines by the direct action of agents such as PGE2 and sympathomimetic amines (DA) in primary nociceptores (Cunha et al., Citation2005). Pharmacological treatment is based on two strategies: the first is by inhibiting the formation of inflammatory agents such as NSAIDs (Cunha et al., 1992) and the second option is blockade-established hypernociception induced by PGE2, such as by the opiate drugs (Cunha et al., Citation2010). But unfortunately, the therapies currently available are associated with important side effects and low efficacy, especially for chronic diseases.
For this reason, many studies had been conducted in the search for new therapeutic options for treating painful conditions. In this context, it can include natural products (Bonjardim et al., Citation2011; Paixão et al., Citation2013). It can highlight the monoterpenos within the natural products, which are the main chemical constituents of the essential oils of plants with therapeutic properties (Edris, Citation2007; Guimarães et al., Citation2013; Silva et al., Citation2007). These molecules and their derivatives exhibit several types of pharmacological properties, such as anxiolytic (Silva et al., Citation2007), analgesic (Guimarães et al., Citation2010, Citation2013; Quintans-Júnior et al., Citation2011a, Citationb), anti-inflammatory (Guimarães et al., Citation2012), sedative (De Sousa et al., Citation2006a; Elisabetsky et al., Citation1995), antidepressant (De Sousa et al., Citation2006b), anticonvulsant (De Sousa et al., Citation2006c, Citation2007; Passos et al., Citation2009) and anti-inflammatory and antibacterial activities (Botelho et al., Citation2009).
Citronellal is the major component of the essential oil of Cymbopogon winterianus Jowitt (Poaceae) (Java citronella) and C. citrates (Lemongrass) (Lenardão et al., Citation2007). In this regard, preliminary pharmacological screening showed that essential oil obtained from C. winterianus (rich in the monoterpenes citronellal, citronellol and citral) demonstrates CNS depressant, anticonvulsant, hypotensive and antinociceptive activities in rodents (Quintans-Júnior et al., Citation2008). It was recently demonstrated by our group that citronellal has antinociceptive and anti-inflammatory effects, which were demonstrated by protocols of writhing induced by acetic acid, formalin, hot plate, and peritonitis; its action is probably mediated by the inhibition of PG synthesis as well as by central inhibitory mechanisms (opioid system; Melo et al., Citation2010, Citation2011). This antinociceptive effect probably occurs through peripheral and central mechanisms, but the exact mechanism of action involved remains to be elucidated.
For this reason, in this study we investigated the effect of citronellal on inflammatory nociception induced by different stimuli and examined the involvement of nitric oxide (NO)-cyclic GMP (cGMP)-ATP-sensitive K+ () channel pathway.
Materials and methods
Drugs and reagents
λ-CG, tumor necrosis factor-alpha (TNF-α), PGE2, DA, Tween 80 and L-NAME were purchased from Sigma (Saint Louis, MO); Indomethacin, dipyrone and glibenclamide were obtained from União Química (Fortaleza, Brazil); and citronellal (citronellal, 97% purity, Dierberger, São Paulo, Brazil). Tween 80 0.02% dissolved in a saline solution of 0.9% was used as vehicle to dilute the test drugs. In these protocols, the agents were injected intraperitoneally (ip) at volumes of 0.1 mL/10 g, except for the algesic chemicals: λ-CG, TNF-α, PGE2 and DA, which were injected subcutaneously into the plantar region of the hind paws. All doses and routes of administration of the citronellal were chosen according to Melo et al. (Citation2010) and Quintans-Júnior et al. (Citation2010).
Animals
Male Swiss mice (28–32 g), 2–3 months of age, were used in this study. They were housed in appropriate cages at 21 ± 2 °C on a 12 h light/dark cycle (lights on 06:00–18:00 h) with free access to food (Purina®, Brazil) and water. Experiments were carried out between 09:00 am and 14:00 pm in a quiet room. Nociceptive and inflammatory tests were carried out by the same visual observer and all efforts were made to minimize the number of animals used as well as minimize any discomfort. Experimental protocols were approved by the Animal Care and Use Committee (CEPA/UFS 12/08) at the Federal University of Sergipe and handling procedures were in accordance with the International Association for the Study of Pain guidelines for the use of animals in pain research (Zimmermann, Citation1983).
Measurement of mechanical nociception
Mechanical nociception was tested in mice, as reported by Cunha et al. (Citation2004). In a quiet room, mice were placed in acrylic cages (12 × 10 × 17 cm) with wire grid floors 15–30 min before the start of testing. The test consisted of evoking a hind paw flexion reflex with a hand-held force transducer (digital anesthesiometer, Insight®, São Paulo, Brazil) adapted with a polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hind paw with a gradual increase in pressure. The end point was characterized by the removal of the paw followed by clear flinching movements. After the paw withdrawal, the intensity of the pressure was automatically recorded. The animals were tested before the treatments with vehicle, citronellal or control drugs, and at selected times after the injection of the nociceptive agents. The protocol was carried out blindly, where the researcher who performed the measures did not know which group the animal belonged to. The results are presented as the Δ withdrawal threshold (g), calculated by the difference between the values obtained after the treatments and before the treatment (Cunha et al., Citation2004).
Nociception induced by CG, TNF-α, PGE2, or DA
This study was performed according Cunha et al. (Citation2004) and Villarreal et al. (Citation2009). Mice were divided into five groups (n = 6 per group), which were treated with 0.1 mL/10 g per vehicle (saline + Tween 80 0.02%), citronellal (25, 50, or 100 mg/kg, ip), or the positive control group treated with indomethacin (10 mg/kg; ip), or dipyrone (60 mg/kg; ip). Thirty min later, 20 μl of CG (300 µg/paw), TNF-α (100 pg/paw), PGE2 (100 ng/paw) or DA (30 μg/paw) was injected subcutaneously into the plantar region of the hind paws. The degree of mechanical nociception was evaluated 0.5, 1, 2 and 3 h after the injection of nociceptive agents.
Evaluation of the involvement of NO–cGMP– channels pathway on attenuation of mechanical nociception of citronellal
channels pathway on attenuation of mechanical nociception of citronellal
In order to investigate the mechanism of action of citronellal, the effects of this compound were evaluated in the presence of L-NAME (30 mg/kg, ip), a non-selective competitive inhibitor of NO synthases or glibenclamide (5 mg/kg, ip), an inhibitor of channels. These inhibitors were used to evaluate the participation of the NO–cGMP–
channels in the analgesic effects of citronellal, similar to the work by Nguelefack et al. (Citation2010). These antagonists or their respective vehicles were administered 30 min before citronellal (100 mg/kg, ip) or dipyrone (60 mg/kg, ip, positive control).
Statistical analysis
The data obtained were evaluated by the two-way analysis of variance (ANOVA) to compare the groups and doses at all times. If a significant interaction between the factors evaluated (treatment and time) was detected, one-way ANOVA followed by the Tukey test was performed each time in order to differentiate the effect of treatments. In all cases, differences were considered significant if p < 0.05. All statistical analyses were done using Graph Pad Prism 3.02 (Graph Pad Prism Software Inc., San Diego, CA).
Results
Effect of citronellal on the CG-induced mechanical nociception
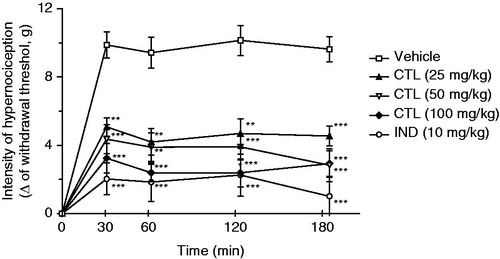
As expected, CG (300 µg/paw) increased the mechanical nociception when injected in the paws of mice. Treatment with citronellal (25, 50, or 100 mg/kg; ip), 30 min before CG administration, exhibited a significant (p < 0.001 and p < 0.01) reduction of the mechanical nociception induced by CG at all evaluated times, when compared with animals of the control group that received only vehicle (). However, this effect was not dose-dependent when compared to doses with each other.
Figure 1. Effect of acute administration of vehicle, citronellal (CTL 25, 50 or 100 mg/kg; ip) or indomethacin (IND; 10 mg/kg) on mechanical hypernociception induced by CG (300 µg/paw). Each point represents the mean ± SEM of the variation of paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. **p < 0.01, and ***p < 0.001 compared with the vehicle-treated group (two-way ANOVA and one-way ANOVA followed by the Tukey test).
Effect of citronellal on the TNF-α-induced mechanical nociception
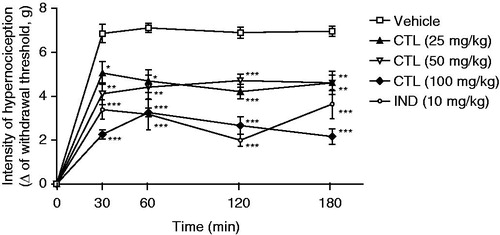
The inhibitory effect of citronellal on the mechanical nociception induced by TNF-α is shown in . Citronellal (25, 50, or 100 mg/kg) reduced significantly (p < 0.001, p < 0.01, and p < 0.05) mechanical nociception induced by TNF-α when compared with animals of the vehicle group ().
Figure 2. Effect of acute administration of vehicle, citronellal (CTL 25, 50 or 100 mg/kg; ip) or indomethacin (IND; 10 mg/kg) on mechanical hypernociception induced by TNF-α (100 pg/paw). Each point represents the mean ± SEM of the variation of paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with the vehicle-treated group (two-way ANOVA and one-way ANOVA followed by the Tukey test).
Effect of citronellal on the DA-induced mechanical nociception
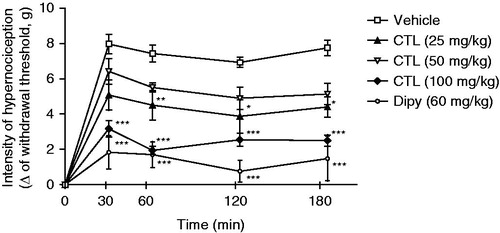
shows the inhibitory effect of citronellal on the mechanical nociception induced by DA. Citronellal at 100 mg/kg was able to reduce (p < 0.001) mechanical nociception induced by DA when compared with animals of the vehicle group (), similar to the treatment with dipyrone. The dose of 25 mg/kg of citronellal also resulted in significant reduction (p < 0.05 and p < 0.01) of mechanical nociception, and the same tendency, although not significant, was observed for the dose of 50 mg/kg of citronellal.
Figure 3. Effect of acute administration of vehicle, citronellal (CTL 25, 50 or 100 mg/kg; ip) or dipyrone on mechanical hypernociception induced by DA (30 µg/paw). Each point represents the mean ± SEM of the variation of paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the vehicle-treated group (two-way ANOVA and one-way ANOVA followed by the Tukey test).
Effects of citronellal on the PGE2-induced mice paw mechanical nociception
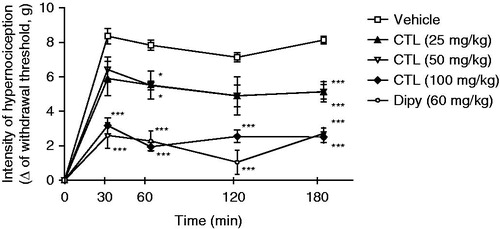
Intraplantar injection of PGE2 (100 ng/paw) induced a marked mechanical hypersensitivity. This nociception was significantly reduced (p < 0.001) by dipyrone (60 mg/kg; ip) and by citronellal at 100 mg/kg (). The groups treated with citronellal doses of 25 and 50 mg/kg also showed the same tendency to lower values, even though it was not significant at all time points evaluated, showing that citronellal has no effect dose dependency.
Figure 4. Effect of acute administration of vehicle, citronellal (CTL 25, 50 or 100 mg/kg; ip) or dipyrone on mechanical hypernociception induced by PGE2 (100 ng/paw). Each point represents the mean ± SEM of the variation of paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. *p < 0.05 and ***p < 0.001 compared with the vehicle-treated group (two-way ANOVA and one-way ANOVA followed by the Tukey test).
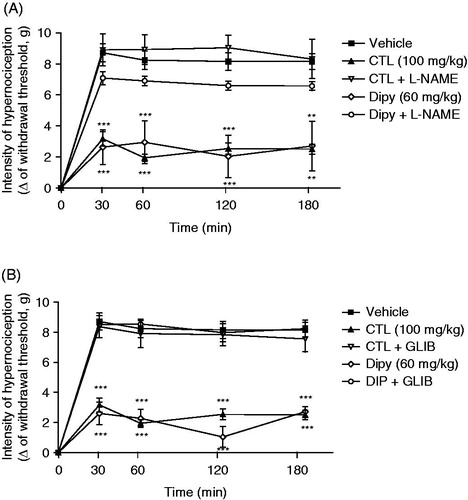
Evaluation of the involvement of NO–cGMP– channels pathway in attenuation of mechanical nociception effect of citronellal
channels pathway in attenuation of mechanical nociception effect of citronellal
In order to investigate the role of NO and channels in the attenuation of mechanical nociception induced by citronellal, groups of mice were pretreated with L-NAME (30 mg/kg) or glibenclamide (10 mg/kg). The pretreatment of animals with L-NAME reversed the effect of citronellal (100 mg/kg) or dipyrone (60 mg/kg) on PGE2-induced mechanical nociception (), as did the previous treatment with glibenclamide ().
Figure 5. Effect of acute administration of L-NAME (30 mg/kg; ip, panel A) or glibenclamide (5 mg/kg; ip, panel B) on the mechanical antinociceptive activities of citronellal (CTL 100 mg/kg; ip) or dipyrone (60 mg/kg; ip) in mechanical hypernociception induced by i.pl. injection of PGE2 (100 ng/paw). L-NAME was injected 30 min before the CTL or dipyrone and 30 min after PGE2. Each point represents the mean ± SEM of the paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. **p < 0.01 and ***p < 0.001 compared with vehicle-treated group (two-way ANOVA and one-way ANOVA followed by the Tukey test).
Discussion
Many compounds obtained from natural sources are able to modulate the inflammatory response by interfering in the production or action of some mediators important to this response (De Sousa et al., Citation2010; Silva, Citation2003). Citronellal, as a compound found in the essential oils of some plants, possesses a high potential to treat inflammatory and painful diseases. In this way, this study was undertaken to evaluate the attenuation of mechanical nociception effect of citronellal in models of acute inflammatory nociception in mice. In addition, we further evaluated some of the mechanisms underlining the effect of this compound.
Intraplantar injection of CG is largely known to produce inflammation and nociception through a cytokine cascade released by resident or migrating cells, mainly initiated by production of bradykinin (Verri et al., Citation2007). This cascade of signalization is believed to lead to the release of prostanoids and sympathomimetic amines (Cunha et al., Citation2005). CG-induced nociception is associated with non-immune reactions that produce inflammatory mediators, including arachidonic acid products (PGE2), mast cell products (histamine, serotonin), neuropeptides, cytokines, such as interleukin-1β (IL-1β), TNF-α, NO, nerve growth factor, leukotriene B4 and transcription factor activation as the nuclear factor-κB (Morris, Citation2003). In agreement with these evidences, in our study we observed that the i.p. administration of CG induced a nociceptive response characterized by the decrease of the intensity of the stimulus to cause a paw withdrawal behavior (expressed as variation of the intensity), as previously shown by other studies conducted by Cunha et al. (Citation2004) and Lima et al. (Citation2010).
Citronellal was able to reverse the response in the nociception induced by GC, suggesting a possible inhibitory effect on the inflammatory cascade, corroborating with the previous studies of Melo et al. (Citation2010), which confirmed the hypothesis of the citronellal involvement on the inhibition of PG synthesis can be the mechanism involved in the nociceptive process or by the release of arachidonic acid metabolites via cyclooxygenase and PG biosynthesis (Duarte et al., Citation1988). Quintans-Júnior et al. (Citation2011c) also described the ability of the citronellal in inhibit leukocyte mobilization into the peritoneal cavity. Both responses support the idea that citronellal could act on the inhibition of cycloxygenase.
Cytokine TNF-α is recognized as a potent pro-inflammatory endogenous substance, which is rapidly produced in large quantities by macrophages in response to inflammatory stimuli, such as bacterial infection (Brown et al., Citation2006). TNF-α interacts with target cells through high-affinity membrane receptors as TNF receptor Type 1 (TNFR1 or p55) and Type 2 (TNFR2 or p75) (Verri Jr et al., Citation2006). This mainly triggers the secretion of IL-1β (Cunha et al., Citation2008a), which induces the expression of COX-2 responsible for various prostanoid biosynthesis, such as PGE2 (Cunha et al., Citation1992). Thus, TNF-α-induced nociception is mediated by prostanoids and sympathetic amines (Cunha et al., Citation2005). We have demonstrated that this nociceptive response induced by the injection of TNF-α into mice paws was blocked by treatment with citronellal as observed with the treatment with indomethacin.
Additionally, we also demonstrated that citronellal inhibited the nociceptive effect induced by PGE2 and DA, suggesting that its action is not only at the inflammatory level, but also points out the involvement of neuronal pathways. In fact, PGE2 and DA mainly activate receptors present in nociceptor membranes, EP2 and D1, respectively, triggering their sensitization (Cunha et al., Citation1992). Therefore, nociception elicited by administration of PGE2 and DA is independent of the production of other inflammatory mediators or recruitment of cells, such as neutrophils (Cunha et al., Citation2008b). Hence, we cannot exclude the possibility that citronellal may interfere with one of the above receptors as well as other types of EP or dopamine receptors, and consequently their pathways to induce the antinociceptive activity.
Evidence points out that NO--cGMP pathway is important for the antinociceptive effect of many drugs (Hernández-Pacheco et al., Citation2008; Rodrigues & Duarte, Citation2000). The increase of cGMP via the guanylyl cyclase enzyme activation mediated by NO leads to an opening of potassium channels, and in this way the cell hyperpolarizes which in turn induces analgesia (Rodrigues & Duarte, Citation2000). Thus, NO production is known to reduce hyperalgesia due to the activation of this pathway, and this mechanism has been demonstrated for analgesics, such as morphine and dipyrone (Sachs et al., Citation2004).
In this study, we evaluated whether the effects of citronellal involve NO--cGMP-- channel pathway activation through the administration of L-NAME, an inhibitor of NO synthases and glibenclamide, a blocker of
channels. Both treatments reversed the attenuation of mechanical nociception effects of citronellal, indicating that this signaling pathway is involved in the actions of this compound. These drugs also affected the response of dipyrone, corroborating with previous findings obtained by Alves and Duarte (Citation2002) who demonstrated NO--cGMP--
channel pathway activation as the possible mechanism of action of dipyrone.
As is well known, peripheral or central opioid receptor activation could lead to the decrease of the pain sensation to an inflammatory stimulus, and NO--cGMP-- channel pathway activation is involved in this effect, as demonstrated for morphine (Sachs et al., Citation2004). Additionally, it was demonstrated that citronellal inhibited the formalin-induced nociceptive response in the orofacial region that was reversed by the opioid antagonist naloxone (Quintans-Júnior et al., Citation2011c), suggesting that the opioid system may also contribute, at least partly, to the effects observed with the administration of citronellal in mice during this study.
Another potential mechanism for citronellal action that cannot be ruled out is the involvement of the glutamatergic system. The activation of glutamate receptors (e.g., NMDA) can stimulate the production of a variety of intracellular secondary messengers, such as NO, and pro-inflammatory cytokines, such as TNF-α and IL-1β, which act synergistically in the excitation of neurons (Ribas et al., Citation2008). As demonstrated by the study of Quintans-Júnior et al. (Citation2010), citronellal inhibited the nociception induced by glutamate and induced partial blockade of the voltage-dependent Na+ channels that could influence the observed antinociceptive response of citronellal in the experimental models used in this study, which may contribute to the attenuation of mechanical nociception observed in our study. Studies suggested that CNS depression and non-specific muscle relaxation effect can reduce the motor coordination response and might invalidate the results of the behavior tests (Almeida et al., Citation2004; De Sousa et al., Citation2006a,Citationb). However, the previous study of our group (Quintans-Júnior et al., Citation2010, Citation2011c) showed that citronellal, at these doses, did not cause any performance alteration in the rota-rod test, validating our results.
Conclusions
In summary, the data reported in this work suggest that acute administration of citronellal has an important attenuation of mechanical nociception property, probably involving activation of the NO--cGMP-- channel pathway. Thus, citronellal may be an interesting candidate for the development of new therapeutic options for the treatment of painful conditions associated with inflammation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by grants from the National Council of Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq/Brazil [grant number 305783/2010-6 and 470774/2011-8]) and the Research Supporting Foundation of State of Sergipe (Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe/FAPITEC-SE/Brazil [grant number 019.203.00860/2009-6]).
References
- Almeida RN, Motta SC, Faturi CB, et al. (2004). Anxiolytic-like effects of rose oil inhalation on the elevated plus-maze test in rats. Pharmacol Biochem Behav 77:361–4
- Alves D, Duarte I. (2002). Involvement of ATP-sensitive K (+) channels in the peripheral antinociceptive effect induced by dipyrone. Eur J Pharmacol 444:47–52
- Bonjardim LR, Silva AM, Oliveira MGB, et al. (2011). Sida cordifolia leaf extract reduces the orofacial nociceptive response in mice. Phytother Res 25:1236–41
- Botelho MA, Martins JG, Ruela RS, et al. (2009). Protective effect of locally applied carvacrol gel on ligature-induced periodontitis in rats: A tapping mode AFM study. Phytother Res 23:1439–48
- Brown KA, Brain SD, Edgeworth JD, et al. (2006). Neutrophils in development of multiple organ failure in sepsis. Lancet 368:157–69
- Coutaux A, Adam F, Willer JC, Le Bars D. (2005). Hyperalgesia and allodynia: Peripheral mechanisms. Joint Bone Spine 72:359–71
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. (1992). The pivotal role of tumor necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107:660–4
- Cunha TM, Verri WA Jr, Vivancos GG, et al. (2004). An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37:401–7
- Cunha TM, Verri WA Jr, Silva JS, et al. (2005). A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Nat Acad Sci USA 102:1755–60
- Cunha TM, Poole S, Ferreira SH, Cunha FQ. (2008a). Role of cytokines in mediating mechanical hypernociception in a model of delayed-type hypersensitivity in mice. Eur J Pain 12:1059–68
- Cunha TM, Verri WA Jr, Schivo IR, et al. (2008b). Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83:824–32
- Cunha TM, Roman-Campos D, Lotufo CM, et al. (2010). Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/K+ATP signaling pathway. Proc Nat Acad Sci USA 107:4442–7
- De Sousa DP, Oliveira FS, Almeida RN. (2006a). Evaluation of the central activity of hydroxydihydrocarvone. Biol Pharm Bull 29:811–12
- De Sousa DP, Gonçalves JCR, Quintans-Júnior LJ, et al. (2006b). Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett 401:231–5
- De Sousa DP, Schefer RR, Brocksom U, Brocksom TJ. (2006c). Synthesis and antidepressant evaluation of three parabenzoquinone mono-oximes and their oxy derivatives. Molecules 11:148–55
- De Sousa DP, Quintans-Júnior LJ, Almeida RN. (2007). Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol 45:69–70
- De Sousa DP, Camargo EA, Oliveira FS, Almeida RN. (2010). Anti-inflammatory activity of hydroxydihydrocarvone. Z Naturforsch C 65:543–50
- Duarte ID, Nakamura M, Ferreira SH. (1988). Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21:341–3
- Edris AE. (2007). Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21:308–23
- Elisabetsky E, Coelho-De-Souza GP, Santos MAC, et al. (1995). Sedative properties of linalool. Fitoterapia 66:407–14
- Guimarães AG, Oliveira GF, Melo MS, et al. (2010). Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin Pharmacol Toxicol 107:949–57
- Guimarães AG, Xavier MA, Santana MT, et al. (2012). Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn-Schmiedeberg's Arch Pharmacol 385:253–63
- Guimarães AG, Quintans JSS, Quintans-Júnior LJ. (2013). Monoterpenes with analgesic activity: A systematic review. Phytother Res 27:1–15
- Hernández-Pacheco A, Araiza-Saldaña CI, Granados-Soto V, Mixcoatl-Zecuat T. (2008). Possible participation of the nitric oxide-cyclic GMP-protein kinase G K+ channels pathway in the peripheral antinociception of melatonin. Eur J Pharmacol 596:70–6
- Lenardão EJ, Botteselle GV, Azambuja F, et al. (2007). Citronellal as key compound in organic synthesis. Tetrahedron 63:6671–712
- Lima FQ, Souza GR, Verri WA Jr, et al. (2010). Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: Involvement of the NO/cGMP/PKG/K+ATP signaling pathway. Pain 151:506–15
- Melo MS, Sena LCS, Barreto FJN, et al. (2010). Antinociceptive effect of citronellal in mice. Pharm Biol 48:411–16
- Melo MS, Guimarães AG, Santana MF, et al. (2011). Anti-inflammatory and redox-protective activities of citronellal. Biol Res 44:363–8
- Morris CJ. (2003). Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225:115–21
- Nguelefack TB, Dutra RC, Paszcuk AF, et al. (2010). Antinociceptive activities of the methanol extract of the bulbs of Dioscorea bulbifera L. var sativa in mice is dependent of NO--cGMP--ATP-sensitive-K(+) channel activation. J Ethnopharmacol 128:567–74
- Obreja O, Rathee PK, Lips KS, et al. (2002). IL-1 beta potentiates heat-activated currents in rat sensory neurons: Involvement of IL-1RI, tyrosine kinase and protein kinase C. FASEB J 16:1497–503
- Passos CS, Arbo MD, Rates SMK, Poser GLV. (2009). Terpenoids with activity in the central nervous system (CNS). Braz J Pharmacogn 19:140–9
- Paixão MS, Melo MS, Oliveira MG, et al. (2013). Hyptis pectinata: Redox protection and orofacial antinociception. Phytother Res DOI: 10.1002/ptr.4869
- Quintans-Júnior LJ, Souza TT, Leite BS, et al. (2008). Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 15:619–24
- Quintans-Júnior LJ, Melo MS, De Sousa DP, et al. (2010). Antinociceptive activity of citronellal in formalin-, capsaicin- and glutamate-induced orofacial pain in rodents and its action on nerve excitability. J Orofac Pain 24:305–12
- Quintans-Júnior LJ, Oliveira MG, Santana MF, et al. (2011a). α-Terpineol reduces nociceptive behavior in mice. Pharm Biol 49:583–6
- Quintans-Júnior LJ, Guimarães AG, Santana MT, et al. (2011b). Citral reduces nociceptive and inflammatory response in rodents. Braz J Pharmacog 21:497–502
- Quintans-Júnior LJ, Rocha RF, Ceregnato FF, et al. (2011c). Antinociceptive action and redox properties of citronellal, an essential oil present in lemongrass. J Med Food 14:630–9
- Ribas CM, Meotti FC, Nascimento FP, et al. (2008). Antinociceptive effect of the Polygala sabulosa hydroalcoholic extract in mice: Evidence for the involvement of glutamatergic receptors and cytokine pathways. Basic Clin Pharmacol Toxicol 103:43–7
- Rodrigues ARA, Duarte IDG. (2000). The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K+ channels. Br J Pharmacol 129:110–14
- Sachs D, Cunha FQ, Ferreira SH. (2004). Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA 101:3680–5
- Silva J. (2003). Analgesic and anti-inflammatory effects of essential oils of Eucaliptus. J Ethnopharmacol 89:277–83
- Silva MIG, Neto MRA, Neto PFT, et al. (2007). Central nervous system activity of acute administration of isopulegol. Pharmacol Bio Chem Behav 88:141–7
- Verri AW Jr, Cunha TM, Parada CA, et al. (2006). Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol Therap 112:116–38
- Verri WA Jr, Cunha TM, Poole S, et al. (2007). Cytokine inhibitors and pain control. Braz J Rheumatol 47:341–53
- Villarreal CF, Funez MI, Figueiredo F, et al. (2009). Acute and persistent nociceptive paw sensitisation in mice: The involvement of distinct signalling pathways. Life Sci 85:822–9
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10
