Abstract
Context: Paracetamol overdose is a predominant cause of hepatotoxicity in both humans and experimental animals.
Objective: In this study, we investigated the protective effect of aqueous artichoke leaf extract (ALE) against paracetamol-induced liver injury in rats using N-acetylcysteine (NAC) as a reference drug.
Materials and methods: Rats were divided into five groups: negative control, paracetamol (2 g/kg, single oral dose), ALE (1.5 g/kg, orally for 14 d), ALE + paracetamol, and NAC (100 mg/kg) + paracetamol. Indices of liver damage (serum alanine aminotransferase and aspartate aminotransferase) were measured. Liver homogenates were analyzed for oxidative stress biomarkers (MDA, malondialdehyde; SOD activity, superoxide dismutase activity; NO, nitric oxide; GSH content, reduced glutathione), glutathione cycling (GR, glutathione reductase), and utilization (GST, glutathione-S-transferase). Apoptosis was assessed using the comet assay.
Results: Paracetamol caused marked liver damage as noted by significant increased activities of serum aminotransferases (p < 0.05) as well as a significant increase in hepatic MDA and NO levels (p < 0.001) compared with the negative control group. GSH content, GR, GST, and SOD activities were decreased significantly (p < 0.001). Comet assay parameters (tail length, percentage of tailed cells, percentage of migrated DNA, and tail moment) were increased (p < 0.05), indicating apoptosis. Histopathological examination showed necrotic areas. Pre-treatment with ALE replenished hepatic GSH, reversed oxidative stress parameters, DNA damage, and necrosis induced by paracetamol.
Discussion and conclusion: These results suggest that ALE may protect from paracetamol-induced liver toxicity via its antioxidant and anti-apoptotic properties.
Introduction
Paracetamol is one of the mostly used analgesics and antipyretics. It is considered to be a safe drug when used at therapeutic levels. However, it is known to cause centrilobular necrosis upon overdose with the potential to progress to liver failure (Davidson & Eastham, Citation1966; Lee, Citation2004). A large portion of a therapeutic dose of paracetamol is directly conjugated with glucuronic acid or sulfate. The remaining part of the dose is metabolized by the P450 system to a reactive metabolite, presumably N-acetyl-p-benzoquinone imine (NAPQI) (Nelson, Citation1990). NAPQI reacts with glutathione (GSH) spontaneously or catalyzed by GSH-S-transferases to form GSH-adduct (Chen et al., Citation2003). Thus, the earliest effect of paracetamol metabolism is a profound depletion of hepatocellular GSH (Mitchell et al., Citation1973), which affects both the cytosolic and the mitochondrial compartments. Once GSH is exhausted, any remaining NAPQI formed will react with alternative targets, in particular cellular proteins (Jollow et al., Citation1973). Covalent binding to mitochondrial proteins may be to a significant degree responsible for the initial mitochondrial dysfunction followed by mitochondrial oxidant stress (Jaeschke et al., Citation2003; Ramsay et al., Citation1989). The formation of peroxynitrite in mitochondria is followed by nuclear DNA fragmentation (Cover et al., Citation2005).
N-Acetylcysteine (NAC) is usually used as an antidote for the prevention of paracetamol-induced hepatotoxicity. However, some cases do not respond and adverse anaphylactoid reactions were reported (Mant et al., Citation1984; Tripathi, Citation2008).
Cynara scolymus L. (Asteraceae), popularly known as artichoke, has multiple pharmacological actions. It was shown to have antitoxic activity (Heidarian et al., Citation2013), antiulcerogenic activity (Nassar et al., Citation2013), and glycemia-lowering effect (Fantini et al., Citation2011). It helped in the management of mild hypercholesterolaemia (Rondanelli et al., Citation2013) and was protective against hepatocellular carcinoma (Metwally et al., Citation2011) and human breast cancer (Mileo et al., Citation2012). It exerted hepatoprotective activity in different models of liver injury in vitro and in vivo (Adzet et al., Citation1987; Gebhardt, Citation1997; Mehmetçik et al., Citation2008; Metwally et al., Citation2011).
However, its protective effect on paracetamol-induced toxicity has not been yet investigated. In this study, we examined the possible protective effect of artichoke leaf extract (ALE) on hepatotoxicity induced by paracetamol in rats.
Materials and methods
Chemicals and drugs
ALE (batch number 12342) was purchased from Western Pharmaceutical Industries, Cairo, Egypt. Paracetamol and NAC were supplied by SEDICO Pharmaceutical Company (Giza, Egypt). Other chemical reagents were purchased from Sigma Aldrich Co. (St. Louis, MO).
Animals
Fifty male Sprague–Dawley rats weighing 180–200 g were housed at cages in a temperature-controlled (25 ± 1 °C) environment and provided free access to pelleted food and purified drinking water ad libitum, and left to accommodate for 1 week before the experiment. The animal experiments described later were approved by the Ethics Committee, Faculty of Pharmacy, Helwan University, Cairo, Egypt.
Experimental design
The animals were divided into five groups, 10 per each group, as follows:
Group I: control group received 10% Tween 80 solution orally by gavage for 14 d.
Group II: rats received 10% Tween 80 solution orally by gavage for 14 d. About 1 h after the last dose, rats received 2 g/kg paracetamol (Galal et al., Citation2012) suspended in 10% Tween 80 solution orally by gavage.
Group III: rats received 1.5 g/kg ALE (Mehmetçik et al., Citation2008) suspended in 10% Tween 80 solution orally by gavage for 14 d.
Group IV: rats received ALE (1.5 g/kg) suspended in 10% Tween 80 solution orally by gavage for 14 d. About 1 h after the last dose, rats received 2 g/kg paracetamol suspended in 10% Tween 80 solution orally by gavage.
Group V: rats received NAC (100 mg/kg) dissolved in 10% Tween 80 solution orally by gavage for 14 d. About 1 h after the last dose, rats received 2 g/kg paracetamol suspended in 10% Tween 80 solution orally by gavage.
Rats were fasted on the 13th day for 18 h before paracetamol administration. Twenty-four hour after receiving paracetamol, blood was collected from animals by retroorbital puncture. Animals were then sacrificed by cervical dislocation. The liver was rapidly removed and washed in ice-cold saline solution. A part was homogenized in phosphate buffer saline (0.1 M PBS, pH 7.4). The homogenates were centrifuged at 10 000 rpm for 30 min at 4 °C, and supernatants were stored at −70 °C until biochemical assays could be performed. The second part of liver was stored in 10% neutral buffered formalin for the histopathological study, and the third was used for apoptosis assessment by the comet assay.
Liver function tests
Serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured as indicators of hepatic injury using standard diagnostic kits (Quimica Clinica Aplicada s.a., Tarragona, Spain).
Determination of lipid peroxide level
Lipid peroxidation was assessed by the measuring MDA level in the liver homogenates supernatants (Mihara & Uchiyama, Citation1978). The principle of the assay depends on a colorimetric determination of pink pigment product, resulting from the reaction of one molecule of MDA with two molecules of thiobarbituric acid (TBA) at low pH (2–3) at 95 °C for 45 min. The resultant color product was extracted by n-butanol and measured at 535 nm spectrophotometrically.
Determination of nitric oxide (NO) level
NO, measured as nitrite, was determined by using the Griess reagent according to the method of Miranda et al. (Citation2001). The amount of nitrite (the stable end product of NO radical) was measured by mixing with the colorless Griess reagent which results in the formation of purple complex. The degree of the color developed is measured spectrophotometrically at 540 nm.
Determination of superoxide dismutase activity
Superoxide dismutase (SOD) activity was determined spectrophotometrically according to Robak and Gryglewski (Citation1988). This assay is based on the generation of superoxide ions from the conversion of xanthine and oxygen to uric acid and hydrogen peroxide by xanthine oxidase. Superoxide anions then convert nitroblue tetrazolium (NBT) to NBT–diformazan, which absorbs light at 550 nm. SOD reduces the superoxide ion concentration and thereby lowers the rate of NBT–diformazan formation. The extent of reduction in the appearance of NBT–diformazan is the measure of SOD activity present in an experimental sample.
Determination of reduced GSH level
GSH was estimated spectrophotometrically by the method of Ellman (Citation1959). Protein in liver homogenate was precipitated with 10% trichloroacetic acid and the contents were centrifuged at 2000 rpm for 5 min. An aliquot of the clear supernatant (0.1 ml) was taken and mixed with 1.7 ml of 0.1 mM potassium phosphate buffer (pH 8) and 0.1 ml of Ellman’s reagent. The optical density was measured at 412 nm.
Determination of GSH reductase activity
The GSH reductase (GR) activity was measured spectrophotometrically by the method of Carlberg and Mannervik (Citation1985), where GSH reductase together with its co-factor (NADPH) catalyzes the reduction of oxidized GSH to reduced GSH. The oxidation of NADPH to NADP+ is monitored as a decrease in the absorbance at 340 nm. The rate of decrease is directly proportional to the GR activity in the sample
Determination of GSH-S-transferase (GST) activity
GST activity was determined spectrophotometrically by the method of Habig et al. (Citation1974) using 1-chloro-2,4-dinitrobenzene as a substrate, with reduced GSH. The conjugation is accompanied by an increase in the absorbance at 340 nm. The rate of increase is directly proportional to the GST activity in the sample.
Determination of total protein
The protein content was measured using bovine serum albumin (BSA) as a standard (Lowry et al., Citation1951). Different concentrations of BSA (0.01–0.1 mg/ml) were used to plot a standard curve. The principle of the assay is based on the copper that reacts with protein molecule in alkaline media, and then the Folin–Ciocalteu reagent is reduced by the copper–protein complex resulting in a blue purple color. The final color developed at room temperature was measured at 500 nm.
Comet assay (single cell gel electrophoresis)
The comet assay was performed according to the method developed by Singh et al. (Citation1988). Briefly, 1 g of liver tissue was transferred to 1 ml ice-cold PBS. This suspension was stirred for 5 min and filtered. The cell suspension (100 μl) was mixed with 600 μl of low-melting agarose (0.8% in PBS). This mixture (100 μl) was spread on pre-coated slides. The coated slides were immersed in lysis buffer [0.045 M Tris–borate–EDTA (TBE), pH 8.4, containing 2.5% sodium dodecyl sulphate (SDS)] for 15 min. The slides were placed in electrophoresis chamber containing the same TBE buffer, but devoid of SDS. The electrophoresis conditions were 2 V/cm for 2 min and 100 mA. Slides were stained with ethidium bromide (EtBr, 20 μl/ml) at 4 °C. The observation was with the samples still humid, the DNA fragment migration patterns of 100 cells for each sample were evaluated with a fluorescence microscope (with excitation filter 420–490 nm [issue 510 nm]). For visualization of DNA damage, EtBr-stained DNA was observed using a 400× magnification using a fluorescent microscope. Comet 5 image analysis software developed by Kinetic Imaging, Ltd. (Liverpool, UK) linked to a charge-coupled device (CCD) camera was used to assess the qualitative and quantitative extent of DNA damage in the cells by measuring the length of DNA migration (tail length, TL in μm), the percentage of tailed cells (%T) and the percentage of migrated DNA (% DNA). Finally, tail moment was calculated (TM = TL × % DNA).
Histopathological examination
Autopsy samples taken from liver of rats in different groups were fixed in 10% neutral buffered formalin. Serial dilutions of alcohol (methyl, ethyl, and absolute ethyl) were used. Specimens were cleared in xylene embedded in paraffin at 56 °C in hot air oven for 24 h. Paraffin bees wax tissue blocks were prepared for sectioning at 4 μm thickness by a sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by hematoxylin and eosin stain for histopathological examination through the light microscope.
Statistical analysis
Results are expressed as the mean ± SEM, and different groups were compared using a one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test for multiple comparisons.
Results
Effect of ALE on paracetamol-induced alteration in liver function tests
Administration of a single dose (2 g/kg) of paracetamol (group II) increased ALT and AST activities by 51% and 48%, respectively, compared with the control group (group I). Pre-treatment of the paracetamol-injected group with ALE (1.5 g/kg) (group IV) reduced these activities significantly by 51% and 22%, respectively, as compared with group II. Pre-treatment with NAC (100 mg/kg) (group V) showed a significant reduction in ALT and AST activities by 57% and 33%, respectively ().
Table 1. Effect of artichoke leaf extract (ALE) on paracetamol-induced alterations of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in rats.
Effect of ALE on paracetamol-induced alterations in hepatic lipid peroxidation, NO level, and SOD enzyme activity
Oral administration of paracetamol induced a significant increase in liver lipid peroxides measured as malondialdehyde (MDA) and NO levels by 112% and 92%, respectively, as compared to the control group (group I). Pre-treatment with ALE and NAC significantly reduced the level of MDA by 34% and 19%, while that of NO was reduced by 26% and 16%, respectively. In contrast, paracetamol administration significantly reduced SOD activity as compared with group I. Pre-treatment with ALE and NAC increased its activity by 51% and 22%, respectively. Furthermore, hepatic SOD activity in rats treated with ALE (group III) was significantly higher than group I by 59% ().
Table 2. Effect of artichoke leaf extract (ALE) on paracetamol-induced alterations of lipid peroxidation (MDA), SOD activity and NO level measured in rats livers.
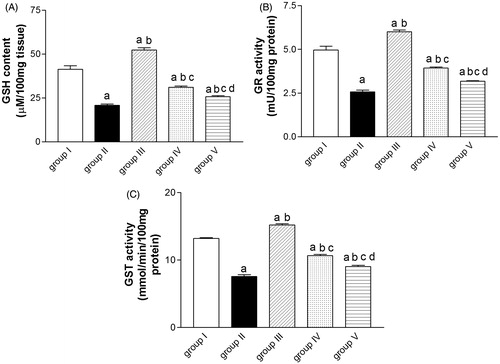
Effect of ALE on paracetamol-induced alteration in reduced GSH level, GR, and GST activities
Compared with control group, paracetamol injected group showed a significant decrease in reduced GSH level, GR, and GST activities as compared with the control group (group I). These alterations had been significantly reversed due to pre-treatment with ALE by 50, 54 and 41%, respectively. Administration of NAC prior to paracetamol significantly increased the GSH level and the GR activity by 24% for each, and the GST activity by 20% as compared with group II. Moreover, administration of ALE alone (group III) significantly increased GSH level, GR, and GST activities by 27, 21, and 15%, respectively, as compared with group I ().
Figure 1. Effect of artichoke leaf extract (ALE) on paracetamol-induced alterations of reduced glutathione (GSH) (A), glutathione reductase (GR) (B), and glutathione-S-transferase (GST) (C) measured in rats livers. Group I: treated with vehicle; group II: treated with saline + 10% Tween 80 orally for 14 d followed by paracetamol (2 g/kg, orally); group III: treated with 1.5 g/kg of ALE orally for 14 d; group IV: treated with 1.5 g/kg of ALE orally for 14 d followed by paracetamol (2 g/kg, orally); group V: treated with 100 mg/kg of N-acetylcysteine (NAC) orally for 14 d followed by paracetamol (2 g/kg, orally). Results are expressed as the mean ± SEM (n = 10) in each group. (a) Significantly different from group I at p < 0.001. (b) Significantly different from group II at p < 0.05. (c) Significantly different from group III at p < 0.001. (d) Significantly different from group IV at p < 0.05.
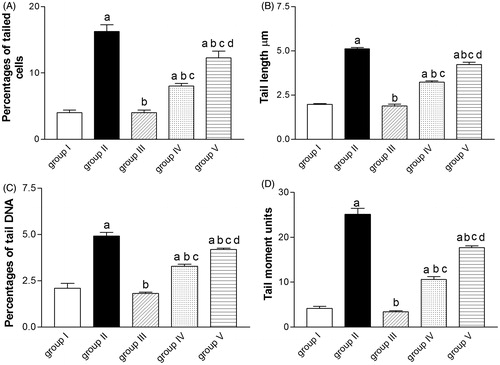
Effect of ALE on paracetamol-induced DNA damage
A significant increase in different comet assay parameters (%T, TL, % DNA in tail, and TM) has been shown in animals receiving paracetamol (group II) compared with control animals (group I). Pre-treatment with either ALE or NAC significantly reduced DNA damage indicated by reduction in %T, TL, % DNA in tail and TM ().
Figure 2. Effect of artichoke leaf extract (ALE) on paracetamol-induced DNA damage assessed by the comet assay in rats livers. Group I: treated with vehicle; group II: treated with saline + 10% Tween 80 orally for 14 d followed by paracetamol (2 g/kg, orally); group III: treated with 1.5 g/kg of ALE orally for 14 d; group IV: treated with 1.5 g/kg of ALE orally for 14 d followed by paracetamol (2 g/kg, orally); group V: treated with 100 mg/kg of N-acetylcysteine (NAC) orally for 14 d followed by paracetamol (2 g/kg, orally). Results are expressed as the mean ± SEM (n = 6) in each group. (a) Significantly different from group I at p < 0.05. (b) Significantly different from group II at p < 0.05. (c) Significantly different from group III at p < 0.05. (d) Significantly different from group IV at p < 0.01.
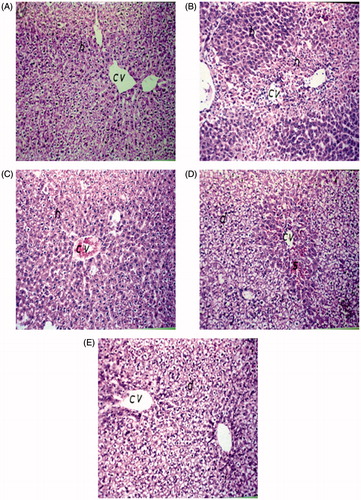
Histopathological findings
Histopathological examination of liver sections from the control group revealed normal structure of the central vein (cv) and surrounding hepatocytes (h) (). Similarly, no histopathological changes were recorded in liver sections of rats treated with ALE (). Liver sections of paracetamol-administered animals revealed severe histopathological alterations appearing as centrolobular necrosis (n) in the hepatocytes surrounding the central veins all over the hepatic parenchyma (). Liver sections of rats pre-treated with either ALE or NAC restored many of normal hepatic structure and the centrilobular area revealed lesser degree of degeneration ()
Figure 3. Hematoxilin- and eosin-stained sections showing the effect of artichoke leaf extract (ALE) on histological liver changes induced by paracetamol. Original magnification, ×40. (A) Group I: treated with vehicle; (B) group II: treated with saline + 10% Tween 80 orally for 14 d followed by paracetamol (2 g/kg, orally); (C) group III: treated with 1.5 g/kg of ALE orally for 14 d; (D) group IV: treated with 1.5 g/kg of ALE orally for 14 d followed by paracetamol (2 g/kg, orally); (E) group V: treated with 100 mg/kg of N-acetylcysteine (NAC) orally for 14 d followed by paracetamol (2 g/kg, orally). cv, central vein; h, hepatocytes; d, degeneration; n, necrosis; s, sinusoids.
Discussion
In the present study, we examined the effects of ALE on paracetamol-induced liver toxicity. Increase in serum ALT and AST activities following paracetamol administration have been attributed to the loss of hepatocytes membrane integrity and cell damage resulting in enzymes release into circulation (Gupta & Misra, Citation2006). Pre-treatment with ALE (1.5 g/kg) reduced serum ALT and AST activities raised by paracetamol, indicating preservation of structural integrity of hepatocellular membrane. This result was also observed in rats treated with NAC (100 mg/kg), used as a positive control. These findings were confirmed by histopathological studies revealing that ALE and NAC were able to ameliorate hepatic cells necrosis produced by paracetamol administration.
Oxidative stress is an important mechanism that has been postulated to be important in the development of paracetamol toxicity. Thus, increased formation of superoxide would lead to hydrogen peroxide and peroxidation reactions (James et al., Citation2003). SODs are the first and most important line of antioxidant enzymes against reactive oxygen species (ROS) and, particularly, superoxide anion radicals (Zelko et al., Citation2002). Enhanced generation of superoxide in the presence of NO will lead to the formation of the potent oxidant and nitrating agent peroxynitrite (Squadrito & Pryor, Citation1998).
In the present study, paracetamol administration caused increased hepatic lipid peroxidation. On one hand, we observed also a significant reduction in hepatic SOD activity, indicating a decrease in the antioxidant capacity. This may be attributed to the consumption of SOD in ROS detoxification (Mladenović et al., Citation2009). It was also shown that SOD is inhibited by oxygen-derived free radicals (Pigeolet et al., Citation1990). On the other hand, we found that NO liver content increased significantly. This is in accordance with previous studies reporting that toxic doses of paracetamol led to increase NO synthesis and the formation of nitrotyrosine–protein adducts due to nitration of tyrosine by peroxynitrite (Hinson et al., Citation1998).
ALE is rich in caffeoylquinic acids and flavonoids giving it a powerful antioxidant activity (Li et al., Citation2004). Our results demonstrate that pre-treatment with ALE-reduced MDA content and augmented SOD activity indicating a decrease of oxidative damage. This was accompanied by a significant reduction in the NO level compared with paracetamol-treated rats. Our findings, which are in parallel with previous studies (Gurel et al., Citation2007; Metwally et al., Citation2011), may be due to the reduction of inducible NO synthase (iNOS) expression leading to decreased NO production by the caffeoylquinic acid content of ALE (Olmos et al., Citation2008).
In case of paracetamol overdose, the increased production of NAPQI depletes GSH and inhibits GSH synthesis (Lauterburg & Mitchell, Citation1982). This may explain the significant decrease of the hepatic GSH content in the paracetamol-treated group compared with the control group. We found also that paracetamol reduced the activity of GST (involved in GSH utilization) and GR (involved in GSH redox cycling). The reduction of GST effectiveness may be due to its inactivation by reactive nitrogen species (Wong et al., Citation2001) or the decrease in GSH concentration (Czeczot et al., Citation2006). Concerning GR activity impairment, it may be due to the action of ROS or the conjugate between NAPQI and GSH (Roušar et al., Citation2010). Pre-treatment of rats given paracetamol with ALE increased significantly the hepatic GSH content as well as GST and GR activities compared with rats administered paracetamol only. This may be due to the antioxidant capacity of ALE which is rich in polyphenolic compounds known to act as free radical scavengers.
It is worthy of note that in the present study, the effect of ALE was significantly higher than that of NAC concerning the restoration of GSH and NO levels as well as antioxidant enzymes (SOD, GST, and GR) activities.
Interestingly, we found that ALE alone increased the normal activity of enzymatic antioxidants and the normal level of GSH significantly. This may attribute to its hepatoprotective effect.
It is well known that increased formation of ROS and peroxynitrite inside the mitochondria with impaired detoxification, due to extensive depletion of cytosolic and mitochondrial GSH levels, leads to mitochondrial DNA damage and nuclear DNA fragmentation (Ramachandran et al., Citation2011).
In the present study, paracetamol administration increased the frequency of tailed nuclei, tail length, percentage of DNA in tail, and tail moment in the liver, indicating DNA damage and apoptosis. This is in accordance with previous studies reporting genotoxicity of paracetamol (Dybing et al., Citation1984; Oshida et al., Citation2008). Pre-treatment with ALE and NAC decreased DNA damage. This may be due to recovery of mitochondrial GSH levels and restoration of the free radicals scavenging activity.
Conclusion
ALE protected against hepatotoxicity induced by paracetamol in rats. The protection exerted by ALE may be due to its antioxidant and anti-apoptotic properties.
Declaration of interest
The authors report no declarations of interest.
References
- Adzet T, Camarasa J, Laguna JC. (1987). Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J Nat Prod 50:612–17
- Carlberg I, Mannervik B. (1985). Glutathione reductase. Meth Enzymol 113:485–90
- Chen C, Hennig GE, Manautou JE. (2003). Hepatobiliary excretion of acetaminophen glutathione conjugate and its derivatives in transport-deficient (TR-) hyperbilirubinemic rats. Drug Metab Dispos 31:798–804
- Cover C, Mansouri A, Knight TR, et al. (2005). Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–87
- Czeczot H, Ścibior D, Skrzycki M, Podsiad M. (2006). Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta Biochim Pol 53:237–41
- Davidson DG, Eastham WN. (1966). Acute liver necrosis following overdose of paracetamol. Br Med J 2:497–9
- Dybing E, Holme JA, Gordon WP, et al. (1984). Genotoxicity studies with paracetamol. Mutat Res 138:21–32
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Fantini N, Colombo G, Giori A, et al. (2011). Evidence of glycemia-lowering effect by a Cynara scolymus L. extract in normal and obese rats. Phytother Res 25:463–6
- Galal RM, Zaki HF, Seif El-Nasr MM, Agha AM. (2012). Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch Iran Med 15:674–80
- Gebhardt R. (1997). Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara scolymus L.) against hydroperoxide-induced oxidative stress in cultured rat hepatocytes. Toxicol Appl Pharmacol 144:279–86
- Gupta AK, Misra N. (2006). Hepatoprotective activity of aqueous ethanolic extract of chamomile capitula in paracetamol intoxicated albino rats. Am J Pharmacol Toxicol 1:17–20
- Gurel E, Caner M, Bayraktar L, et al. (2007). Effects of artichoke extract supplementation on gonads of cadmium-treated rats. Biol Trace Elem Res 119:51–9
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–9
- Heidarian E, Rafieian-Kopaei M. (2013). Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm Biol 51:1104–9
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. (1998). Nitrotyrosine protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11:604–7
- Jaeschke H, Knight TR, Bajt ML. (2003). The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144:279–88
- James LP, Mayeux PR, Hinson JA. (2003). Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31:1499–506
- Jollow DJ, Mitchell JR, Potter WZ, et al. (1973). Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187:195–202
- Lauterburg BH, Mitchell JR. (1982). Toxic doses of acetaminophen suppress hepatic glutathione synthesis in rats. Hepatology 2:8–12
- Lee WM. (2004). Acetaminophen and the U.S. acute liver failure study group: Lowering the risks of hepatic failure. Hepatology 40:6–9
- Li H, Xia N, Brausch I, et al. (2004). Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J Pharmacol Exp Ther 310:926–32
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Mant TG, Tempowski JH, Volans GN, Talbot JC. (1984). Adverse reactions to acetylcysteine and effects of overdose. Br Med J 289:217–19
- Mehmetçik G, Ozdemirler G, Koçak-Toker N, et al. (2008). Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress. Exp Toxicol Pathol 60:475–80
- Metwally NS, Kholeif TE, Ghanem KZ, et al. (2011). The protective effects of fish oil and artichoke on hepatocellular carcinoma in rats. Eur Rev Med Pharmacol Sci 15:1429–44
- Mihara M, Uchiyama M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–8
- Mileo AM, Di Venere D, Linsalata V, et al. (2012). Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J Cell Physiol 227:3301–9
- Miranda KM, Espey MG, Wink DA. (2001). A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
- Mitchell JR, Jollow DJ, Potter WZ, et al. (1973). Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–17
- Mladenović D, Radosavljević T, Ninković M, et al. (2009). Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem Toxicol 47:866–70
- Nassar MI, Mohamed TK, Elshamy AI, et al. (2013). Chemical constituents and anti-ulcerogenic potential of the scales of Cynara scolymus (artichoke) heads. J Sci Food Agric 93:2494–501
- Nelson SD. (1990). Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10:267–78
- Olmos A, Giner RM, Recio MC, et al. (2008). Interaction of dicaffeoylquinic derivatives with peroxynitrite and other reactive nitrogen species. Arch Biochem Biophys 475:66–71
- Oshida K, Iwanaga E, Miyamoto-Kuramitsu K, Miyamoto Y. (2008). An in vivo comet assay of multiple organs (liver, kidney and bone marrow) in mice treated with methyl methanesulfonate and acetaminophen accompanied by hematology and/or blood chemistry. J Toxicol Sci 33:515–24
- Pigeolet E, Corbisier P, Houbion A, et al. (1990). Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–97
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. (2011). The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 251:226–33
- Ramsay RR, Rashed MS, Nelson SD. (1989). In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys 273:449–57
- Robak J, Gryglewski RJ. (1988). Flavonoids are scavengers of superoxide anions. Biochem Pharmacol 37:837–41
- Rondanelli M, Giacosa A, Opizzi A, et al. (2013). Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int J Food Sci Nutr 64:7–15
- Roušar T, Pařík P, Kučera O, et al. (2010). Glutathione reductase is inhibited by acetaminophen-glutathione conjugate in vitro. Physiol Res 59:225–32
- Singh NP, McCoy MT, Tice RR, Schneider EL. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–91
- Squadrito GL, Pryor WA. (1998). Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite and carbon dioxide. Free Radic Biol Med 25:392–403
- Tripathi KD. (2008). Essentials of Medical Pharmacology. 6th ed. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd
- Wong PS, Eiserich JP, Reddy S, et al. (2001). Inactivation of glutathione-S-transferases by nitric oxide-derived oxidants: Exploring a role for tyrosine nitration. Arch Biochem Biophys 394:216–28
- Zelko IN, Mariani TJ, Folz RJ. (2002). Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–49

