Abstract
Context. Brassica juncea (BJ; Linnaeus) Czern & Coss (Brassicaceae), commonly known as Indian mustard, are enriched in redox-active polyphenols with antidiabetic activities. Diverse other health benefits of this edible plant have been described in classical Ayurvedic texts.
Objective: The reported experiments were designed to assess therapeutic potential of a methanol extract of BJ leaves for treatment of cognitive disorders associated with diabetes or caused by central cholinergic dysfunctions.
Materials and methods: Elevated plus-maze and active- and passive-avoidance tests were used to assess anti-amnesic potentials of BJ (100, 200 and 400 mg/kg/day, p.o., for 10 days) in alloxan diabetic or scopolamine-challenged rats. Treatment effects on brain acetylcholinesterase (AChE), superoxide dismutase (SOD) and catalase (CAT) activities were quantified in behavioral tested animals.
Results: Anti-amnesic efficacy of all three tested BJ doses against scopolamine-induced amnesia was almost equal in all behavioral tests. Such efficacy of the extract in diabetic rats was increased always with its increasing doses. All treatments of BJ dose dependently decreased the elevated level of AChE, and significantly increased the SOD and CAT levels in brain homogenates of scopolamine-challenged and diabetic rats. Minimal effective oral daily doses of BJ in all tests were 100 mg/kg/day for 10 consecutive days.
Discussion and conclusion: Our observation indicates that BJ could be a therapeutic option for treatment of cognitive disorders associated with diabetes, or caused by cholinergic deficit and brain oxidative status. They also indicate that the bioactive constituents or mode of actions involved in observed effects of the extract in scopolamine-challenged or diabetic rats are most probably not the same.
Introduction
Diabetes mellitus is an important risk factor in mild cognitive impairment and late onset Alzheimer disease (Cheng et al., Citation2011; Luchsinger et al., Citation2007; Strachan et al., Citation2008). Available therapy for diabetes is generally associated with an increased risk of severe hypoglycemia, neuroglycopenic consequences, and other side effects (Cryer, Citation2007; Holemans et al., Citation2001). Epidemiological studies have reconfirmed that consumption of higher amounts of vegetables and fruits could have beneficial effects against the spread of diabetes and dementia (Carter et al., Citation2010; Hughes et al., Citation2010). Brassica juncea (BJ, Linnaeus) Czern & Coss (Brassicaceae), commonly known as Indian mustard, is an agriculturally important plant commonly used for production of edible mustard oil and other condiments. Diverse medicinal uses of this plant are well known to Ayurvedic medical practitioners (Manohar et al., Citation2009), and broad spectra of therapeutically interesting bioactivities of BJ seed and leaf are also known (Kumar et al., Citation2011). Edible green BJ leaves (mustard green) are consumed as vegetables and salad in India and many other countries. Mustard leaves are rich not only in vitamins, but also in numerous polyphenolics and other structurally diverse phytochemicals with medicinally interesting bio-activities (Bouayed, Citation2010; Kumar & Andy, Citation2012). Critical analysis of available information on bioactive phytochemicals of BJ leaves led us to speculate that they could also be useful for combating diverse mental health problems often associated with diabetes and other metabolic disorders (Bouayed, Citation2010; Kumar & Andy, Citation2012; Kumar et al., Citation2011, Citation2012). This speculative working hypothesis is in agreement with our recent observations revealing antihyperglycemic, anxiolytic-like (Thakur et al., Citation2013) and antidepressant activities of a methanol BJ leaf extract in alloxan-diabetic rodents (under publication). Since co-morbidities of anxiety, depression and dementia are often encountered in diabetic as well as in other patients suffering from diverse chronic non-communicable diseases, it was of interest to tests the possible effects of BJ on cognitive functions. Results of the very first set of experiments to verify such possibilities are described and discussed in this communication.
Materials and methods
Plant material, extraction and analysis
BJ leaves were collected during November, from a local agricultural area in Varanasi (U.P., India). Botanical authentication was done by Prof. N. K. Dubey in Herbarium of Department of Botany, Banaras Hindu University (Voucher specimen number: Dubey 12/Nov/2009). Leaves were dried at room temperature, powdered and exhaustively extracted with 90% v/v methanol (ratio 90:10, MeOH:H2O) until the extraction solvent became colorless. The total filtrate was concentrated and dried in vacuum dryer at 40 °C. A well-validated high-performance liquid chromatography (HPLC) method was used for characterization of phyto-constituents in the extract (Chandrasekaran et al., Citation2009). Briefly, a Shimadzu LC 2010HT HPLC system equipped with a quaternary pump, UV detector, degasser and an auto sampler with “Lab solution” software was used. Mobile phase was acetonitrile: phosphate buffer (pH 2.5–2.8). Column was C18-ODS (octadecyl silane) 5 µm size, 250 × 4.6 mm, wavelength: 280 nm, flow rate: 1.5 ml/min, injection volume: 20 µl, and run time was 40 min. All reference standards used for HPLC analysis were purchased from Sigma, Bangalore, India. A spectrophotometric method was used for quantifying sinapic acid in the tested extract (Malgorzata & Aleksander, Citation2010).
Animals
Adult Charles Foster rats (160 ± 20 g) of both sexes were obtained from Central Animal House of Institute of Medical Sciences, Banaras Hindu University, Varanasi. They were housed in groups of six in polypropylene cages at an ambient temperature of 25 ± 1 °C and 45–55% relative humidity, with a 12:12 h light/dark cycle. Except as stated otherwise, animals were provided with commercial food pellets and water ad libitum. All animals were acclimatized to laboratory conditions for at least 1 week before using them for the experiments. Principles of laboratory animal care (NIH publication 85–23, revised in 1985) guidelines were followed. Prior approval (Dean/10-11/283 dated 19.10.2010) from the Central Animal Ethical Committee of the University was obtained.
Diabetes induction
Diabetes mellitus was induced in overnight fasted rats by a single i.p. injection of alloxan monohydrate (120 mg/kg; Sigma, St. Louis, MO) in normal saline. On the 3rd and 7th days after the alloxan challenge, hyperglycemia was confirmed by fasting blood glucose level measurement using a blood glucose estimation kit (Beacon Diagnostic Pvt. Ltd, India). Preselected hyperglycemic rats (fasting blood glucose levels >250 mg/dl on 7th day after alloxan challenge) were used for the experiments.
Animal grouping and drug administration
Ten experimental groups consisting of three males and three females each were used in behavioral studied. They were: groups i and ii, nondiabetic control treated with vehicle; group iii, BJ treated (100 mg/kg/day) nondiabetic; group iv, BJ (200 mg/kg/day) nondiabetic; group v, BJ (400 mg/kg/day) nondiabetic; group vi, vehicle-treated diabetic control; group vii, BJ (100 mg/kg/day) treated diabetic; group viii, BJ (200 mg/kg/day) treated diabetic; group ix, bj (400 mg/kg/day) diabetic and group x, piracetam (100 mg/kg/day) treated diabetic. Drug treatments were started on the 7th day after induction of diabetes (day 1 of treatment) for 10 consecutive days in the form of suspension in 0.3% carboxymethyl cellulose (CMC). Dose levels of BJ were selected based on our pilot study and previous reported study on BJ (Rahmatullah et al., Citation2010). Scopolamine (1 mg/kg; Sigma, St. Louis, MO) was subcutaneously administered only once in nondiabetic rats (group ii–v) on the 2nd day of the experiment (immediately after the training session on this day) to induce amnesia. All behavioral experiments were conducted after 1 h of BJ, piracetam, or the vehicle treatments.
Modified elevated plus maze test
This learning and memory test was conducted according to the procedure standardized and routinely used in our laboratories (Itoh et al., Citation1990; Kumar et al., Citation2000). Transfer latencies (TL) were observed on day 2, day 3 and on the last treatment day (transfer latency on day 10) by an observer in blind manner as per protocol.
Passive avoidance test
This test for amnesia was originally developed by King and Glasser (Citation1970), and has been well standardized in our laboratories. Details of the apparatus and experimental procedures used for this test have been published elsewhere (Kumar et al., Citation2000). According to the protocol, latencies to step through to the dark chamber were observed (in second) on training day (day 2), on the following day (24 h retention interval), and on day 10 (i.e., after a gap of one week) by blinded observer.
Active avoidance test
Detailed descriptions of the apparatus and experimental procedure used have been reported elsewhere (Kumar et al., Citation2000; Spignoli & Pepeu, Citation1986). Like in other behavioral tests used in this study, the rats were subjected to the training schedule on day 2 of experiments, and were retested for their performances in the avoidance test box on the next day and on the day 10.
Acetylcholinesterase activity
After the active avoidance test, rats were decapitated and their brains was dissected out, frozen and stored at −70 °C until analysis. The whole brain acetylcholinesterase (AChE) activity was quantified in 20% of brain homogenate in phosphate buffer (0.1 M; pH 8) by the method described by Ellman et al. (Citation1961). The rate of formation of thiocholine from acetyl(thio)choline iodide in the presence of brain cholinesterase was measured in duplicate for 3 min at regular intervals of 30 s by using microplate absorbance reader (iMark-Bio-Rad Laboratories, Hercules, CA). Protein content of brain homogenates was quantified by the Lowry et al. (Citation1951) method. Brain AChE activity is expressed as µmole substrate hydrolyzed/min/mg/protein.
Superoxide dismutase and catalase activity
After active avoidance test, the rats were sacrificed and their brains were stored at −70 °C until analysis. Superoxide dismutase (SOD) and catalase (CAT) activities were quantified in the supernatants of brain homogenates in duplicate by using microplate absorbance reader (iMark-Bio-Rad Laboratories, Hercules, CA). For brain SOD activity, the method described by Kakkar et al. (Citation1984) was followed, and these results were expressed as units of SOD activity/mg protein. For CAT activities the standard method described elsewhere was used (Luck, Citation1963). CAT activities are expressed as µmol H2O2 decomposed/min/mg protein.
Statistical analysis
Mean ± standard errors of mean (SEM) were calculated for the observed values in each experimental group. Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Student–Newman–Keuls multiple comparison test. GraphPad Prism 5 was used for statistical analysis. A p value less than 0.05 were considered as statistically significant.
Results
Plant extract and phytochemical testing
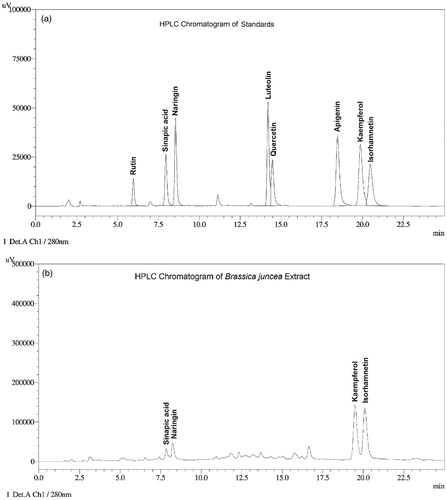
Yield of the BJ leaves extract was 11% of the weight of dried leaves. Quantitatively BJ was found to contain kaempferol 0.37% w/w and isorhamnetin 0.29% w/w of total flavanoids. Representative HPLC chromatograms of a standard mixture of polyphenols and that of a hydrolyzed BJ sample are shown in . Sinapic acid content of BJ was 5.0% (w/w) of total phenolics.
Modified elevated plus maze test
Mean TL of all nondiabetic groups on day 2 (i.e., on learning day) were not statistically different from each other, whereas those of the vehicle treated diabetes group was significantly higher than that of the vehicle treated nondiabetic group. The TLs of all BJ treated diabetic groups on this day were significantly lower than that of the diabetic control group. This effect of extract treatment was dose dependent. Qualitatively the observed effect of piracetam (100 mg/kg/day) treatment was higher than that of the highest dose of BJ tested ().
Figure 2. Effect of BJ in transfer latency test on elevated plus maze in scopolamine amnesia model and alloxan induced diabetic rats. *p < 0.05 versus nondiabetic control; ¥p < 0.05 versus scopolamine control; $p < 0.05 versus diabetic control; ap < 0.05 versus the values of the same group on day 2.
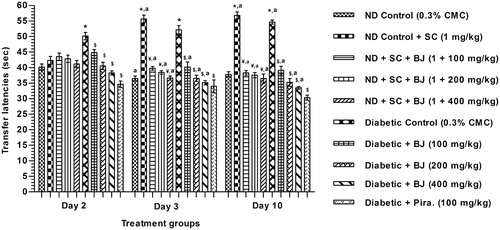
On day 3 (i.e., on the first retention test day), mean TLs of the nondiabetic control group was significantly lower than that of the same group on the learning day. Opposite was the case for the nondiabetic control group challenged with scopolamine after the training session. This amnesic effect of scopolamine were absent in all the three BJ treated nondiabetic groups, as the TLs of these BJ groups were significantly lower than those of the same control group on the learning day (day 2). TL of the diabetic group remained elevated on day 3, whereas those of the BJ treated diabetic ones were further lowered on day 3 (as compared to day 2). The effect of piracetam (100 mg/kg/day) in diabetic rats on this day was qualitatively similar that of the highest dose of BJ tested (400 mg/kg/day).
In comparison to the day 2 TL values, those of the scopolamine induced nondiabetic control, and vehicle-treated diabetic groups on day 10 were higher. Opposite were the cases for all BJ treated and the piracetam treated groups. Taken together, these observations clearly reveal that learning deficits in diabetic animals, as well as amnesic effects of scopolamine treatment are completely antagonized even by the lowest oral BJ dose (100 mg/kg/day) tested.
Passive avoidance test
On the first training day (i.e., on day 2 of the experiment) the mean step through latencies of all nondiabetic groups did not significantly differ from each other, whereas that of the diabetic control group was significantly lower than that of the nondiabetic control group. This hyperglycemia associated deficit was quantitatively less severe in all BJ treated and the piracetam treated diabetic groups. The mean step through latencies of diabetic control group remained lower on the subsequent retest days 3 and 10. However, unlike in the diabetic control group the mean TL of the BJ, or piracetam, treated diabetic groups increased further on these 2 days. These results clearly reveal piracetam-like beneficial effects of BJ on performance deficits caused by of alloxan-induced diabetes ().
Figure 3. Effect of BJ extract on passive avoidance retention deficits in scopolamine induced amnesia model and alloxan induced diabetic rats. *p < 0.05 versus nondiabetic control; ¥p < 0.05 versus scopolamine control; $p < 0.05 versus diabetic control; ap < 0.05 versus the values of the same group on day 2.
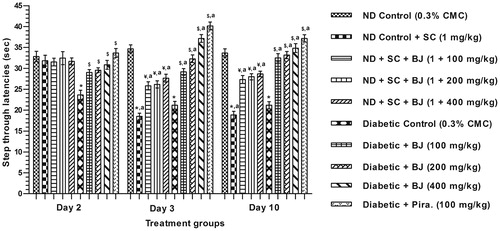
Results also reveal that the amnesic effect of scopolamine is not as severe in the BJ treated nondiabetic groups. As compared to the mean step through latencies of the scopolamine challenge nondiabetic control group on days 3 and 10, those of the BJ treated nondiabetic ones on these test days were much higher. This anti-amnesic effect of BJ on both these days was highest after its lowest dose tested, and did not increase further with its increasing doses or treatment days.
Active avoidance test
Performance deficit of diabetic control animals were apparent already on day 2 of the experiment, and this deficit persisted (or deteriorated a little) on days 3 and 10. Such deficits were less severe in the BJ or piracetam treated diabetic groups, and piracetam-like beneficial effects of BJ in diabetic rats were already apparent after its lowest dose tested, i.e., 100 mg/kg/day. This efficacy of BJ was only marginally increased with its increasing doses and days of treatments ().
Figure 4. Effect of BJ extract on active avoidance test namely: (a) shocked trials (b) total trials and (c) total time in scopolamine-induced amnesia model and in alloxan-induced diabetic rats. *p < 0.05 versus nondiabetic control; ¥p < 0.05 versus scopolamine control; $p < 0.05 versus diabetic control; ap < 0.05 versus the values of the same group on day 2.
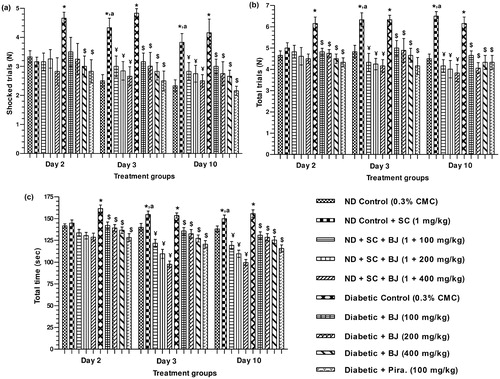
As judged by all parameters quantified, no significant differences in the performances of the nondiabetic groups were observed on day 2, i.e., the learning day. However, on days 3 and 10, the performance of the scopolamine challenged nondiabetic control group deteriorated considerable. Such deteriorations were not observed in the BJ treated scopolamine challenged groups, and again the observed effects of the extract treatment did not considerably increase with its increasing doses or days of treatments. These observations suggest that its 100 mg/kg/day doses of BJ are high enough for obtaining its cognitive benefits.
AChE, SOD and CAT enzyme activity
In comparison to that observed in nondiabetic controls, AChE activity in the brain homogenates of scopolamine challenged, or diabetic animals were significantly higher. Such elevations of AChE levels were less severe in BJ treated groups, and magnitude of these effects of extract treatment increased with its increasing daily doses ().
Table 1. Effect of BJ on AChE enzymes, SOD and CAT activity in scopolamine-induced nondiabetic amnesic and alloxan-induced diabetic rats.
SOD and CAT activities in brain homogenates of scopolamine challenged or alloxan-induced diabetic animals were significantly lower than those of the vehicle treated nondiabetic controls. Such reductions in the activities of both the antioxidative enzymes were not as severe in the BJ treated groups. Observed effects of BJ in both, scopolamine challenged and diabetic animals were dose dependent. Both SOD and CAT activities of the brain homogenates of all BJ treated groups were significantly higher than the corresponding vehicle treated control groups ().
Discussion
Broad spectra of pathologies accompany diabetes mellitus, and many of them can be studied in animal models (Biessels & Gispen, Citation2005). The fact that deregulated oxidative process involving CAT and SOD accompanying cognitive psychopathologies in alloxan-diabetic rats is well established (Ceretta et al., Citation2012). Clear dose-dependent reversal of the reduced activities of both these enzymes in brains of cognitively deficient alloxan-diabetic and scopolamine challenged rats were observed in BJ treated animals. These effects of BJ treatments were accompanied with behavioral improvements in all the three behavioral tests used in this study for assessing cognitive functions. Daily oral BJ doses of 100 mg/kg/day were high enough for partially compensating all quantified behavioral deficits in alloxan-diabetic as well as in scopolamine challenged rats. It was interesting to note that efficacy of BJ treatment in scopolamine-challenged rats did not increase much with its increasing daily doses, whereas such were not the cases in diabetic animals. These observations suggest that either the pathological mechanisms involved in cognitive deficits of diabetic and scopolamine challenged animals are not identical or that cognitive changes caused by deregulated brain oxidative system in alloxan diabetic and scopolamine challenged are differentially modulated by BJ extract treatments.
Dysregulation of oxidative processes in alloxan-diabetic rats are brain region specific (Ceretta et al., Citation2012), and such pathologies influence not only brain functions, but also diverse other biological processes involved in hyperglycemia and other central as well as peripheral pathologies (Maritim et al., Citation2003). Scopolamine, on the other hand, is a centrally acting agent affecting cognitive functions by its inhibitory effects on muscarinic receptors. Our observations reveal that long lasting cognitive disturbances observed in scopolamine challenged rats accompany reduced SOD and CAT activities and increased AChE activities in their brains. Since BJ treatments antagonize scopolamine-induced amnesia as well as reverse the effects of the anti-muscarinic agent, it could as well be that beneficial effects of the extract are due to elevated functioning of the central muscarinic receptors. Earlier observations in our laboratories have revealed that although BJ treatments dose dependently increases brain dopamine levels in nondiabetic rats, it does not alter brain norepinephrine and serotonin contents (under publication). Thus, it seems reasonable to assume that BJ treatments alter that processes and mechanisms involved in central dopaminergic–cholinergic interaction dictating cognitive and other functions of the central nervous system (CNS).
Screening experiments described in this communication on CNS function modulating potentials of BJ were conducted under our newly initiated project directed toward defining psychopharmacological activity profiles of Ayurvedic plants. For such purposes, a medicinal phytochemistry-based holistic pharmacological strategy is used in our laboratories (Chatterjee & Kumar, Citation2012). The observations reported in this article not only point out the usefulness of this strategy for identifying novel therapeutic possibilities offered by vegetables and fruits but also add further experimental evidence indicating that BJ leaves could be useful for combating mental health problems commonly associated with diabetes and other chronic diseases (Bouayed, Citation2010; Kumar et al., Citation2011). However, further efforts to identify the bioactive principles of BJ and their modes of action(s) will be necessary for understanding the psychopharmacological benefits offered by BJ leaves.
Conclusion
Regular consumption of BJ leaves as vegetables could be a dietary alternative for combating cognitive disorders often encountered in diabetic patients. Modulation of central oxidative mechanisms and cholinergic processes could be involved in the observed cognitive function improving effects of the tested extract of dried BJ leaves.
Declaration of interest
Ajit Kumar Thakur is grateful to the University Grants Commission, New Delhi, India for providing the financial assistance. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors acknowledge analytical support from Natural Remedies Pvt. Ltd., Bangalore, for characterization of BJ leaves extract.
References
- Biessels GJ, Gispen WH. (2005). The impact of diabetes on cognition: What can be learned from rodent models? Neurobiol Aging 26:S36–41.
- Bouayed J. (2010). Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci 6:13–18
- Carter P, Gray LJ, Troughton J, et al. (2010). Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. Br Med J 341:c4229. doi: 10.1136/bmj.c4229
- Ceretta LB, Reus GZ, Abelaira HM, et al. (2012). Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp Diabetes Res 2012:302682. doi: 10.1155/2012/302682
- Chandrasekaran CV, Thiyagarajan P, Sundarajan K, et al. (2009). Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmColdTM). Food Chem Toxicol 47:1892–902
- Chatterjee SS, Kumar V. (2012). Holistic psychopharmacology and promiscuous plants and principles of ayurveda. Am J Plant Sci 3:1015–21
- Cheng D, Noble J, Tang MX, et al. (2011). Type 2 diabetes and late-onset Alzheimer's disease. Dement Geriatr Cogn Disord 31:424–30
- Cryer PE. (2007). Hypoglycemia, functional brain failure, and brain death. J Clin Invest 117:868–70
- Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Holemans X, Dupuis M, Misson N, Vanderijst JF. (2001). Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med 18:761–3
- Hughes TF, Andel R, Small BJ, et al. (2010). Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish twins. Am J Geriatr Psychiatry 18:413–20
- Itoh J, Nabeshima T, Kameyama T. (1990). Utility of an elevated plus maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 101:27–33
- Kakkar P, Das B, Viswanathan PN. (1984). A modified spectrophotometric assay of super oxide dismutase. Indian J Biochem Biophys 21:130–2
- King RA, Glasser RL. (1970). Duration of electroconvulsive shock-induced retrograde amnesia in rats. Physiol Behav 5:335–9
- Kumar S, Andy A. (2012). Health promoting bioactive phytochemicals from Brassica. Int Food Res J 19:141–52
- Kumar V, Singh PN, Muruganandam AV, Bhattacharya SK. (2000). Effect of Indian Hypericum perforatum Linn. on animal models of cognitive dysfunction. J Ethnopharmacol 72:119–28
- Kumar V, Thakur AK, Barothia ND, Chatterjee SS. (2011). Therapeutic potentials of Brassica juncea: An overview. TANG: Int J Genuin Tradit Med 1:e2. 1–e2.17
- Kumar V, Thakur AK, Chatterjee SS. (2012). Obesity, cancer and psychopathology: Can vegetarian diet be of help? In: Shankar S, Srivastava RK, eds. Nutrition, Diet and Cancer. Dordrecht, Heidelberg, London, New York: Springer, 459–91
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Luchsinger JA, Reitz C, Patel B, et al. (2007). Relation of diabetes to mild cognitive impairment. Arch Neurol 64:570–5
- Luck H. (1963). Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. Weinheim, NY: Verlag Chemie Academic Press, 885–8
- Malgorzata NK, Aleksander S. (2010). Changes of phenolic content in rapeseed during preliminary drying. J Oilseed Brassica 1:33–8
- Manohar PR, Pushpan R, Rohini S. (2009). Mustard and its uses in Ayurveda. Indian J Tradit Knowl 8:400–4
- Maritim AC, Sanders RA, Watkins JB III. (2003). Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol 17:24–38
- Rahmatullah M, Shefa TF, Hasan L, et al. (2010). A study on antinociceptive and anti-hyperglycemic activity of methanol extract of Brassica juncea (L.) Czern. leaves in mice. Adv Nat Appl Sci 4:221–5
- Spignoli G, Pepeu G. (1986). Oxiracetam prevents electroshock-induced decrease in brain acetylcholine and amnesia. Eur J Pharmacol 126:253–7
- Strachan MW, Reynolds RM, Frier BM, et al. (2008). The relationship between type-2 diabetes and dementia. Br Med Bull 88:131–46
- Thakur AK, Chatterjee SS, Kumar V. (2013). Anxiolytic-like activity of leaf extract of traditionally used Indian-mustard (Brassica juncea) in diabetic rats. TANG: Int J Genuin Tradit Med 3:e7. 1–e7.7