Abstract
Context: There is increasing evidence that Vitamin D (Vit D) and its metabolites, besides their well-known calcium-related functions, may also exert antiproliferative, pro-differentiating, and immune modulatory effects on tumor cells in vitro and may also delay tumor growth in vivo.
Objective: The aim of this review is to provide fresh insight into the most recent advances on the role of Vit D and its analogues as chemopreventive drugs in cancer therapy.
Methods: A systematic review of experimental and clinical studies on Vit D and cancer was undertaken by using the major electronic health database including ISI Web of Science, Medline, PubMed, Scopus and Google Scholar.
Results and conclusion: Experimental and clinical observations suggest that Vit D and its analogues may be effective in preventing the malignant transformation and/or the progression of various types of human tumors including breast cancer, prostate cancer, colorectal cancer, and some hematological malignances. These findings suggest the possibility of the clinical use of these molecules as novel potential chemopreventive and anticancer agents.
Introduction
The clinical use of cytotoxic drugs has had a significant impact on neoplastic diseases. However, their therapeutic effectiveness is limited due to their narrow therapeutic index and the onset of chemoresistance. Therefore, many efforts are currently being directed to finding new therapeutic options that may overcome these problems. In this scenario, chemoprevention, i.e., the use of natural, synthetic, or biological substances to reverse, suppress, or prevent the development and progression of malignant diseases, holds great promise (Davis & Wu, Citation2012). In this context, a consistent body of investigation provides evidence that Vitamin D (Vit D) and its metabolites, in addition to its well-known involvement in calcium homeostasis, also appears to be effective in preventing the malignant transformation and the progression of various types of human tumors (Krishnan & Feldman, Citation2010; Nagpal et al., Citation2005; Vuolo et al., Citation2012). On one hand, experimental evidence indicates that these effects appear to be likely due to the antiproliferative, proapoptotic, and immunomodulatory activities with which these molecules are endowed (Krishnan & Feldman, Citation2010; Vanoirbeek et al., Citation2011). On the other hand, numerous epidemiological observations based on geographic variation in the incidence of cancer and\or mortality in relation to 18 different types of human cancer clearly demonstrate that Vit D exerts chemopreventive effects on at least three types of solid tumors at high risk of mortality, namely breast cancer (Khan et al., Citation2010), prostate cancer (Swami et al., Citation2011), and colorectal cancer (Pereira et al., Citation2012) and on squamous cell carcinoma (SCC) and some hematological malignances (Kim et al., Citation2012; Reddy, Citation2013). The aim of this paper is to provide insight into the most recent advances on the role of Vit D as chemopreventive drug in cancer therapy.
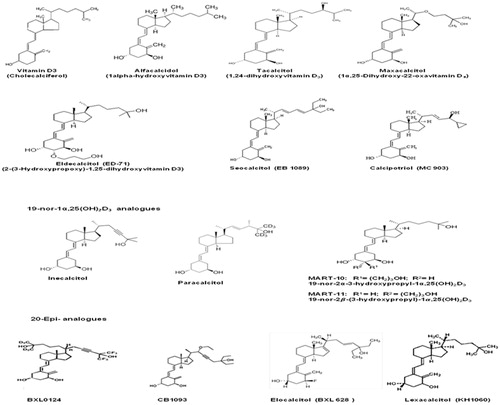
Vit D chemical structure, biological functions, and metabolism
The Vit D complex includes a group of fat-soluble pro-hormones that contribute to maintaining calcium and phosphate homeostasis and bone and muscle integrity (Bouillon et al., Citation2008). On one hand, Vit D is known to be essential for the absorption of calcium and phosphate ions in the small intestine, their mobilization from the bone tissue, and their resorption in the kidney (Bouillon et al., Citation2008). These effects suggest an indirect involvement of this molecule in the regulation of the normal function of different tissues such as muscle contraction, nerve conduction, bone metabolism, and blood clotting (Bouillon et al., Citation2008). Interestingly, emerging evidence suggests that Vit D also appears to be implicated in the regulation of other important biological processes such as cell proliferation and differentiation (Gocek & Studzinski, Citation2009), immune response (Hewison, Citation2011), and insulin secretion (Teegarden & Donkin, Citation2009). Vit D is available in two main distinct forms: i.e., Vitamin D2 (Vit D2) (ergocalciferol) and Vitamin D3 (Vit D3) (cholecalciferol) (). Vit D2 is synthesized in plants, yeasts, and fungi whereas Vit D3 is of animal origin (Bouillon et al., Citation2008; Jäpelt & Jakobsen, Citation2013) (). Vit D2 is derived from ergosterol, which is turned into viosterol by ultraviolet (UV) light and then converted into ergocalciferol. In this form, Vit D2 can be ingested from the diet and from supplements (). On the other hand, the exposure of skin to ultraviolet B radiation (UVB; 290–320 nm) converts 7-dehydrocholesterol (DHCR7) to pre-vitamin D3 (1,25-dihydroxycholecalciferol or calcitriol) which isomerizes to Vit D3 () (Bouillon et al., Citation2008; Jäpelt & Jakobsen, Citation2013). In this form, Vit D3 binds to Vit D-binding protein (DBP) and is then transported into the liver. Vit D complex molecules are known as secosteroids, namely steroids in which one of the rings of its cyclopentanoperhydrophenanthrene structure has a broken carbon–carbon bond. Vit D2 and Vit D3 differ in their side chains in that the side chain of the D2 form additionally contains a double bond between carbons 22 and 23 and a methyl group on carbon 24 (). The biosynthetic pathway of Vit D3 involves the hepatic hydroxylation of the carbon atom in position 25 by four cytochrome P450 isoenzymes, i.e., CYP2R1, CYP2J2, and CYP3A4 isoforms and the mitochondrial CYP27A1 isoform () (Jones et al., Citation2014; Schuster, Citation2011). This first hydroxylation generates the main circulating form of Vit D, namely 25-hydroxyvitamin D3 [25(OH)D] or calcidiol (). Normal serum values of 25(OH)D are comprised between 25 and 130 nmol/L depending on the geographic location (Ross et al., Citation2011). This form reflects dietary sources as well as Vit D production by UV light on the skin (Ross et al., Citation2011). A second hydroxylation in the 1-α position occurs in the kidney, at the tubule proximale levels, where it leads to the formation of 1α,25(OH)2D3 (calcitriol) which is the most biologically active form of Vit D () (Jones et al., Citation2014; Schuster, Citation2011). Once synthesized in the kidney, via CYP27B1 (25-hydroxyvitamin D3 1-α-hydroxylase), this active form enters the bloodstream and it is then transported, by specific binding proteins (VDBP) to distant target tissues (Bouillon et al., Citation2008) (). Vit D activity is tightly regulated by metabolic processes mediated by the CYP24A1 isoform (1,25-dihydroxyvitamin D3 24-hydroxylase) that converts 1α,25(OH)2D3 into 1,24,25-trihydroxycholecalciferol [1α,24,25(OH)3D3] which has a lower affinity for Vit D receptors (VDR) (Schuster, Citation2011). This molecule, then, undergoes a further metabolization to generate calcitroic acid which is excreted in this form (). In contrast, expression levels of renal 1-α hydroxylase (CYP27B1), namely the enzyme which converts 1,25-hydroxycholecalciferol into 1,25-dihydroxycholecalciferol, are positively regulated by high calcium and phosphate levels parathyroid hormone (PTH), calcitonin, growth hormone, and insulin-like growth factor-I (IGF-I) (Henry, Citation2011). Conversely, low calcium and phosphate levels, fibroblast growth factor 23 (FGF23), and 1,25(OH)2D3 itself function as negative regulators of this enzyme (Fukumoto, Citation2014). However, PTH and 1α,25(OH)2D3 have no effect on the expression and/or activity of extrarenal 1α-hydroxylase. Other rate-limiting steps in Vit D metabolism involve the modulation of CYP2R1 (Vit D 25-hydroxylase) activity, which is induced by decreased level of 25(OH)D and that of CYP24A1 which is induced by 25(OH)D and 1,25(OH)2D3 (Bouillon et al., Citation2008; Schuster, Citation2011).
Figure 1. Synthesis and metabolism of secosteroids Vitamin D3 and Vitamin D2. In humans, cholecalciferol (Vitamin D3) is synthesized from 7-dehydrocholesterol upon sunlight exposure. Vitamin D may also be obtained from dietary sources or supplements as ergocalciferol or Vitamin D2. Vitamin D3 binds to Vitamin D-binding protein (DBP) in the bloodstream and then is transported to the liver where it is first converted by the enzyme 25-hydroxylase (CYP2R1) to 25-hydroxyvitamin D [25(OH)D]. This molecule is converted by the renal enzyme 1-α hydroxylase (CYP27B1) to 1,25 dihydroxycholecalciferol (calcitriol), which is the active form of Vitamin D. The rate limiting step in catabolism is the degradation of 25(OH)D3 and 1,25(OH)2D3 to 24,25(OH)D3 and 1,24,25(OH)2D3, respectively, which occurs through 24-hydroxylation by mitochondrial 1,25-dihydroxyvitamin D3 24-hydroxylase, (CYP24A1). 24,25(OH)D3 and 1,24,25(OH)2D3 are excreted in this form.
![Figure 1. Synthesis and metabolism of secosteroids Vitamin D3 and Vitamin D2. In humans, cholecalciferol (Vitamin D3) is synthesized from 7-dehydrocholesterol upon sunlight exposure. Vitamin D may also be obtained from dietary sources or supplements as ergocalciferol or Vitamin D2. Vitamin D3 binds to Vitamin D-binding protein (DBP) in the bloodstream and then is transported to the liver where it is first converted by the enzyme 25-hydroxylase (CYP2R1) to 25-hydroxyvitamin D [25(OH)D]. This molecule is converted by the renal enzyme 1-α hydroxylase (CYP27B1) to 1,25 dihydroxycholecalciferol (calcitriol), which is the active form of Vitamin D. The rate limiting step in catabolism is the degradation of 25(OH)D3 and 1,25(OH)2D3 to 24,25(OH)D3 and 1,24,25(OH)2D3, respectively, which occurs through 24-hydroxylation by mitochondrial 1,25-dihydroxyvitamin D3 24-hydroxylase, (CYP24A1). 24,25(OH)D3 and 1,24,25(OH)2D3 are excreted in this form.](/cms/asset/169a6ccd-3115-4a79-8b0d-752f806c6585/iphb_a_988274_f0001_b.jpg)
Vit D receptor: structure and functions
The biological effects induced by the active form of Vit D and its semisynthetic analogues are mediated by the vitamin D receptor (VDR), also known as NR1I1 receptor (Wang et al., Citation2012). This receptor belongs to the superfamily of the nuclear receptor (NR) that includes receptors for steroid hormone, retinoids, and thyroid hormones. VDR is located in the nucleus of a variety of target cells including cells of the immune system (Wang et al., Citation2012). Similar to other nuclear receptors, VDR shows a domain structure which is homologous to that of these receptors (Bouillon et al., Citation2008; Haussler et al., Citation2011). This domain can be functionally divided into three regions with well-characterized functions, i.e., (a) an aminoterminal region factor that binds a short N-terminal activation-function 1 (AF-1) domain (A/B) and that plays an important role in the VDR-mediated transactivation; (b) a central region that contains a DNA-binding domain (DBD) which interacts directly with the cellular DNA at the level of Vit D-response elements (VDREs). This region contains two Zn2+-finger (C) portions consisting of four cysteine residues that coordinate a zinc atom; (c) a carboxy-terminal region that encompasses a multifunctional domain named ligand-binding domain (LBD), which may interact with different ligands such as retinoid X receptor (RXR) and the transcriptional regulatory factor AF-2 (Bouillon et al., Citation2008).
Vit D and regulation of gene expression
The effects induced by Vit D may occur in three different ways through the modulation of the expression of specific genes responsive to Vit D (Haussler et al., Citation2011, Citation2013; Kriebitzsch et al., Citation2009). In particular, the transcription of genes that are involved in the regulation of bone-remodeling processes, such as receptor activator of NF-kB ligand (RANKL), carbonic anydrase II, osteocalcin, osteopontin, or other genes, such as phospholipase C (PLP C), 24-hydroxylase or CYP3A4, β3 integrin, tumor suppressor p21, insulin growth factor-binding protein-3 (IGFB-3), can be positively regulated by a direct interaction with vitamin D response elements (VDREs) present in their promoter regions (Cao et al., Citation1993; Haussler et al., Citation2011, Citation2013). Conversely, Vit D, through the interaction with negative VDREs, may negatively regulate the expression of gene encoding for several pro-inflammatory cytokines such as interleukin-2 (IL-2) and interleukin-12 (IL-12), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), and/or growth factors and receptors such as epidermal growth factor receptor (EGFR), c-myc, and hormones involved in calcium homeostasis including parathyroid hormone (PTH), parathyroid hormone-related peptide (PTHrP), and rel-B (Haussler et al., Citation2011, Citation2013). Gene transcription may also be inhibited by the expression of genes that antagonize the effects of specific transcription factors, such as (NF)-aT and NF-kB (Haussler et al., Citation2013).
Vit D signal transduction pathways
To date, two major signal transduction pathways activated by Vit D in target cells have been identified, namely the so-called “genomic pathway,” where the Vit D nuclear receptor plays a major role, and the “non-genomic signal transduction pathway” (Haussler et al., Citation2011). This latter pathway triggers those responses mediated by Vit D that are faster than those induced following changes in gene expression. In this case, Vit D is supposed to interact directly with a receptor present in the plasma membrane (mVDR). This interaction induces rapid changes in intracellular calcium concentrations, alterations in membrane phospholipid metabolism, and activation of several signaling transduction pathways (Haussler et al., Citation2011). In order to ensure the full biological activity of 1,25(OH)2D3 both pathways needed to be activated. Following the binding of 1,25(OH)2D3 to VDR, the receptor is phosphorylated. In this form, VDR may promote the recruitment of its preferred dimerization partner, namely the nuclear receptor for 9-cis retinoic acid (RXR), thus forming a heterodimer that, in turn, binds to VDR responsive elements (VDREs) (Haussler et al., Citation2011). In the absence of its ligand, most of the Vit D receptors are located in the cytoplasm. However, upon interaction with 1,25(OH)2D3 and the subsequent heterodimerization the complex migrates from the cytoplasm into the nucleus. The 1,25(OH)2D3-VDR–RXR complex then interacts with DNA at VDREs level that is located in the classic promoter regions of responsive genes, near the transcription start site of the gene (Haussler et al., Citation2011, Citation2013). Downstream targets of these genes are implicated in mineral metabolism and in the regulation of other metabolic pathways including those involved in the immune response and cancer.
Effects of vitamin D on tumor progression
The prophylactic and therapeutic activities of Vit D toward the most common types of cancer have been extensively investigated either in vitro or in vivo (Khan et al., Citation2010; Leyssens et al., Citation2013; Pereira et al., Citation2012; Swami et al., Citation2011). The most striking results have been obtained following studies on breast cancer, prostate cancer, and colorectal cancer (Krishnan & Feldman, Citation2010; Leyssens et al., Citation2013; McCulloug et al., Citation2009). Experimental observations suggest that the chemopreventive effects of Vit D appear to be mainly due to its modulating activity on important biological functions such as cell proliferation, cell differentiation, growth factors gene expression, signal transduction, and apoptosis (Gocek & Studzinski, Citation2009; Haussler et al., Citation2013; Samuel & Sitrin, Citation2008) (). The inhibiting effects of Vit D on tumor cell growth were first described by Colston et al. (Citation1981) who showed for the first time a dose-dependent decrease of cell proliferation in melanoma cells treated with 1,25(OH)2D3. The growth inhibiting activity of this molecule was subsequently observed in other tumor cell lines including breast, prostate, and colon cancer cells (Welsh, Citation2012). These studies also highlighted the presence of specific receptors with high affinity for 1,25(OH)2D3 that appeared to be essential for the growth inhibitory activity exerted by Vit D (Welsh, Citation2012). In line with these observations, other in vitro studied reported that antisense oligonucleotides, which decreased the intracellular levels of VDR, reduced the sensitivity of tumor cells to the antiproliferative effects of 1,25(OH)2D3 (Hedlund et al., Citation1996; Welsh, Citation2012). On the contrary, VDR overexpression resulted in a potentiation of cell growth arrest (Hedlund et al., Citation1996; Welsh, Citation2012; Zhuang et al., Citation1997). Interestingly, recent studies have shown that 1,25(OH)2D3 may also affect ovarian cancer cells proliferation by decreasing human telomerase reverse transcriptase (hTERT) mRNA through a small non-coding RNA (Ikeda et al., Citation2003; Kasiappan et al., Citation2012). Consistent with these observations a recent experimental investigation has shown that, in ovarian tumor and ovarian cancer cell lines, microRNA-498 (miR-498) induced by 1,25OH2D3 decreased hTERT mRNA expression, fostered cell death, and suppressed tumor growth (Kasiappan et al., Citation2012). Conversely, the ability of 1,25OH2D3 to decrease hTERT mRNA and to suppress ovarian cancer growth was compromised in the absence of miR-498, following its depletion in cell lines and in tumor-bearing mice (Kasiappan et al., Citation2012). Finally, Vit D has been reported to foster several types of malignant cells to undergo differentiation toward more mature phenotypes or to induce cell death by triggering apoptosis according to the cell type (Gocek & Studzinski, Citation2009).
Table 1. Proposed mechanisms underlying chemopreventive effects of Vitamin D.
Effects of Vit D on cyclin/cycline-dependent kinase system
Many investigations undertaken with the aim of assessing a direct effect of 1,25OH2D3 on the expression levels of genes encoding for intracellular inhibitors of the cell cycle demonstrate that this molecule may increase the expression of cyclin-dependent protein kinase (CDK) inhibitors p21 and p27, while it decreases the expression of cycline regulatory proteins such as cyclin-dependent kinase 2 (Cdk2) (Colston & Hansen, Citation2002; Hager et al., Citation2001; Wang et al., Citation1996; Yang & Burnstein, Citation2003). These phenomena ultimately lead to a growth arrest of cells in the G0/G1 phase. In addition, Vit D has been shown to inhibit human breast cancer and prostate cancer cell-cycle progression by blocking cells in G1/S transition (Istfan et al., Citation2007; Jensen et al., Citation2001). This effect appears to be due to the conversion of the retinoblastoma gene (Rb), which is a direct target of cyclin-CDK complexes, in its active hypophosphorylated form (Jensen et al., Citation2001). Furthermore, other in vitro studies on SCC cells show that a 30 h exposure of these cells to 1,25(OH)2D3 induces the overexpression of p18 tumor suppressor gene but not that of p27 or p19 gene, which regulates G1 progression, by forming a stable complex with CDK4 or CDK6 and by preventing the activation of CDK kinases (Gedlicka et al., Citation2006). However, in LNCaP human prostate cancer cell line, 1,25(OH)2D3 induces a marked increase of p21 gene while, in RWPE-1 prostate epithelial cells, VDR may epigenetically regulate p21 gene expression by generating histone modifications in the promoter (Flores et al., Citation2010). Moreover, 1,25(OH)2D3 may also regulate p21 expression levels by modulating miR-106b expression (Thorne et al., Citation2011). Conversely, in MCF-7 human breast cancer cells 1,25(OH)2D3 show minimal effects on the expression levels of mRNA coding for p21 (Verlinden et al., Citation1998). These findings suggest that, according to the cell types, the growth inhibitory effect of 1,25(OH)2D3 does not appear to be related to an activation of VDR- mediated p21 gene transcription. In contrast, Swami et al. (Citation2003) highlighted the fact that, in MCF-7 MDA-MB-231 estrogen receptor α positive [ERα(+)] and estrogen receptor α negative [ER α(−)] human breast cancer cells, the treatment with 1,25(OH)2D3 induces different profiles of gene expression with a few overlapping genes. These findings further support the hypothesis that different cellular pathways regulated by 1,25(OH)2D3 may be involved in the growth inhibitory effects in different tumor cells.
Vit D-mediated regulation of the forkhead box O (FoxO) proteins
The forkhead box O (FoxO) proteins belong to a family of transcription factors that plays an important role in tumor suppression by upregulating target genes involved in cell-cycle arrest and apoptosis (An et al., Citation2010). In particular, FoxO1 (FKHR), FoxO3A (FKHRL1), FoxO4 (AFX), and FoxO6 regulate cell proliferation and differentiation. They are inhibited by phosphatidylinositol-3-kinase (PI3K), which stimulates their Akt-dependent phosphorylation and nuclear export. The biological functions of several members of the FoxO family are inhibited by phosphorylation induced by mitogen-activated protein kinases (MAPKs) such as ERK and p38. Interestingly, recent findings show that the interaction between 1,25(OH)2D3 and VDR induces post-translational modifications and functional alterations of FoxO proteins (An et al., Citation2010). In fact, in vitro studies report that the treatment of human head and neck SCC (HNSCC) with 1,25(OH)2D3 potentiates the binding of FoxO3A and FoxO4 proteins to FoxO promoter target genes and causes a block in the mitogen-induced FoxO protein nuclear export (An et al., Citation2010). Furthermore, in vitro investigations on human neuroblastoma cells show that a 4 h exposure of these cells to 1,25(OH)2D3 induces the deacetylation and the dephosphorylation of FoxO. Consistent with these observations, the arrest of cell-cycle progression induced by Vit D is not observed in cells lacking FoxO3 and FoxO4 (An et al., Citation2010). These findings further indicate that FoxO proteins appear to play a key role as mediators of the anti-proliferative effects of Vit D in some human tumors.
Insulin growth factor modulation by Vit D
Experimental evidence shows that Vit D may also negatively affect cell proliferation by interfering with several growth factors. In particular, Vit D appears to be implicated in the regulation of insulin growth factor (IGF) and in that of certain IGF-binding proteins including the major binding protein IGFBP-3 (Boyle et al., Citation2001; Matilainen et al., Citation2005; Peng et al., Citation2004; Teegarden & Donkin, Citation2009). These observations are in line with the results from in vitro studies on MCF-7 and Hs578T human breast cancer cell lines showing that two Vit D analogues EB1089 and CB1093 may inhibit the stimulating effects of IGF-I on cell growth and may enhance the production of IGFBP-3 which, in turn, regulates the promoting activity of IGF-I and IGF-II on cell proliferation (Colston et al., Citation1998). Similar effects were observed in prostate cancer cells following their exposure to Vit D or its analogues (Huynh et al., Citation1998; Sprenger et al., Citation2001). The role of IGFBP-3 as a critical mediator of the antiproliferative activity of Vit D has been further highlighted by other studies which show that antisense oligonucleotides against IGFBP-3 antagonize the growth-inhibiting effects of Vit D in androgen-responsive LNCaP human prostate cancer cells (Boyle et al., Citation2001; Krishnan et al., Citation2004). On one hand, in agreement with these data, Peng et al. (Citation2008) have recently shown that, in the LNCaP human prostate cancer cell line, high concentrations of androgens exert growth inhibitory effects at least partially through the IGFBP-3-p21/p27 pathway. On the other hand, in vivo studies by Nickerson and Huynh (Citation1999) show that the administration of Vit D analog EB1089 to rats for 14 d increases the expression of several isoforms of IGFBPs, including IGFBP-3, in the prostatic tissue and that these effects were associated with a reduction of prostate volume. As IGFBP-3 has been shown to possess pro-apoptotic, antimetastatic, and anti-angiogenic activities against prostate cancer cells (Massoner et al., Citation2009), it is conceivable to hypothesize that the modulation of IGFP-3 expression by Vit D may be a possible effective therapeutic option in the clinical treatment of prostate cancer.
Transforming growth factor-β modulation by Vit D
Transforming growth factor-β (TGF-β) is a member of growth factor of the namesake superfamily of growth factors which is implicated in the regulation of several important biological processes such as cell proliferation, differentiation, motility, adhesion, organization, and programmed cell death (Massagué, Citation2008). TGF-β is known to inhibit the proliferation of normal epithelial cells and the early steps of carcinogenesis while it fosters the later steps of cancer progression, e.g., cell motility, invasion, and metastasis (Massagué, Citation2008). Vit D and TGF-β share similar effects on cell growth and differentiation (Daniel et al., Citation2007; Wu et al., Citation1998). Experimental studies stress that, according to the cell type, Vit D may increase the expression levels of TGF-β and that of its receptors (Chen et al., Citation2002; Daniel et al., Citation2007; Koli & Keski-Oja, Citation1995; Tu et al., Citation2013; Wu et al., Citation1997a,Citationb, Citation1998; Yanagisawa al., Citation1999) or its secretion (Bizzarri et al., Citation2003; Koli & Keski-Oja, Citation1995). These effects, which may in part, account for the anti-proliferative effects of Vit D, were also described as occurring in various breast cancer cell lines such as MCF-7, MDA-MB-231, or MCF10CA (Lee et al., Citation2006; Swami et al., Citation2003; Wu et al., Citation1998; Yang et al., Citation2001) and in prostate cancer cells (Murthy & Weigel, Citation2004; Peehl et al., Citation2004). In particular, some of these studies show that short-term exposure (<12 h) to 1,25(OH)2D3 or to its analog EB1089 results in an increased expression level of TGF-β and/or TGF-β receptors in breast cancer cells (Yang et al., Citation2001). In addition, other in vitro observations on LNCaP prostate cancer cells show that the growth-inhibiting effects of 1,25(OH)2D3 on these tumor cells appear to be associated with the increased expression and secretion of the growth differentiation factor-15 (GDF-15), another member of the TGF-β superfamily of growth factors (Lambert et al., Citation2006). Interestingly, the effects of a long-term treatment with 1,25(OH)2D3 on the mRNA levels encoding different members of the family TGF-β are also reported on other tumor cell types such as colorectal cancer cells or squamous carcinoma cells (Lin et al., Citation2002; Pálmer et al., Citation2003).
Vit D interaction with Wnt/β-catenin-signaling pathways
Another possible mechanism by which Vit D may halt cell proliferation involves the inhibition of some of the numerous functions mediated by the Wnt/β-catenin-signaling pathway. To this end, in vitro observations show that in colon cancer cell lines 1,25(OH)2D3 can block the transcriptional regulation mediated by β-catenin by decreasing the formation of the transcriptional complex TCF4-β-catenin (Larriba et al., Citation2013). Consistent with this hypothesis, Xu et al. (Citation2010) have demonstrated that administration of 1,25(OH)2D3 or that of its analogues for 12 weeks reduces the number of polyps in the colon mucosa and that, in the small intestine and in the colon, this effect is associated with a reduced expression of target genes for β-catenin. The effects mediated by Vit D may also indirectly affect the function of β-catenin through an increased production of E-cadherin, a membrane protein that binds β-catenin, thus preventing its nuclear localization and transactivation (Pálmer et al., Citation2001). However, there is evidence that 1,25(OH)2D3 may also inhibit the growth of many different cells without affecting cadherin expression. These findings indicate that the up-regulation of E-cadherin is just one of the mechanisms by which Vit D may negatively affect the β-catenin signaling pathway (Shah et al., Citation2006). These observations also indicate that the effects of 1,25(OH)2D3 on the growth and differentiation of many different epithelial cancer cells may be, in part, explained by its ability to differentially regulate the activity of VDR, E-cadherin, and β-catenin/TCF pathways (Beildeck et al., Citation2009; Shah et al., Citation2006). 1,25(OH)2D3 may also interact with the Wnt/β-catenin-signaling pathway by affecting the expression of Wnt regulators, for instance, by up-regulating the expression of the Wnt antagonist Dickkopf-1 (DKK-1) protein (Pendás-Franco et al., Citation2008b). However, whether all the DNA binding sites for β-catenin are equally inhibited by VDR and whether the link of β-catenin in different sites is equally influenced by 1,25(OH)2D3 and VDR remain still unraveled. Further studies may better define these interactions.
Vit D and apoptosis
The induction of apoptosis is an additional, important mechanism by which Vit D appears to exert its chemopreventive effects on cancer cell growth (Vanoirbeek et al., Citation2011). Vit D has been shown to promote apoptosis in breast cancer, prostate cancer, colon cancer, and SCC cells (Gocek & Studzinski, Citation2009). However, this phenomenon is not univocal. For instance, Zhang et al. (Citation2005) have reported that, in ovarian cancer cells, Vit D may inhibit apoptosis. These findings are in agreement with the results of several studies showing that Vit D may positively or negatively modulate the expression of anti-apoptotic or pro-apoptotic factors according to the cell type (Díaz et al., Citation2000; Pereira et al., Citation2012). Although the mechanisms by which Vit D may promote apoptosis remain to be fully clarified, experimental evidence highlights the fact that 1,25(OH)2D3 can trigger the intrinsic pathway of programmed cell death 1,25(OH)2D3 (Guzey et al., Citation2002). In this context, in vitro studies on colorectal cancer cells show that 1,25(OH)2D3 and its analogue EB1089 may promote apoptosis by a p53-independent mechanism (Díaz et al., Citation2000). These investigations also show that these molecules may inhibit apoptosis by down-regulating the expression of anti-apoptotic and pro-survival proteins such as Bcl-2, Bcl-XL, or by increasing the expression of pro-apoptotic proteins such as Bax, Bak, and Bad (Díaz et al., Citation2000). Additionally, these studies also show that the increased expression of Bak and the reduced expression of BCl-2 in response to EB1089 were more marked compared with that induced by 1,25(OH)2D3 (Díaz et al., Citation2000). In line with these findings, Blutt et al. (Citation2000) demonstrate that continuous 6-d exposure of LNCaP cells to 1,25(OH)2D3 induces apoptosis and that this phenomenon was associated with the down-regulation of anti-apoptotic proteins Bcl-2 and Bcl-XL and with the up-regulation of pro-apoptotic protein Bax. More recently, Pan et al. (Citation2010) have shown that 1,25(OH)2D3 may promote apoptosis also in the HCG-27 gastric cancer cell line. This effect appears to be the result of the up-regulation of PTEN, a tumor suppressor gene that negatively regulates the anti-apoptotic activity of protein kinase B (Akt), mediated by VDR. Furthermore, in the MCF-7 cell line, the treatment with 1,25(OH)2D3 induced an increase in the level of the pro-apoptotic Death Associated Protein-3 (DAP-3), Fas-Associated Death Domain (FADD), and the caspases-3, -4, -6, and -8 (Swami et al., Citation2003). Consistent with these observations in vitro studies on squamous cell carcinoma SCC25 cells and colon cancer SW480-ADH cells show that Vit D may potentiate its pro-apoptotic effects by increasing the gene expression of the pro-apoptotic protein G0–G1 switch 2 (G0S2) (Pálmer et al., Citation2003) or by activating caspase effector molecules (Pálmer et al., Citation2001). Furthermore, more recent in vitro studies from Sergeev (Citation2012) suggest that, in breast cancer cells, Vit D can act as an apoptotic initiator that directly recruits Ca(2+)-dependent apoptotic effectors such as Ca(2+)-dependent μ-calpain and Ca(2+)/calpain-dependent caspase-12 which are capable of executing apoptosis. Finally, Kasiappan et al. (Citation2012) have recently reported that, in OVCAR3 ovarian cancer cells, 1,25(OH)2D3 destabilizes telomerase reverse transcriptase (TERT) mRNA, inducing apoptosis through telomere attrition and the down-regulation of telomerase activity. The multiple mechanisms underlying Vit D-mediated apoptosis observed in different tumor cell lines may be, in part, explained by the need for tumor cells to develop different mechanisms that may be useful for escaping the pro-apoptotic effects induced by Vit D.
Vit D and autophagy
Autophagy or autophagocytosis is a catabolic process by which cells may degrade cytosolic macromolecules and intracellular components through the lysosomal machinery (Singletary & Milner, Citation2008). This process plays a key role in the regulation of several important biological processes such as cell growth, development, and homeostasis, by maintaining a balance among the synthesis, degradation, and subsequent recycling of cellular products. Although autophagy is generally regarded as a survival strategy or a mechanism that protects cells from stressful situations such in the case of lack of energy reserves or oxidative stress, it can also be modulated to determine the death of cancerous cells (Singletary & Milner, Citation2008). Therefore, unlike apoptosis, autophagy, in response to stressful stimuli, can contribute either to cell survival or to cell death (Morselli et al., Citation2009; Singletary & Milner, Citation2008). Many food components such as selenium, resveratrol, curcumin, and Vit D itself have been reported to promote autophagy (Singletary & Milner, Citation2008; Wu & Sun, Citation2011). The first evidence regarding the permissive effect of 1,25(OH)2D3 on autophagy was reported by Mathiasen et al. (Citation1999). These authors showed that 1,25(OH)2D3 and two analogues EB1089 and CB1093 induced growth arrest in MCF-7 breast cancer cells expressing the tumor suppressor gene p53 and in T47D breast cancer cell lines line lacking p53. Surprisingly, the same studies also highlighted the fact that the growth-inhibiting effects of Vit D and its analogues were also caspase independent and that the overexpression of the anti-apoptotic protein Bcl-2, completely protected tumor cells from autophagy induced by these molecules (Mathiasen et al., Citation1999). Consistent with these data, other in vitro studies reported that Vit D analog EB1089 induced tumor cell death by a mechanism not related to caspase activation and which consisted in the induction of chromatin condensation and DNA fragmentation (Høyer-Hansen et al., Citation2005). In particular, these investigations showed that, in MCF-7S1 tumor cells, autophagic activity could be increased by protein Beclin-1, also known as autophagy-related gene ATG6. Beclin-1 is a Bcl-2-interacting protein that promotes, in association with its binding partner class III phosphoinositide 3-kinase (PI3K), autophagosome formation (Høyer-Hansen et al., Citation2010). It may also function as a brake for autophagy and autophagic cell death when associated with Bcl-2 (Høyer-Hansen et al., Citation2010). Conversely, this phenomenon was inhibited by the mammalian target of rapamycin protein (mTOR) (Høyer-Hansen et al., Citation2007). These findings are consistent with those of Wang et al. (Citation2008) showing that, in HL-60 human myeloid leukemia cells, Vit D triggered autophagy by up-regulating Beclin-1 and by down-regulating mTOR levels. Furthermore, additional evidence showed that Vit D-induced autophagy may be mediated by CDK inhibitors. For instance, Tavera-Mendoza et al. (Citation2006) showed that 1,25(OH)2D3 contribute to make SCC25 cells knocked down for p19(INK4D) gene expression more susceptible to cell death by autophagy as this gene protects cells from autophagy-induced death (Høyer-Hansen et al., Citation2005). This effect was also noted in MCF-7 human breast cancer cells (Høyer-Hansen et al., Citation2005). However, 1,25(OH)2D3 has been shown to decrease the circulating levels of TNF-α, a phenomenon that may lead to a decreased autophagic activity induced by this molecule (Stubbs et al., Citation2010). In addition, Vit D may also inhibit the release of IFN-γ from macrophages and peripheral blood mononuclear cells (Wu & Sun, Citation2011). This effect may result in an inhibition of IFN-γ induced activation and potentiation of lysosomal activity of macrophages, recruitment of autophagic proteins and, ultimately, may lead to a decrease of autophagy (Wu & Sun, Citation2011). Another possible target for the chemopreventive activity of Vit D on cancer progression is the nuclear factor kappa B (NF-κB), a nuclear transcription factor involved in the regulation of many genes implicated in inflammation, growth regulation, apoptosis, autophagy, carcinogenesis, and malignant progression (Aggarwal, Citation2004; Baldwin, Citation2012). In this context, Tse et al. (Citation2010) reported that Vit D3 inhibited NF-κB activity in human breast cancer cells. Likewise, a similar effect was observed by other authors on colorectal cancer cells (Schwab et al., Citation2007) and prostate cancer cells (Krishnan & Feldman, Citation2010). Nevertheless, as opposing results have been also reported on the effects of Vit D on NF-kB expression levels (Bao et al., Citation2010; Janjetovic et al., Citation2011; Krishnan et al., Citation2007) further studies may better define the role of NF-kB in autophagy and, consequently, the potential therapeutic impact of Vit D in modulating this phenomenon in cancer cells.
Vit D and cell differentiation
Experimental studies show that Vit D may also induce differentiation in normal and neoplastic cells which, in some case, may be associated with a reduced proliferation rate (Gocek & Studzinski, Citation2009). The differentiating activity of Vit D is associated with the increased expression and/or activation of several intracellular signaling pathways. This may, in part explain, why the mechanisms underlying Vit D-induced differentiation may somehow differ according to the cell types (Gocek & Studzinski, Citation2009). For instance Geng et al. (Citation2011) showed that CYP27B1/1α-hydroxylase is required for osteoblast differentiation of human marrow stromal cells. Recent studies suggest that the anti-apoptotic effects of 1,25(OH)2D3 on osteoblasts and osteocytes are mediated by Src, PI3K, and JNK kinases (Gocek & Studzinski, Citation2009). The association of VDR with other proteins appears to be important in Vit D-induced osteoblast differentiation (Gocek & Studzinski, Citation2009; van Driel et al., Citation2006; Woeckel et al., Citation2013). 1,25(OH)2D3 may also regulate keratinocytes differentiation by increasing intracellular calcium levels through the induction of the expression of calcium receptor (CaR) and phospholipase C (PLC) which are critical for calcium to stimulate keratinocyte differentiation (Bikle, Citation2012). Additionally, Vit D has been shown to increase, via AP-1 activation, the expression of several genes involved in the regulation of keratinocytes differentiation such as involucrin, transglutaminase, loricrin, and filaggrin and that of cornified envelope formation while inhibiting the proliferation of keratinocytes (Bikle, Citation2012). Moreover, time-dependent changes in the expression of VDR co-activators were noted during cell differentiation. It has been hypothesized that these changes may contribute to the temporal sequence of Vit D-mediated gene expression during keratinocytes differentiation (Bikle, Citation2012). 1,25(OH)2D3 has also been shown to facilitate myogenic differentiation by increasing the expression of IGF-II and Follistatin (Lee et al., Citation2010) and by decreasing the expression of the insulin growth factor I (IGF-I) and that of myostatin, a negative regulator of skeletal muscle mass (Garcia et al., Citation2011; Lee et al., Citation2010). In contrast, in human colon and breast cancer cells, Vit D appears to foster tumor cell differentiation by increasing the expression levels of proteins such as β-catenin and E-cadherin (Lopes et al., Citation2012; Pendás-Franco et al., Citation2008a). In particular, on one hand, the binding of β-catenin to VDR may cause the loss of this molecule from the transcriptional complex TCF-4-β-catenin in the nucleus. This phenomenon ultimately results in a decreased cell proliferation (Larriba et al., Citation2013). One of the proposed mechanisms that may account for the reduced cell proliferation associated with cell differentiation induced by Vit D may be related, as emphasized in Caco-2 cells, to the marked inhibitory effects of this molecule on the expression of EGFR at both mRNA and protein levels (Gocek & Studzinski, Citation2009). On the other hand, in vitro studies on MDA-MB-453 human breast cancer cells have shown that their treatment with 1,25(OH)2D3 resulted in accumulation of integrins, paxillin, and focal adhesion kinase and their phosphorylation (Pendás-Franco et al., Citation2007). Conversely, the mesenchymal marker N-cadherin and the myoepithelial marker P-cadherin resulted down-regulated. These findings suggest that 1,25(OH)2D3 may revert the myoepithelial phenotype associated with more aggressive forms of human breast cancer. However, not all breast cancer cell lines show a similar response to 1,25(OH)2D3. The difference appears, in part, to be due to the lack or decrease of VDR expression or function (Gocek & Studzinski, Citation2009; Valrance et al., Citation2007). However, alterations in 1,25(OH)2D3 metabolizing enzymes, which can decrease Vit D levels below its effective concentration, cannot be ruled out (Byrne & Welsh, Citation2007; Gocek & Studzinski, Citation2009). For instance, in ER(+) breast cancer cell lines, 1,25(OH)2D3 may facilitate cell differentiation by converging VDR and estrogen receptor pathways to regulate BRCA-1, a tumor suppressor gene that encodes a nuclear phosphoprotein that plays a role in maintaining genomic stability (Campbell et al., Citation2000; Roy et al., Citation2011). This effect contributes to regulating the balance between differentiation and proliferation signaling (Campbell et al., Citation2000; Gocek & Studzinski, Citation2009). Likewise breast cancer, experimental observations provide evidence that 1,25(OH)2D3 may also induce differentiation in prostate cancer cells (Gocek & Studzinski, Citation2009). To this end, in vitro studies demonstrated that the treatment of LNCaP cells with 1,25(OH)2D3 up-regulates the expression of the androgen receptor (AR) and increases the secretion of prostate-specific antigen (PSA), a differentiation marker for epithelial prostate cells (Gocek & Studzinski, Citation2009). The up-regulation of AR may cause, in turn, an increase in the expression levels of VDR which selectively enhances the AR-mediated androgenic pro-differentiating effects but not the proliferation activity. In contrast, microarray analysis by Krishnan et al. (Citation2004) demonstrates that in LNCAP tumor cells 1,25(OH)2D3 increases the expression of insulin-like growth factor-binding protein-3 (IGFBP-3), which functions as an inhibitor of cell proliferation, by up-regulating p21/Cip1 (Boyle et al., Citation2001). In addition, Vit D treatment may also cause the up-regulation of a “prostate differentiation factor,” a member of the bone morphogenetic protein (BMP) family, which is generally involved in growth and differentiation of embryonic and adult tissues (Lambert et al., Citation2006). Interestingly, these studies also revealed that 1,25(OH)2D3 regulates certain androgen-responsive genes as well as genes that encode enzymes involved in androgen catabolism. Prostate cancer cells are also known to undergo “trans-differentiation” to a neuroendocrine phenotype which is an aggressive form of prostate cancer. Recent evidence suggests a key role for NF-κB, as well as IL-6, in this process (Mori et al., Citation2009). In this context, Vit D up-regulates the expression of CCAAT/enhancer-binding protein beta (C/EBP β), a transcriptional activator that regulates genes involved in immune and inflammatory responses, and which cooperates with NF-κB in regulation of the secretion of IL-6 in neuroendocrine human prostate cancer cells (Xiao et al., Citation2004). These data suggest that 1,25(OH)2D3 may be promising as a potential therapeutic agent in the treatment of this aggressive form of prostate cancer. Experimental findings show that Vit D may induce leukemic cells to differentiate. In particular, in vitro studies show that the exposure of human myeloid leukemia cells to physiological concentrations of 1,25(OH)2D3 for 36–48 h induces their differentiation into functional monocytes (Hughes et al., Citation2010). The differentiating activity of Vit D is associated with the increased expression and/or activation of different intracellular pathways such as protein kinase C (PKC), PI3K/AKT pathway, p42 extracellular-regulated kinase (p42-ERK), p38-ERK, and the c-Jun N-terminal kinases (JNK) families of mitogen-activated protein kinases (MAPKs) (Hughes et al., Citation2010). Pharmacological or genetic blockade of these pathways may abrogate 1,25(OH)2D3-driven monocytic differentiation.
Antioxidant defense and DNA
On one hand, free radicals, also known as reactive oxygen species (ROS), in concert with reactive nitrogen species (RNS) may play a dual role in cell homeostasis since they may function as a second messenger in controlling cell proliferation and differentiation. Furthermore, ROS may also foster cellular senescence (Dröge, Citation2003) and apoptosis (Circu & Aw, Citation2010). The cumulative production of ROS and RNS in response to endogenous or exogenous insults, i.e., the “oxidative stress”, is a typical phenomenon that can be observed in many types of cancer cells (Valko et al., Citation2006). A redox imbalance occurring within these cells may ultimately facilitate oncogenic stimulation. On the other hand, the induction of antioxidant defense mechanisms may reduce the biological impact of ROS (Valko et al., Citation2006). In line with these observations, several in vitro and in vivo studies highlight the fact that 1,25(OH)2D3 exerts antioxidative activities on colorectal cancer (Nair-Shalliker et al., Citation2012). In particular, it has been shown that DNA damage induced by oxidative stress, as measured by the amount of 8-hydroxy-2′-deoxyguanosine, is high in the epithelium of the distal colon of VDR-knockout mice and it is reduced in the epithelium of human colon after a daily supplement of 800 IU (international units) of Vit D (1 IU is the biological equivalent of 0.025 µg cholecalciferol or ergocalciferol) (Fedirko et al., Citation2010). These findings further suggest that Vit D may protect against oxidative stress-induced DNA damage in humans. In line with this hypothesis, Banakar et al. (Citation2004) report that the treatment of rats with calcitriol increases the expression of VDR and markedly reduces the levels of malondialdehyde. However, no substantial evidence has been obtained so far regarding a direct relationship between Vit D and prevention of DNA damage at a population level. Nevertheless, the clinical and epidemiological observations that suggest a correlation between deficient levels of calcidiol and increased incidence of diseases associated with increased levels of DNA damage in humans warrant further extensive investigation.
Induction of antioxidant enzymes
1,25(OH)2D3 is known to increase the expression of numerous enzymes of the antioxidant defense system in humans (Fleet et al., Citation2012). For instance, in vitro studies show that the exposure of prostate cancer cells and MCF-7 breast cancer cells to 1,25(OH)2D3 or its analogues induce the expression of thioredoxin reductase 1 (TXNRD1), an enzyme that converts thioredoxin to its reduced form needed to perform its antioxidant function (Kovalenko et al., Citation2010; Peehl et al., Citation2004; Swami et al., Citation2003). In addition, 1,25(OH)2D3 has been shown to increase the production of superoxide dismutase 1 (SOD1) and 2 (SOD2) in prostate epithelial cells (PECs) and in androgen-sensitive prostate cancer cells (LNCaP), respectively (Lambert et al., Citation2006; Peehl et al., Citation2004). Furthermore, other in vitro observations show that the treatment of the human prostate epithelial cell line RWPE-1, and that of BPH-1 benign prostatic hyperplasia (BPH) epithelial cell line or OVCAR3 ovarian carcinoma cell line, with 1,25(OH)2D3, increases the intracellular levels of glucose-6-phosphate dehydrogenase (G6PDH), an enzyme which regulates the intracellular levels glutathione (Bao et al., Citation2008; Kovalenko et al., Citation2010; Zhang et al., Citation2005). This effect ultimately protects cells from apoptosis induced by H2O2. These findings are in line with experimental evidence showing that the expression levels of G6PDH in prostatic epithelial cells are modulated by 1,25(OH)2D3 through VDRE located in the first intron of the gene coding for G6PDH (Bao et al., Citation2008). However, this phenomenon was not observed in DU145 and CWR22 prostate cancer cells (Bao et al., Citation2008). The different responses of these cell lines to 1,25(OH)2D3 treatment may be, in part explained, with the loss of AR expression, which is a characteristic of tumor cells less susceptible to Vit D treatment (Stewart & Weigel, Citation2004; Ting et al., Citation2007a,Citationb). Furthermore, the protection from oxidative stress mediated by Vit D, may also be indirectly due to the induction of the nuclear factor erythroid-derived 2-Like 2 (NFE2L2), a transcription factor that controls the gene expression of several enzymes of the antioxidant systems such as glutathione peroxidase 3 (GPX-3), heme oxygenase 1 (HMOX-1), and aldo-keto reductase 1C2 (AKR1C2) (Kovalenko et al., Citation2010). The effects of Vit D on the oxidative system further support the clinical benefit of this molecule in cancer chemoprevention.
Regulation of proteins involved in DNA repair
Experimental in vivo observations show that VDR-deficient mice are more susceptible to the development of skin tumors either induced by chemical carcinogens such as 7,12-dimethylbenzanthracene (DMBA) or by chronic UVR exposure (Bikle, Citation2012). These studies suggest that 1,25(OH)2D3 may protect the skin from malignant transformation by controlling keratinocyte proliferation and differentiation, by facilitating DNA repair, and by suppressing the activation of the hedgehog (Hh) pathway following UVB exposure (Bikle, Citation2012). In particular, recent studies show that 1,25(OH)2D3 may protect DNA by regulating the expression of genes coding for DNA repair enzymes (Krishnan et al., Citation2004; Nair-Shalliker et al., Citation2012). In this context, Akhter et al. (Citation1997) have reported that in SCC cells, Vit D analog EB1089 induces the overexpression of the growth arrest and DNA-damage-inducible α (GADD45α) gene, a p53 target gene whose products are involved in DNA repair. It has also been shown that the treatment of ovarian cancer cells with 1,25(OH)2D3, causes cell-cycle arrest at the G2/M transition through p53-independent induction of GADD45α (Jiang et al., Citation2003a,Citationb). The role of GADD45α induction in eliciting the chemopreventive effects of Vit D is supported by the findings that cell-cycle arrest in G2 or in M induced by 1,25(OH)2D3 does not occur following GADD45α deletion (Akter et al., Citation1997; Jiang et al., Citation2003a). Furthermore, microarray analyses performed in MCF-7 breast cancer cells show that the treatment of these cells with 1,25(OH)2D3 increases the mRNA expression levels of other molecules involved in DNA repair such as p53 and proliferating cell nuclear antigen (PCNA) (Swami et al., Citation2003). Additionally, more recent studies show that 1,25(OH)2D3 treatment can protect BPH-1 human prostate epithelial cells from carcinogen-induced genotoxic stress via VDR-mediated transcriptional upregulation of DNA repair genes, ATM and RAD50, thereby facilitating DNA double-strand break repair (Ting et al., Citation2012). Interestingly, on one hand, recent findings stress that in BRCA1-deficient breast cancer cells, Vit D prevents the degradation of the DNA repair protein 53BP1 mediated by cysteine proteinases Cathepsin L (Gonzalo, Citation2014), a lysosomal endopeptidase which is involved in tumor cell proliferation, invasion, and metastasis (Gonzalo, Citation2014; Lankelma et al., Citation2010; Leto et al., Citation2010). On the other hand, recent observations report that the gene encoding the BRCA1 protein is a critical downstream target of Vit D. Consistent with these data Campbell et al. (Citation2000) show that treatment of MCF-7 cells with calcitriol results in a near 6-fold increase in BRCA1 protein and that VDR expression is directly correlated with induction of BRCA1.
Effects of Vit D on the synthesis and metabolism of prostaglandins
It is well established that prostaglandings (PGs) may promote cancer cell proliferation and progression (Wang & Dubois, Citation2010). Since experimental findings demonstrate that 1,25(OH)2D3 may act as a negative modulator of the synthesis and activity of prostaglandins (PGs) (Krishnan & Feldman, Citation2010; Moreno et al., Citation2006), these effects may also, in part, account for the chemo-preventive activity of Vit D on tumor progression. In support of this hypothesis, recent studies have better defined the role of prostaglandin–endoperoxide synthase, a key enzyme of prostaglandin synthesis and more widely known as cyclooxygenase (COX), on carcinogenesis (Wang & Dubois, Citation2010). Increasing experimental and clinical observations show that the inducible isoform of this enzyme, namely COX-2, is overexpressed in many human tumors and in cancer cell lines. Moreover, these findings also show positive relationship between COX-2 overexpression and tumor progression (Cordes et al., Citation2012; Krishnan & Feldman, Citation2011; Thill et al., Citation2012). Alterations in the expression of COX-2 and its product, prostaglandin E2 (PGE2), have been observed in breast cancer and colorectal cancer where these molecules appear to be involved in several key steps of malignant progression such as tumor initiation, tumor cell proliferation, and metastasis formation (Thill et al., Citation2012; Wang & Dubois, Citation2010). Thus, COX-2 may be considered an appropriate target for cancer chemoprevention and treatment. To this end, some in vitro studies carried out on LNCaP and PC-3 prostate cancer cells have shown that the treatment with 1,25(OH)2D3 decreases the expression levels of COX-2 and that of prostaglandin receptors EP2 and FP, whereas it increases the expression of 15-hydroxyprostaglandin-d dehydrogenase (15-PGDH) a NAD+-dependent enzyme involved in the degradation of PGE2 (Krishnan & Feldman, Citation2010; Moreno et al., Citation2005). Interestingly, Thill et al. (Citation2012) have recently reported that VDR and COX-2 expressions are inversely correlated in malignant breast cell lines. This phenomenon has also been observed in ovarian cancer tissues (Cordes et al., Citation2012). These findings support the hypothesis that 1,25(OH)2D3 may inhibit tumor cell proliferation by reducing the intracellular levels of biologically active prostaglandins. In line with these observations, more recent in vitro studies by Yuan et al. (Citation2012) report that a 72-h exposure of MCF-7 breast cancer cell to 1,25(OH)2D3 results in a significant decrease of COX-2 mRNA expression levels and in that of PGE2 in cell culture supernatant. These data suggest a possible therapeutic effectiveness of the association calcitriol with non-steroidal anti-inflammatory drugs (NSAIDs) in the prevention and treatment of breast and prostate cancers (Krishnan & Feldman, Citation2010; Moreno et al., Citation2005). This drug association may also have the vantage of lowering the dose of NSAIDs thus reducing their toxic effects (Moreno et al., Citation2005).
Target cells of Vit D
Effects of Vit D on cancer stem cells
Vit D is one of the molecules involved in the regulation of stem cell homeostasis. 1,25(OH)2D3 exerts its important biological effects on both adult stem cells (ASC) (Cianferotti et al., Citation2007; Zhou et al., Citation2010) and cancer stem cells (CSCs) (Feldman et al., Citation2014; Maund et al., Citation2011; So et al., Citation2011). DNA repair and protection from oxidative damage are processes that mainly affect ASCs, while cell-cycle arrest and induction of apoptosis limit the expansion of the CSCs population. Extensive research has recently been carried out to evaluate the direct effects of 1,25(OH)2D3 on stem cells (Fleet et al., Citation2012). Most of the experimental data regarding the molecular mechanisms underlying the inhibiting effects of 1,25(OH)2D3 on CSC growth and differentiation have been obtained following investigations carried out on primary cancer cell cultures or on established cancer cell lines (Pervin et al., Citation2013). These studies highlighted the fact that in mice the proliferation of prostate stem cells was inhibited by 1,25(OH)2D3. Further experiments performed to better clarify the possible mechanisms underlying this effect showed that the interaction between Vit D and VDR stimulates the production of interleukin-1α (IL-1α) (Maund et al., Citation2011). This pro-inflammatory cytokine, in turn, mediated the anti-proliferative effects of 1,25(OH)2D3 in adult prostate progenitor/stem cells (PrP/SC) by promoting cell-cycle arrest and senescence (Maund et al., Citation2011). Furthermore, Fedirko et al. (Citation2009) have recently shown that 1,25(OH)2D3 treatment and calcium supplements decrease the expression of the human telomerase reverse transcriptase (hTERT) in cells of the upper portion of the colon. These observations indicate that Vit D may indirectly inhibit the expansion of this cell population and protect it from potential genetic mutations. In line with these observation, Kasiappan et al. (Citation2012) described how 1,25(OH)2D3 decreases the mRNA expression of hTERT by inducing the expression of non-coding small RNA microRNA-498 (miR-498) in ovarian tumor cells. These effects ultimately result in the suppression of ovarian cancer growth. Finally, 1,25(OH)2D3, and its analogues have been reported to regulate the expression of CD44, a specific marker of breast cancer stem cells, in human breast cancer cells in vitro (Parvin et al., Citation2013; So et al., Citation2011). These studies provide a basis for preclinical and clinical evaluations of Vit D and its analogues for chemoprevention of cancer stem cells. These observations warrant more extensive studies to assess the impact of Vit D on cancer stem cells.
Effects of vitamin D on vascular cells and angiogenesis
Growing evidence indicates that Vit D may play an important role in the inhibition of tumor angiogenesis (Vanoirbeek et al., Citation2011; Xu et al., Citation2013). This peculiar function has been defined with the term “angioprevention” (Tosetti et al., Citation2002). On one hand, experimental studies suggest that the preventive activity of 1,25(OH)2D3 on tumor angiogenesis might be the consequence of the effects of this molecule on vascular endothelial cells (EC) (Furigay & Swamy, Citation2004; Mantell et al., Citation2000). On the other hand, in vitro observations have highlighted the presence of VDR on cultured bovine aortic endothelial cells, in human capillary and in venous endothelial cells (Chung et al., Citation2006, Citation2009; Merke et al., Citation1989). In addition, the expression of the enzyme 1α-hydroxylase, a key enzyme involved in the biosynthesis of 1,25(OH)2D3, has also been reported in these cells (Chung et al., Citation2009; Merke et al., Citation1989; Suzuki et al., Citation2009; Zehnder et al., Citation2002). Early experimental investigations by Mantell et al. (Citation2000), aimed at evaluating the effect of Vit D on angiogenesis, show that 1,25(OH)2D3 may inhibit the expression of the vascular endothelial growth factor (VEGF) and the formation of new blood vessels in mice transplanted with MCF7 human breast cancer, a tumor which expresses high levels of VEGF. In prostate cancer, 1,25(OH)2D3 has been reported to decrease the expression of VEGF through transcriptional repression of the hypoxia-inducible factor 1(HIF-1) (Ben-Shoshan et al., Citation2007). Furthermore, in SCC tumor cells, 1,25(OH)2D3 has been observed to suppress the expression of the proangiogenic factor IL-8 via NF-kB-dependent pathway thus leading to the inhibition of endothelial cell migration and tube formation (Bao et al., Citation2006). The specific mechanisms underlying this phenomenon consist in a reduced translocation of the p65 subunit of NF-kB to the nucleus that results in a decreased transcription of IL-8 gene mediated by NF-kB. Furthermore, Chung et al. (Citation2009) have highlighted the fact that, in VDR wild-type (WT) or VDR knockout mice inoculated with transgenic adenocarcinoma of the mouse prostate (TRAMP), the tumor vessels are enlarged and their volume increased in KO mice thus suggesting a negative regulation of VDR-Vit D on tumor angiogenesis. These investigations additionally showed that VDR knockout mice had increased expression levels of pro-angiogenic factors such as HIF-1, VEGF, angiopoietin-1 (Ang-1), and platelet-derived growth factor (PDGF). Vit D can increase VEGF mRNA levels in vascular smooth muscle cells (Cardús et al., Citation2006) while in SW480-ADH human colon tumor cells, this molecule has been shown to upregulate the mRNA levels of thrombospondin-1 (THSD1), a potent anti-angiogenic factor (Fernandez-Garcia et al., Citation2005). Other studies aimed at investigating the effect of 1,25(OH)2D3 on normal endothelial cells and tumor-derived endothelial cells (TDECs) showed effects of 1,25(OH)2D3 greater than those elicited in the normal aortic endothelial cells or yolk sac endothelial cells (MYSECs) (Chung et al., Citation2009). Furthermore, Vit D analogues, EB1089, Ro 25-6760, and ILX23-7553, also showed a potent antiproliferative activity against TDECs (Bernardi et al., Citation2002). It has been also demonstrated that the Vit D-dexamethasone association was more effective in inhibiting TDECs growth than each single agent (Chung et al., Citation2009). Other in vitro observations reported that, in TDECs, Vit D increased the intracellular levels of VDR and that of the pro-apoptotic protein p27 which reduces the concentration of signal molecules for angiogenesis, including angiopoietin-2 (Bernardi et al., Citation2002; Flynn et al., Citation2006). Interestingly, on one hand, Chung et al. (Citation2006) compared the effects of calcitriol on SCC TDECs and endothelial cells derived from matrigel (MDECs). These authors pointed out that both these cell types expressed VDR and the interaction with 1,25(OH)2D3 resulted in a 47% growth inhibition of TDECs and in a 12.3% growth inhibition of MDECs. Furthermore, in TDECs, Vit D caused cell-cycle arrest in the G0/G1 phase and a decrease of the number of cells in the S phase, due to the induction of p27 and the down-regulation of p21. These data indicated that TDECs are more susceptible than MDECs to the anti-proliferative effects of 1,25(OH)2D3. On the other hand, Flynn et al. (Citation2006) showed that 1,25(OH)2D3 regulated the expression of several proteins involved in TDEC differentiation and apoptosis. These authors reported that although VDR is present in TDECs and MYSEC and Vit D upregulated VDR in these cells, a 48-h exposure of the cells to dexamethasone further increased VDR expression. Finally, no increase in the intracellular levels of CYP24A4, the predominant enzyme involved in the catabolic inactivation of 1,25(OH)2D3 in normal tissues, was found in TDECs. Conversely this phenomenon occurred in MYSECs (Flynn et al., Citation2006). In line with these findings, Chung et al. (Citation2006) have demonstrated that TDECs may be more sensitive to calcitriol due to novel epigenetic silencing of CYP24A1. Therefore, the direct effects of calcitriol on endothelial cells may play a role in the calcitriol-mediated antitumor activity observed in vivo in animal tumor models. Furthermore, in vitro studies on RWPE1 prostatic epithelial cells highlighted treatment with 1,25(OH)2D3 as causing growth arrest (Kovalenko et al., Citation2010). The subsequent genomic analysis revealed a decrease in the expression level of genes coding for NF-kB and IGF-1. Additionally, the inhibition of the transcription of pro-inflammatory cytokines, including IL-1, IL-6, and IL-17, was noted as occuring after about a 6-h exposure. The same studies also showed that 1,25(OH)2D3 caused a reduction of VEGF and VEGF receptors mRNA levels, including the kinase insert domain receptor (KDR) and neuropilin 1 (NRP1) and the induction of anti-angiogenic factors and that of molecules involved in the protection of cells from oxidative stress and in the homeostasis of cellular redox (Kovalenko et al., Citation2010). Finally, it was shown that, in RWPE1 cells, 1,25(OH)2D3 induced the expression of numerous isoforms of semaphorins, including SEMA 3B, 3F, and 6D (Kovalenko et al., Citation2010). As these molecules may antagonize the proangiogenetic effects of VEGF by a competitive binding to receptor NRP1, this may also partly account for the growth-inhibiting effects of Vit D on prostate cancer cells. An additional mechanism of the preventive effects of Vit D on tumor angiogenesis involves its ability to inhibit COX-2 expression levels (Aparna et al., Citation2008). COX-2 has been shown to exert indirectly its promoting effects on tumor angiogenesis by increasing the synthesis of HIF-1α protein in cancer cells (Sahin et al., Citation2009). Therefore, the inhibition of this enzyme may result in a decreased growth activity of tumors. Moreover, 1,25(OH)2D3 strongly represses DICKKOPF-4 (DKK4), a weak WNT antagonist that promotes invasion and angiogenesis in cultured colorectal cancer (CRC) cells and that is up-regulated in human colon tumors (Pendás-Franco et al., Citation2008b). All these effects may also, in part, account for the significant inhibition of metastasis observed in murine models of prostate and lung cancer treated with Vit D.
Regulation of immune function by vitamin D
Vit D and the immune system
The role of Vit D, as an immunomodulator, was well established nearly 30 years ago (Rook et al., Citation1986). The effects of Vit D on immune system appear to be closely linked to the chemopreventive effects on tumors (Fleet et al., Citation2012). The possible role of Vit D in the regulation of immune responses is strongly supported by the findings that almost all immune cells, including T cells, B cells, monocytes, neutrophils, platelets, macrophages, and dendritic cells express Vit D receptors and that Vit D appears to modulate the activity of these cells (Hewison, Citation2011). The ligand for VDR also showed a synergic activity with Vitamin A, Vitamin K2, and certain chemotherapeutic agents (Funato et al., Citation2002; James et al., 1999). Interestingly, only naive T cells display very low VDR levels, while this receptor is abundantly present upon T cell activation (Hewison, Citation2011). However, the differentiation of monocyte into macrophages or dentritic cells (DCs) has been shown to be associated with a decrease in VDR-expression, making these cells less sensitive to 1,25(OH)2D3 (Hewison, Citation2011). In this context, 1,25(OH)2D3 has been recognized as an important mediator of innate immune responses, enhancing the antimicrobial properties of immune cells such as monocytes and macrophages (Hewison, Citation2011). There is evidence that Vit D modulates the expression of many genes in cells of the innate immune system which encode for proteins that are crucial for autophagy and for the antimicrobial activity (Hewison, Citation2011). In particular, one of these studies reported that Vit D increased the expression levels of genes encoding for the antimicrobial peptides human cathelicidin (CAMP) and β defensin-1 (DEFB1) in isolated human keratinocytes, monocytes, and neutrophils (Wang et al., Citation2004). These molecules form the first line of host defence against microbial pathogens. Another study showed that 1,25(OH)2D3 markedly induced the expression of CAMP and, to a lessen extent, that of defensin β2 (DEFB2/HBD2) in different cell types such as colon cancer, acute myeloid leukemia, keratinocytes, human bone marrow cells-derived macrophages, and bone marrow cells (Gombart et al., Citation2005). These effects occurred following the interaction of Vit D with a specific VDRE present in the promoter region. In particular, in vitro studied showed that 1,25(OH)2D3 stimulates the expression of the pattern recognition receptor NOD2/CARD15/IBD1 gene in primary human monocytic and epithelial cells. As a consequence of the NOD2 downstream signaling activation, a stimulation of NF-κB transcription factor function occurs. This effect, in turn, induces the expression of the gene-encoding antimicrobial peptide defensin β2 (DEFB2/HBD2) (Wang et al., Citation2010a). Furthermore, numerous cytokines can modulate the metabolism of Vit D in macrophages, monocytes and dendritic cells (Hewison, Citation2011). The pro-inflammatory cytokines such as IFN-γ and TNF-α stimulate the synthesis of 1,25(OH)2D3 by increasing the expression of CYP27B1 in monocytes (Zehnder et al., Citation2002). On the other hand, several inflammatory cytokines and agonists of toll-like receptor (TLR), which are transmembrane proteins involved in recognizing and defending against invading pathogens, may increase the expression of CYP27B1 and that of VDR in dendritic cells (Széles et al., Citation2009). In contrast, IL-4 produced by type-2 T-helper lymphocytes potentiates CYP24 gene expression in monocytes, leading to the formation of inactive metabolite 24,25-dihydroxyvitamin D3 (Edfeldt et al., Citation2010). These effects may alter the intracellular levels Vit D metabolites which, in turn, can modulate the function of other immune cells in the microenvironment. Numerous studies have demonstrated that 1,25(OH)2D3 is also involved in the regulation of cell functions of the adaptive immune system (Hewison, Citation2011). Consistent with these findings, Vit D deficiency has been associated with the development of several autoimmune diseases, including ulcerative colitis, Crohn's disease, and also infectious diseases (Cantorna, Citation2012; Meeker et al., Citation2014). However, a very limited number of studies have been performed to assess the role of the immune regulatory function of Vit D in cancer. In this context, Krishnan et al. (Citation2007) reported that calcitriol exhibits anti-inflammatory effects that may account for its inhibitory activity in prostate cancer. More recently, Bessler and Djaldetti (Citation2012) showed that Vit D alters the relationship between immune and cancer cells leading to a significant decrease in the pro-inflammatory cytokines TNF-α and IL-6. On the basis of these results, these authors hypothesized that the reduced production of pro-inflammatory cytokines induced by Vit D may lead to a suppression of tumor development. Furthermore, recent studies by Young and Day (Citation2013) showed that the time elapsing before cancer recurrence following surgical treatment was increased by over 3-fold in head and neck patients receiving 1,25(OH)2D3 as compared with untreated patients. This phenomenon was associated with, and increased, differentiation of blood-derived CD34+ cells into dendritic cells and to a decrease in the peripheral blood and intratumoral levels of immunosuppressive CD34+ cells.
Chemopreventive effects of Vit D on specific tumors: possible mechanisms of action
Effects of vitamin D on breast cancer cells
In vitro and in vivo studies carried out in order to assess the effects of 1,25(OH)2D3 and its semisynthetic analogues on breast cancer cell proliferation and malignant progression showed that different ligands of the VDR are equally effective in inhibiting the growth of ER(+) breast cancer cell lines such as MCF-7, T-47-D, ZR-75-1, SKBR-3 (Krishnan et al., Citation2012), and ER(−) breast cancer cell lines such as BT-20, MDA-MB-435, MDA-MB-231, and SUM-159PT (Flanagan et al., Citation2003; Mehta et al., Citation2012). These data are in agreement with the clinical observations that describe the therapeutic benefit of Vit D and its analogues in both ER(+) and ER(−) breast cancer (Krishnan et al., Citation2012; Lee et al., Citation2008; Li & Brown, Citation2009; Mehta et al., Citation2012). Although the exact mechanisms by which Vit D may exert its growth inhibitory activity on breast cancer cells has not yet been fully understood, in vitro observations indicate that this molecule may affect tumor cell proliferation by causing cell-cycle arrest in the G0/G1 phase, by promoting apoptosis and by inhibiting tumor angiogenesis (Li et al., Citation2005; Nagpal et al., Citation2005; Vanoirbeek et al., Citation2011). The inhibiting effects of Vit D on cell-cycle arrest in G0/G1 entry appears to be due to an increase of the expression of cyclin kinase inhibitors CDKIs, including p21 and p27 which inhibit cell-cycle progression by blocking the activity of CDK complexes with VDR ligands (Jensen et al., Citation2001; Krishnan et al., Citation2012). Furthermore, studies carried out on the MCF-7 cell line showed that 1,25(OH)2D3 reduced, in a time-dependent fashion, the intracellular levels of CDK2, CDK4, cyclin D1, and cyclin A (Jensen, Citation2001; Lowe et al., Citation2003; Verlinden et al., Citation1998). In particular, 1,25(OH)2D3 was shown to prevent the activation of the cyclin D1-CDK4 complex, to decrease cyclin D3 expression, and to inhibit the E2F transcription factor thus decreasing the expression of cyclin A (Jensen et al., Citation2001). However, on one hand, the antiproliferative effects of Vit D on breast cancer cells also appears to be mediated by the induction of TGF-β (Colston & Hansen, Citation2002; Koli & Keski-Oja, Citation1995; Proietti et al., Citation2011) and by the suppression of the protoncogene c-myc expression (Jensen et al., Citation2001; Lopes et al., Citation2012; Saunders et al., Citation1993). In addition, Vit D can block the proliferative activity of insulin and IGF-1, most likely by increasing the expression of IGFBP-3 and IGFBP-5 (Colston et al., Citation1998; Lee et al., Citation2006; Rozen et al., Citation1997). On the other hand, the promoting effect of Vit D on apoptosis in breast cancer cells appears to be the result of decreased levels of Bcl-2, a redistribution of Bax, a release of cytochrome c, and DNA fragmentation (Nagpal et al., Citation2005; van den Bemd & Chang, Citation2002). Furthermore, it was demonstrated that 1α,25(OH)2D3 is involved in the inhibition of transcriptional activity of NF-κB in breast cancer cells (Tse et al., Citation2010). In addition to their direct growth-inhibiting effects, VDR ligands may also inhibit angiogenesis and decrease the invasive and metastatic potential of breast cancer cells in vitro and in vivo (Hansen et al., Citation1994; Mantell et al., Citation2000; van den Bemd & Chang, Citation2002; Vanoirbeek et al., Citation2011). These results support the concept that the combination of VDR ligands with the most common clinically available antitumor agents used in breast cancer treatment might result in a more effective therapeutic response (Krishnan & Feldmann, Citation2011). This hypothesis has been further sustained by in vitro studies which demonstrated that 1,25(OH)2D3 enhances the cytotoxic effects of doxorubicin, paclitaxel, adriamycin, cisplatin, and those induced by irradiation on tumor cells (Chaudhry et al., Citation2001; Lawrence et al., Citation2013; Sundaram et al., Citation2000). Interestingly, recent observations show that Vit D prevents genomic instability due to the Cathepsin L-mediated degradation of DNA repair protein 53BP1 in BRCA-1-negative breast cancer cells (Gonzalo, Citation2014). These results suggest that Vit D may be of clinical relevance in the treatment of aggressive form of breast cancer such as triple-negative BRCA-1-deficient ones.
Vit D on breast cancer: clinical studies
The epidemiologic studies regarding the association between Vit D and breast cancer risk have generated conflicting results so far (Engel et al., Citation2010; Freedman et al., Citation2007; Khan et al., Citation2010; Maalmi et al., Citation2014; Mehta et al., Citation2012; Mohr et al., Citation2011; Rose et al., Citation2013). For instance recent clinical observations showed a significant inverse association between 1,25(OH)2D3 serum concentration and risk of breast cancer with a more pronounced decrease in risk in premenopausal than in perimenopausal women (Engel et al., Citation2010; Maalmi et al., Citation2014; Mohr et al., Citation2011; Mehta et al., Citation2012). In line with these findings, other clinical investigations reported that women with 1,25(OH)2D3 serum concentrations greater than 52 ng/ml showed a significant decrease (50%) in the risk of breast cancer compared with women with circulating levels of 1,25(OH)2D3 lower than 13 ng/ml (Bertone-Johnson et al., Citation2005; Garland et al., Citation2007; Lowe et al., Citation2005). Furthermore, women with 1,25(OH)2D3 serum concentrations above 27 ng/ml showed a decreased risk of breast cancer by 27%, compared with those with circulating levels of this molecule lower than 19.8 ng/ml (Engel et al., Citation2010). These data also showed that the preventive effects of a high plasma concentration of 1,25(OH)2D3 on the onset of breast cancer were more pronounced in women with normal body mass index (BMI), i.e., lower than 25 kg/m2 (Engel et al., Citation2010). These findings also indicate that, in order to maintain plasma concentrations of 1,25(OH)2D3 higher than 30 ng/ml, women leading a sedentary life style and who are exposed for a short period of time to sunlight require an intake of at least 2000 IU/d (Garland et al., Citation2007). Alternatively, it was calculated that 12 min per day of continuous exposure to sunlight and the exposure of at least the 50% skin surface is approximately equivalent to an oral administration of 3000 IU of Vit D (Engel et al., Citation2010; Holick, Citation2013). However, the optimal cut-off levels of Vit D and the threshold values below which a subject may be considered Vit D deficient are still controversial. The optimal dose currently suggested to be administered in order to prevent deleterious consequences due to hypercalcemia is 30 ng/ml (Khan et al., Citation2010). Foods naturally rich in Vit D content more widely consumed by the population are fish (55%), meat (23.5%), cheese (6.8%), and eggs (5.6%). However, no consistent association of breast cancer risk with the consumption of meat, eggs, or dairy foods have been highlighted so far, while the correlation between fish intake and breast cancer incidence still remains debatable (Engeset et al., Citation2006; Kim et al., Citation2009; Pala et al., Citation2009). The geographical area of residence, such as in the case of northern and southern Italy, has been shown to be an important factor in influencing the endogenous synthesis of Vit D (Rossi et al., Citation2009). In fact, an inverse association between Vit D and breast cancer was observed to be much higher in women in southern Italy compared with women in the northern part of the country because of longer exposure to sunlight (Rossi et al., Citation2009). However, conflicting results on the relationship between skin pigmentation and Vit D synthesis were obtained following a study carried out in United States to assess the risk of breast cancer when comparing women of Latin American origin (Hispanic women) and non-Hispanic white women (John et al., Citation2007; Rollison et al., Citation2012). Overall these studies showed an inverse relationship between Vit D intake and incidence of breast cancer either in premenopausal women or in post-menopausal women (Anderson et al., Citation2010; Rollison et al., Citation2012; Shin et al., Citation2002). In addition, circulating levels of Vit D were shown to be associated with increased breast cancer mortality (Goodwin et al., Citation2009; Mohr et al., Citation2011; Yao & Ambrosone, Citation2013). On one hand, ethnic difference and the degree of skin pigmentation have provided important evidence that exposure to sunlight is the main source of Vit D and that this phenomenon stimulates the synthesis and increases the concentrations of circulating 1,25(OH)2D3 (Armas et al., Citation2007; John et al., Citation2007; Knight et al., Citation2007; Rollison et al., Citation2012; Yao & Ambrosone, Citation2013). On the other hand, discrepant results regarding the relationship between existing pigmentation of the skin and ability to synthesize Vit D have been obtained from other studies (Bogh et al., Citation2010; John et al., Citation2007; Rollison et al., Citation2012). Increased skin pigmentation reduced the dose of UV exposure, consequently it may cause a decreased synthesis of Vit D. The Hispanic women showed lower levels of circulating Vit D than white women exposed to the same amount of sunlight and, consequently, an increased incidence of breast cancer and mortality (Mohr et al., Citation2011; Yao & Ambrosone, Citation2013). The integration of Vit D in one’s diet, in particular among Hispanic women, had a considerable impact on Vit D circulating levels and on the incidence of breast cancer (John et al., Citation2007; Mohr et al., Citation2011; Rollison et al., Citation2012; Yao & Ambrosone, Citation2013). Overall these studies do not fully clarify whether Vit D is associated with a reduced risk of breast cancer. Further investigation may better define the clinical impact of vitamin supplementation in breast cancer development and treatment.
Effects of vitamin D on prostate cancer cells
Experimental and clinical studies provide evidence of a positive correlation between Vit D deficiency and prostate cancer (Swami et al., Citation2011). The findings showing that VDRs are expressed in normal prostate tissue, in benign prostate hyperplasia (BPH), and in prostate cancer cells suggest that BPH and prostate cancer (PCa) may represent potential targets for VDR ligands (Adorini et al., Citation2007; Crescioli et al., Citation2002; Hendrickson et al., Citation2011; Krill et al., Citation2001; Munetsuna et al., Citation2011; Swami et al., Citation2011). Several experimental observations have highlighted the fact that VDR ligands may exert numerous effects on prostate cancer cells including cell-cycle arrest in the G0/G1 phase, apoptosis, and interaction with androgen-mediated signaling (Guzey et al., Citation2002; Nagpal et al., Citation2005; Zhuang & Burnstein, Citation1998). VDR has also been shown to repress COX-2 and enhance expression of hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD), the combined effects of which can serve to limit prostate cancer cell growth through an overall reduction in prostaglandin activity (Moreno et al., Citation2005). Consistent with this hypothesis, earlier studies by Zhao et al. (Citation1997) showed that the androgen antagonist Casodex suppresses the antiproliferative effect of 1,25(OH)2D3 in LNCaP cells, indicating that the AR is involved in 1,25(OH)2D3-mediating signaling. Experimental evidence shows that the growth inhibition mediated by VDR ligands appears to be the result of a decrease in CDK2 activity and an increase in the expression of p21, p27, IGFBP-3, IGFBP-5, and E-cadherin (Drivdahl et al., Citation1995; Guzey et al., Citation2002; Huynh et al., Citation1998; Krishnan et al., Citation2004; Zhuang & Burnstein, Citation1998). In addition, in some prostate cancer cells, Vit D has been shown to down-regulate some anti-apoptotic genes such as Bcl-2 (Chen & Holick, Citation2003; Guzey et al., Citation2002). Recent cDNA analysis of normal prostate cells and LNCaP prostate cancer cells treated with 1,25(OH)2D3 contributed to identifying the target genes and to clarifying, in part, the mechanism of Vit D-induced tumor cell growth inhibition (Krishnan et al., Citation2004; Peehl et al., Citation2004). These studied have reported the overexpression of the gene-encoding 24-hydroxylase, the enzyme which catalyzes the first step of the catabolic degradation of 1,25(OH)2D3 (Chen et al., Citation2012; Krishnan et al., Citation2004; Muindi et al., Citation2007, Citation2010). These observations imply that the use of 24-hydroxylase inhibitors may increase the inhibitory activity of 1,25(OH)2D3 and that of its synthetic analogues (Muindi et al., Citation2010). Other possible mechanisms underlying the growth-inhibiting activity of Vit D on prostate cancer cells include stimulation of differentiation, modulation of growth factor activity, and inhibition of tumor angiogenesis, invasion, and metastasis (Krishnan & Feldman, Citation2011; Marchiani et al., Citation2006; Stio et al., Citation2011). Moreover, it has been also reported that, in normal and malignant prostate cells, 1,25(OH)2D3 may induce the expression of enzymes implicated in the maintenance of redox balance and protection of cells from oxidative damage such as TXNRD1 and SOD-2 (Peehl et al., Citation2004). Although a direct inhibiting effect of these enzymes in prostate cell growth inhibition appears unlikely, it is reasonable to hypothesize that they may indirectly mediate the chemopreventive effects of 1,25(OH)2D3 by preventing DNA damage caused by ROS. Finally, recent finding by Hsu et al. (Citation2011) show that Vit D may indirectly affect tumor cell invasion and metastasis by facilitating prostate cancer cell aggregation through the increase of E-cadherin expression. However, Ajibade et al. (Citation2014) recently reported that prolonged treatments with calcitriol in homozygous male TRAMP mice resulted in the development of a resistant and more aggressive form of prostate cancer associated with increased distant organ metastasis. Although the possible mechanism(s) facilitating these effects were not defined, these results support the concept that Vit D compounds may be effective in slowing or preventing progression of prostate cancer of earlier stages.
Clinical studies with prostate cancer
The data obtained following epidemiological studies on the effects of 1,25(OH)2D3 on prostate cancer are not univocal (Gilbert et al., Citation2012; Hendrickson et al., Citation2011; Krishnan & Feldman, Citation2011; Kristal et al., Citation2014; Swami et al., Citation2011). In fact, whereas some studies demonstrated a strong inverse association between Vit D serum concentrations and risk of prostate cancer (Gilbert et al., Citation2012; Tretli et al., Citation2009), other investigations show no significant correlation between Vit D circulating levels and serum PSA (Gilbert et al., Citation2012). More recently Kristal et al. (Citation2014) reported that low and high Vit D concentrations were associated with increased risk of prostate cancer. This association resulted stronger for high-grade disease. Other clinical studies have highlighted the effective therapeutic potential of Vit D when administered alone or in combination with other cytostatic agents (Beer, Citation2008; Hershberger et al., Citation2001; Krishnan & Feldman, Citation2011; Ma et al., Citation2010; Nagpal et al., Citation2005; Swami et al., Citation2011; Wigington et al., Citation2004). In addition, these studies have provided the rationale for a combination calcitriol–taxanes therapy in patients with prostate cancer (Beer et al., Citation2008; Ting et al., Citation2007a,Citationb). On the basis of these considerations, many clinical studies have been undertaken in androgen-independent prostate cancer patients where Vit D3 was often combined with standard cancer therapies. However, although these drug associations were well tolerated and the addition of Vit D3 did not result in any additional toxicity, when compared with the standard therapies alone, most of these investigations reported no beneficial effect of Vit D in these patients (Leyssens et al., Citation2013).
Effects of vitamin D on colon cancer cells
A consistent number of investigations provide evidence that 1,25(OH)2D3 and its semisynthetic analogues may play a key role in the prevention and treatment of colorectal cancer (Byers et al., Citation2012; Leyssens et al., Citation2013; Pereira et al., Citation2012). Numerous studies have shown that colorectal cancer cells express VDR and the enzyme 1α-hydroxylase that converts 25-hydroxyvitamin D3 [25(OH)D3] into the active metabolite of vitamin D, 1,25(OH)2D3 (Matusiak et al., Citation2005). The activation of VDR elicits antitumor effects by triggering apoptosis and by inhibiting cell proliferation, invasion, and angiogenesis (González-Sancho et al., Citation2006; Krishnan & Feldman, Citation2011; Pendás-Franco et al., Citation2008a; Samuel & Sitirin, Citation2008). In particular, in vitro studies showed that this molecule may inhibit the proliferation of colon tumor cells by blocking the cell cycle in the G1 phase, by promoting apoptosis and cell differentiation and by affecting tumor angiogenesis (Bettoun et al., Citation2002; Pálmer et al., Citation2001; Pendás-Franco et al., Citation2008a; Pereira et al., Citation2012). Furthermore, on one hand, studies on different colon carcinoma cell lines showed that 1,25(OH)2D3 may promote apoptosis by the up-regulation of the proapoptotic protein BAK1 and the down-regulation of the nuclear anti-apoptotic protein BAG1 and by preventing the formation of apoptotic heterodimers Bcl-2-Bax (Pereira et al., Citation2012; Welsh, Citation2012). The negative effects of 1,25(OH)2D3 on cell proliferation and apoptosis are further underlined by the findings that this molecule promotes sensitivity to the chemotherapeutic agent 5-fluorouracil (5-FU) by down-regulating the expression of the anti-apoptotic protein survivin and that of thymidylate synthase, a key enzyme in the biosynthethic pathway of DNA. On the other hand, in vitro studies also reported that 1,25(OH)2D3 promotes cell differentiation by increasing the expression of several components of cell adhesion that are essential for the maintenance of the epithelial phenotype and that of proteins associated with the actin cytoskeleton and intermediate filaments (Pálmer et al., Citation2001; Pereira et al., Citation2012). These findings are consistent with the observations that the treatment of Caco-2 colon cancer cells with 1,25(OH)2D3 induced an increase in the expression of p21, p27, E-cadherin, and other adhesion proteins (ZO-1, ZO-2, and vinculin) and promotes the translocation of β-catenin and ZO-1 from the nucleus to the plasma membrane (Gaschott et al., Citation2002). More specifically, it was shown that the VDR antagonist, ZK-191-732, modulates the differentiation process induced by butyrate on Caco-2 cells (Gaschott et al., Citation2002). Furthermore, it was established that a decrease in the levels of cyclin D1 is essential for the anti-proliferative effects of Vit D (Hofer et al., Citation1999; Maier et al., Citation2009). In vitro studies aimed at assessing the effects of 1,25(OH)2D3 on the gene expression in SW480-ADH cell line showed that Vit D increased the expression of c-Jun, JunD, Jund, FREAC-1/Fox1, ZNF-44/Kox7, G0S2, tumor suppressors normal epithelial-cell-specific gene 1 (NES-1), or kallikrein 10 and protease M (Pálmer et al., Citation2003). This phenomenon further stresses the concept that Vit D appears to play a role in cell growth inhibition, adhesion, differentiation, and apoptosis. These effects ultimately lead to the reversion of the neoplastic phenotype to a normal epithelial phenotype (Gocek & Studzinski, Citation2009; Nagpal et al., Citation2005). Finally, Meeker et al. (Citation2014) by using a model of bacteria-driven colitis and colon cancer when infected with Helicobacter bilis (H. bilis) showed that mice fed high vitamin D diet had a significantly lower incidence of cancer compared with mice fed maintenance diet. These findings further suggest that increased dietary vitamin D is beneficial in preventing inflammation-associated colon cancer through the suppression of inflammatory responses during the initiation of neoplasia or early-stage carcinogenesis.
Colorectal cancer and vitamin D
Clinical studies
Colorectal cancer is the third most common type of cancer in men and women in western countries (Chan & Giovannucci, Citation2010). There is strong evidence on a significant relationship between lifestyle and diet and incidence-rate of this type of cancer (Chan & Giovannucci, Citation2010). Several case–control studies and cohort studies have examined the relationship between Vit D intake (total, with diet, or supplementary) and the risk of colorectal cancer (Giovannucci, Citation2010; Woolcott et al., Citation2010). The consistent body of investigation that analyzed the relationship between the serum 25-hydroxyvitamin D3 [25(OH)D3] level and colorectal cancer risk generally shows an inverse association (Freedman et al., Citation2007). In support of these observations, a large observational-nested case–control study undertaken within the European Prospective Investigation into Cancer and Nutrition showed a strong inverse association between 25(OH)D3 concentrations and colorectal tumor (Jenab et al., Citation2010). Furthermore, a recent meta-analysis of 35 independent studies further confirmed the inverse relationship between circulating levels of 25(OH)D3 and colorectal cancer risk (Gandini et al., Citation2011; Maalmi et al., Citation2014). Finally, a systematic review of 18 prospective studies carried out by Ma et al. (Citation2011) undertaken to assess the association of Vit D intake or 25-hydroxyvitamin D3 serum levels and the risk of colorectal tumor in about 1 000 000 individuals highlighted the fact that vitamin D intake and 25-(OH)D3 blood levels were inversely associated with the risk of colorectal cancer. In particular, some of these studies showed that the intake of 1000 IU/d of Vit D was associated with a 50% decrease in risk of colorectal tumor, while plasma concentrations of 20–29 ng/ml of 25-hydroxyvitamin D3 were associated with an increased risk of developing colorectal cancer. Conversely, concentrations higher than 30–39 ng/ml were associated with a decreased risk (Jenab et al., Citation2010). However, some investigations have reported different results. For instance, on one hand, Wactawski-Wende et al. (Citation2006) failed to show marked effects of Vit D on the incidence of colorectal cancer. Furthermore, the co-administration of calcium and Vit D in women taking estrogen resulted in an increased risk of colorectal cancer. The conclusion of the study was that Vit D supplementation may have a greater impact on mortality, but a lower incidence of colorectal cancer. On the other hand, Ishihara et al. (Citation2008) did not highlight any statistically significant correlation between Vit D intake and risk of colorectal cancer. Only concentrations of 25-(OH)D3 lower than 80 nmol/L were inversely associated with mortality from colorectal cancer, but not with the incidence. The discrepancies in these results were, in part, explained by the fact that the subjects considered in these studies were often taking food high in calories and low in fiber. Moreover, these subjects did not carry out any physical activity, had a high body mass index, a positive family history of colorectal cancer, and were smokers. There was no difference in the amount of Vit D and sunlight exposure between cases and controls. The use of Vit D supplements was more common among the controls, and this was statistically significant among men. Because of these conflicting results further studies are needed to better define the chemopreventive effects of Vit D on colorectal cancer.
Effects of vitamin D on SCC
The presence of VDR in keratinocytes and the ability Vit D to induce differentiation and to inhibit cell proliferation may, in part, account for the therapeutic potential of this molecule and its semisynthetic analogues in the SCC of the head neck (H&NSCC) and aerodigestive tract (Bikle, Citation2012; Ma et al., Citation2013a,Citationb; Nagpal et al., Citation2005). In particular, the inhibition of p21 expression in keratinocytes has been reported to be an essential prerequisite for the induction of cell differentiation (Di Cunto et al., Citation1998). 1,25(OH)2D3 has been shown to inhibit SCC growth in vitro and in vivo (Hershberger et al., Citation2001; Ma et al., Citation2013b). Interestingly, recent in vitro studies have highlighted the additive inhibitory effects of Vit D in combination with 5-FU and or 13-cis-retinoic acid on human oral squamous carcinoma cell growth (Dalirsani et al., Citation2012). In contrast, Gedlicka et al. (Citation2006) have shown that Vit D induced growth inhibition in SCC cell lines of the head and neck by arresting cells in the G0/G1 phase of the cell cycle and that this effect was associated with an upregulation of p18 cell-cycle inhibitor. Further studies demonstrated that 1,25(OH)2D3 inhibits, in vitro, cell motility and invasion of SCC cells while, in vivo, this molecule markedly suppresses the ability of SCC cells to establish pulmonary metastases in tumor-bearing mice (Ma et al., Citation2013a). The potential target genes for Vit D have been identified in cell line SCC25 following treatment with the analogue EB1089 (Lin et al., Citation2002). The expression of genes such as 24-hydroxylase, protease M, cystatin M, amphiregulin, stromelysin, and collagenase I resulted up-regulated whereas the expression levels of CRABP-II, N-cadherin, and SCC antigen were down-regulated (Lin et al., Citation2002). The effects of Vit D and its analogues on the expression of genes involved in cell differentiation, growth inhibition, and immunomodulation, further indicate that SCC cell lines can be driven to differentiation by these compounds (Goceck & Studzinski, Citation2009). To date, limited epidemiologic studies on the effect of Vit D or its metabolites on SCC prevention or treatment in humans have generated conflicting data. Recently, Eide et al. (Citation2011) reported a positive relationship between plasma levels of 25(OH)D3 and non-melanoma skin cancer (NMSC) including SCC and basal cell carcinoma (BCC), in a study of 3223 white health maintenance organization patients who sought advice about the risk of osteoporosis or low bone density. These findings have been confirmed by a recent nested case–control study among women by Liang et al. (Citation2012). Conversely, Tang et al. (Citation2010) highlighted higher serum 25(OH)D3 levels as being associated with a decreased risk of NMSC in older Caucasian men. The discrepancies in these results were explained by the different observation periods and the different types of subject enrolled in these studies. Overall, there is some evidence that Vit D may be of clinical interest in SCC and melanoma prevention. However, additional studies are needed to assess the suitability of topical or oral Vit D for chemoprevention of SCC and BCC in humans (Tang et al., Citation2012).
Effects of vitamin D on hematological malignancies
There is increasing interest in the possible use of Vit D to combat hematological diseases including leukemias, myelodysplastic syndrome (MDS), lymphomas, and multiple myeloma (MM) (Kim et al., Citation2012; Motomura et al., Citation1991; Shanafelt et al., Citation2011). The potential therapeutic role of 1,25(OH)2D3 in the treatment of hematologic malignancies was first highlighted by Abe et al. (Citation2004) who reported that Vit D was able to induce in vitro differentiation of M1 murine myeloid cells. These findings were further confirmed by other experimental in vivo studies which showed that the administration of Vit D increased the survival time of mice inoculated with leukemic cells (Honma et al., Citation1983). The possible therapeutic effectiveness of 1,25(OH)2D3 in the clinical treatment of hematological malignancies relies on the observations that this molecule appears to inhibit the proliferation of hematopoietic precursor cells and to promote their maturation and, ultimately, cell differentiation (Abe et al., Citation2004; Gocek & Studzinski, Citation2009; Honma et al., Citation1983; Kim et al., Citation2012; Shanafelt et al., Citation2011). The biochemical mechanisms through which Vit D and its derivatives induce these effects have been, only in part, elucidated (Kim et al., Citation2012). Experimental observations have highlighted the fact that these mechanisms may be different according to the cell type (Bhatia et al., Citation1995; Hughes et al., Citation2010; Kim et al., Citation2012). For instance, Vit D may induce monocytic differentiation of myeloid leukemia cells. This phenomenon may result in the G1 phase cell-cycle block and, consequently in the cessation of cell proliferation (Bhatia et al., Citation1995). In acute promyelocitic leukemic cells, Vit D appears to activate three types of intracellular signaling pathways, namely PKC pathway, PI3K-AKT pathway, and three different MAPK pathways which have been suggested to intersect at a common nodal point (Raf-1) (Bhatia et al., Citation1995; Goceck & Studzinski, Citation2009; Hughes et al., Citation2010; Kim et al., Citation2012; Wang & Studzinski, Citation1997). Activation of the MAPK and PI3K-AKT pathways has also been implicated in Vit D-mediated VDR synthesis and nuclear translocation (Goceck & Studzinski, Citation2009; Kim et al., Citation2012). However, in the acute promyelocytic leukemia cell line NB4, the monocytic differentiation was induced independently of any VDR/VDRE interaction (Bhatia et al., Citation1996). On the other hand, in U937 leukemic cells, Vit D was shown to activate the transcription of cycline-dependent kinase inhibitors p21Waf1/Cip1 and p27Kip1 and that of the protein HOXA10 (Liu et al., Citation2010). Overexpression of p21 and HOXA10 facilitates the differentiation of U937 cells into monocytes/macrophages cell lineage (Kim et al., Citation2012; Rots et al., Citation1998). Therefore, the therapeutic strategies currently available in the clinical treatment of leukemias and MDS include agents that induce differentiation of hematopoietic precursors (Harrison & Bershadskiy, Citation2012; Kim et al., Citation2012; Nagpal et al., Citation2005; Shanafelt et al., Citation2011). The main hematologic responses were observed in patients with MDS treated with calcitriol and alfacalcidol (Kim et al., Citation2012; Mellibovsky et al., Citation1998). Although the range of response in these studies varied from 44 to 100%, with complete remission in only 6% of patients, the prevention of the progression of MDS is significant. Recent findings have shown that Vit D may induce antileukemic effects by promoting autophagy in leukemic cells via the increase of the intracellular levels of beclin-1 which is known to induce the formation of autophagosomes in mammalian systems (Kim et al., Citation2012; Wang et al., Citation2008). On the contrary, in the K562 chronic myeloid leukemia cell line, which is characterized by a rapid growth rate and lack of differentiation, Vit D was found to induce apoptosis (Kim et al., Citation2012; Wang & Studzinski, Citation1997). Similarly, the treatment of HL-60 cells with Vit D induced an increase in Mcl-1, an anti-apoptotic protein that blocks cytochrome c release in the apoptosis pathway and may also target Raf-1 (Kim et al., Citation2012; Wang & Studzinski, Citation1997). Although experimental studies support a potential clinical benefit of the use of Vit D derivatives in the treatment of hematological malignancies, the partial validation observed in clinical trials against acute myeloid leukemia and MDS implies that the therapeutic effectiveness of Vit D in these malignancies deserves further extensive investigation (Kim et al., Citation2012).
Effects of cancer on vitamin D metabolism
Effects of cancer on VDR levels
Although a consistent body of experimental and clinical research have been undertaken in order to define the therapeutic effectiveness of Vit D on cancer, other studies have been directed towards investigating the impact of cancer on the Vit D system. Some of these studies have shown that VDR may be overexpressed or down-regulated in various human cancers (Friedrich et al., Citation2006; Kure et al., Citation2009; Lopes et al., Citation2012; Motomura et al., 1991; Matusiak et al., Citation2005; Reichrath et al., Citation2004). For instance, Matusiak et al. (Citation2005) demonstrated that the expression levels of VDR were low in normal epithelial cells of the colon while they were increased in aberrant crypts foci, in polyps, and in well-differentiated cancer cells. These findings are in line with those of Kure et al. (Citation2009) who showed that in 233 out of 619 patients the overexpression of VDR was associated with mutations of Ras-MAPK and PI3K-AKT. Conversely, clinical studies in 82 patients with melanoma highlighted a remarkable reduction, or even the absence, of VDR expression in tumor tissue in comparison with normal skin. This phenomenon was correlated with the progression of melanocytic lesions (Brożyna et al., Citation2011). These data suggest that altered expression levels of VDR may be regarded as a potentially useful marker in the follow-up of melanoma patients treated with Vit D or its semisynthetic analogues. Furthermore, studies undertaken to assess the expression level of VDRs in normal mammary cells, in benign lesions, in localized breast cancer, and invasive breast cancer highlighted the presence of these receptors in a variety of breast tissues with some quantitative differences (Lopes et al., Citation2010; Zhang et al., Citation2014). In particular, VDRs were frequently expressed in benign lesions (93.5%) while in carcinoma in situ or in metastatic tumor the rate of expression was lower (56.2%) (Lopes et al., Citation2010). Similar results were obtained from studies which evaluated the expression level of VDR in benign and malignant ovarian tissues (Thill et al., Citation2010). These studies showed that VDR expression levels were significantly lower in malignant tissue as compared with normal tissue. On one hand, it is worth noting that Zhang et al. (Citation2014) recently demonstrated a negative correlation between VDR expression in human breast cancer tissue and metastasis in breast cancer. Furthermore, coculture of VDR-overexpressing breast cancer cells with a macrophage cell line demonstrated that overexpression of VDR alleviated the prometastatic effect of cocultured macrophages on breast cancer cells. On the other hand, Hendrickson et al. (Citation2011), by assessing the level of expression of VDR in tumor tissue from 841 patients with prostate cancer, showed that the high expression of VDR in tumor tissue was associated with a reduced risk of cancer death suggesting an important role of Vit D on prostate cancer progression. Interestingly, Srinivasan et al. (Citation2011) recently described the presence of VDR at nuclear and cytoplasmic levels in lung cancer cells. These authors additionally showed that while the high levels of nuclear VDR were associated with increased survival, no correlation was observed between the survival and the expression levels of cytoplasmic VDR. Furthermore, it has been reported that some gene mutations are strongly associated to cancer progression such as observed in the case of Ha-Ras, in HC-11 mouse mammary epithelial cells (Escaleira & Brentani, Citation1999) or K-Ras in RWPE-2 cells (Zhang et al., Citation2010) or Simian Virus 40 (SV40) in human mammary epithelial (HME) cells (Kemmis & Welsh, Citation2008), and that this phenomenon may result in a decreased expression of VDR. On the other hand, several studies have identified many factors that may influence the expression of VDR in cancer. For instance, it has been shown that the overexpression of SNAIL transcription factors can reduce the expression of the gene encoding VDR in SW-480-ADH, HCT116, Caco-2, LS174T, and HT29 colon cancer cell lines (Pálmer et al., Citation2004). Furthermore, Larriba et al. (Citation2013) showed that SNAIL1 repressed the expression of VDR and also inhibited the migration of nuclear β-catenin induced by 1,25(OH)2D3 in SW-480-ADH colon cancer cells. In addition, SNAIL1 thwarted the inhibitory effects of 1,25(OH)2D3 on cell proliferation. In colon and breast cancer cell lines, SNAIL1 and SNAIL2 can bind E-boxes in the proximal promoter of the gene for VDR and enhance the recruitment of co-repressors that reduce the expression of VDR (Mittal et al., Citation2008; Peña et al., Citation2005). Another protein that regulates the expression of VDR gene is p53. It has been shown that in Saos-2 osteosarcoma cells and human non-small cell lung cancer cells H1299, the overexpression of p53, resulted in an increased expression of VDR (Maruyama et al., Citation2006). Unfortunately, p53 gene is mutated in many human tumors and this may account for the reduced expression of VDR gene in breast and lung cancer cells. Interestingly, the p53 mutant form has been shown to be able to interact with VDR, to increase its accumulation in the nucleus, and to convert Vit D into an antiapoptotic agent (Stambolsky et al., Citation2010). These findings indicate that in tumors with p53 mutant, the therapeutic potential of Vit D or its analogues may be limited (Stambolsky et al., Citation2010). In addition, there is also some evidence that ras activation can decrease the transcriptional activity mediated by Vit D (Fleet et al., Citation2012). This phenomenon was first described by Solomon et al. (Citation1999), who observed that in ras-transformed keratinocytes, the transcriptional activity mediated by VDRs was reduced as a result of phosphorylation on serine 260 of the heterodimeric partners of the VDR, namely RXR. A reduction in the transcriptional activity of the VDR after phosphorylation within the domain of RXR AF-1 was also observed in the RWPE2 cell line (Zhang et al., Citation2010). Overall, these studies suggest that the development of cancer can lead to a reduction of the responses mediated from 1,25(OH)2D3 thus weakening VDR-mediated signaling pathways.
Effects of malignant transformation on 1-α-hydroxylase (CYP27B1) expression levels
Although the presence of the enzyme 1-α-hydroxylase, in many tumor tissues, has been well recognized, studies on the expression of this enzyme during cancer development have had discrepant results (Cross et al., Citation2005, Citation2009; Höbaus et al., Citation2013). In fact, the expression and activity of CYP27B1 have been shown to increase, decrease, or remain unchanged according to the organ, the tumor grade, and the reference tissue (Cross et al., Citation2005; Höbaus et al., Citation2013). In this context, several in vitro studies have shown that the activity of CYP27B1 and its contribution to 1,25(OH)2D3 production is lost in tumors with an aggressive phenotype (Fleet et al., Citation2012). On the other hand, Hsu et al. (Citation2011) reported that CYP27B1 was present in normal prostate epithelia cells (PECs). However, its enzyme activity was reduced in cells isolated from BPH while it was virtually absent in cells isolated from prostate cancer patients. Further, Segersten et al. (Citation2002) reported that this enzyme was overexpressed in a number of parathyroid adenomas of primary hyperthyroidism (HTP) and in hyperplastic glands of secondary HPT while it resulted underexpressed in parathyroid carcinomas, compared with normal parathyroid glands. These findings are consistent with the observations that, unlike cancer cells, normal cells were susceptible to the growth inhibiting effects of 1,25(OH)2D3 treatments. These results were confirmed by another study which showed that the reduced expression of CYP27B1 in LNCaP cells resulted in a reduced growth inhibition induced by 1,25(OH)2D3 (Chen et al., Citation2003). Moreover, CYP27B1 expression was undetectable in metastases from human colon cancer (Matusiak & Benya, Citation2007). More recently, Brożyna et al. (Citation2011) showed an inverse correlation between CYP27B1 expression levels and melanoma progression. However, the relationship between decreased levels of CYP27B1 and malignant phenotype was not univocally observed in all types of cancer (Fleet et al., Citation2012). For instance, Friedrich et al. (Citation2006) showed that the mRNA coding for CYP27B1 increased in breast cancer when compared with normal tissue. Furthermore, Clinckspoor et al. (Citation2012) recently highlighted CYP27B1 expression levels as increasing in malignant thyroid tumors. Conversely, immunohistochemical observations by Lopes et al. (Citation2010, Citation2012) failed to highlight significant differences in the expression of CYP27B1 between normal breast tissue and tumor tissue while discrepant results were obtained with renal carcinoma (Blomberg Jensen et al., Citation2010; Urbschat et al., Citation2013). Because of these conflicting data, no definitive conclusion can be drawn on the consequences of malignant transformation on the expression of CYP27B1.
Effects of malignant transformation on 24-hydroxylase (CYP24A1) expression levels
Similarly, as described for CYP27B1, the level of CYP24A1 expression, an enzyme involved in the degradation of metabolic products of Vit D, may be influenced by the malignant transformation (Cross et al., Citation2011; Hobaus et al., Citation2013). In fact, aberrantly high basal expressions of CYP24A1 have been observed in various tumors (Hobaus et al., Citation2013; Horváth et al., Citation2010). Furthermore, epidemiological studies showed that serum levels of 25(OH)D3, the precursor of 1,25(OH)2D3, below 30 nM were strongly associated with an increased incidence of colorectal cancer (Cross et al., Citation2011). In this context, recent experimental and clinical observations by Cross et al. (Citation2005) and Brozek et al. (Citation2012) reported that the expression levels of CYP24A1 increased dramatically during colorectal cancer progression to a poorly differentiated stage (G3–G4). Furthermore, studies on breast cancer showed that the CYP24A1 gene was overexpressed in this type of tumor and that this phenomenon also accounted for the inhibition of the antiproliferative effects of 1,25-(OH)2D3 on tumor cells (Lopes et al., Citation2010). Consistent with these observations, Anderson et al. (Citation2006) reported an increased expression of CYP24A1 levels in MCF-7, SW-620 breast cancer cells, in breast tumor tissue and in ovary, colon, and lung cancer. Subsequent studies showed that CYP24A1 was present in the nuclei of normal colonic epithelial cells, aberrant cryptic foci and adenomatous polyps (Matusiak & Benya, Citation2007). However, following malignant transformation, the location of this enzyme shifted almost entirely from the nuclear compartment to the cytoplasmic compartment (Matusiak & Benya, Citation2007). Overexpression of CYP24A1 was also demonstrated in prostate cancer, ovarian cancer, cervical cancer, lung cancer, SCC, and BCC (Friedrich et al., Citation2006; Mitschele et al., Citation2004; Muindi et al., Citation2007). In esophageal cancer, high CYP24A1 expression was reported to correlate with a poor prognosis (Mimori et al., Citation2004). Overall, these data highlight the altered metabolism of 1,25-(OH)2D3 as appearing to be a typical feature of advanced cancer and also suggest that one major mechanism responsible for Vit D resistance or reduced sensitivity to calcitriol in VDR-positive cells may be dependent on an increase of 1,25-(OH)2D3 and 25(OH)D3, catabolism via the C-24 hydroxylation pathway. In line with these observations, Muindi et al. (Citation2007) have demonstrated that ketoconazole, an inhibitor of CYP24A1, can restore the activity of growth inhibition exerted by 1,25-(OH)2D3 in prostate cancer cells. More recently, Komagata et al. (Citation2009) found that the levels of CYP24A1 can be reduced at post-transcriptional levels by miR125b, a micro-RNA that can bind the 3′-UTR (untranslated region) mRNA for CYP24A1. In addition, these authors demonstrated that, in breast cancer, CYP24A1 levels were inversely related to miR125b levels suggesting that the lack of this regulatory RNA may account for the increased expression levels of CYP24A1 in cancer cells. These findings might, in part, account for the limited therapeutic effects of 1,25(OH)2D3 observed in some clinical trials. Thus the inhibition of CYP24A1 by pharmacological means may lead to a new approach in Vit D-based treatment of neoplastic diseases.
The semi-synthetic analogues of Vit D
The numerous epidemiological and preclinical investigations suggesting a role of Vit D in the prevention and treatment of several human tumors support the clinical use of 1α,25(OH)2D3 and Vit D analogues as potential preventive and therapeutic anticancer agents (Brown & Slatopolsky, Citation2008; Trump et al., Citation2010). However, the hypercalcemic effects induced by 1,25(OH)2D3 have strongly limited its therapeutic use (Ma et al., Citation2010; Mehta, Citation2012). Therefore, many efforts are currently being directed towards synthesizing new Vit D analogues with the goal of improving the biological profile of the natural hormone, which retains the therapeutic activity the analogues being endowed with a lower calcemic effect (Byers et al., Citation2012; Chen & Kittaka, Citation2011; Fleet et al., Citation2012; Trump et al., Citation2010). Vit D analogues are semi-synthetic molecules, since they share the basic structure of 1,25(OH)2D3 but differ in the presence of a characteristic functional group (R) (Brown & Slatopolsky, Citation2008; Trump et al., Citation2010; Unten et al., Citation2004) (). To date, nearly 1500 Vit D analogues have been synthesized and evaluated, in vitro and in vivo, for their therapeutic effectiveness, in a variety of carcinogenesis and human cancer models (Leyssens et al., Citation2014; Mehta, Citation2012). However, of these compounds, only a few have been approved for further evaluation in clinical trials in leukemia patients, breast cancer patients, prostate cancer patients, and colon cancer patients (Agoston et al., Citation2006; Hussain et al., Citation2003; Kim et al., Citation2012; Leyssens et al., Citation2014; Mehta, Citation2012). The chemical structure of some of the Vit D analogues endowed with cancer chemo-preventive activity is shown in . Analogues that are structurally unrelated to Vit D but are able to interact with VDR have also been synthesized (Byers et al., Citation2012; Choi & Makishima, Citation2009). More recently a new class of Vit D analogues, characterized by two side-chains linked to carbon-20 (Gemini) and with deuterium substituted on one side-chain, have been synthesized (Maehr et al., Citation2013; Okamoto et al., Citation2014; Spina et al., Citation2007). Although these analogs do not show any adverse calcemic effects from Vit D, they may induce other toxic effects unrelated to calcemia (Mehta et al., Citation2012).
Alfacalcidol and doxercalciferol
Alfacalcidol (1α,25(OH)D3) and doxercalciferol (1α,25OHD2) () are Vit D analogues endowed with chemopreventive effects. Early reports from Iseki et al. (Citation1999) showed that a long-term administration of high doses of alfacalcidol significantly reduced the incidence of colon cancer in rats. In line with these observations, Kikuchi et al. (Citation2007) showed that treatment with alfacalcidol prevented the ulcerative colitis and the development of colon cancer in mice. Similar results were reported from experiments on female Sprague–Dawley rats with 7,12-dimethylbenz[a]anthracene(DMBA)-induced mammary tumor where the administration of alfacalcidol suppressed the growth of tumors in a dose-dependent fashion (Iino et al., Citation1992). Hara et al. (Citation2001) showed that the oral administration of alfacalcidol to mice transplanted with Dunn osteosarcoma significantly suppressed tumor growth and metastasis formation. The same study reported that the co-administration of alfacalcidol and doxorubicin resulted in additive antitumor and antimetastatic effects. Although the mechanisms of the antitumor effects of alfacalcidol have not yet been fully elucidated, experimental evidence indicates that, in particular, this molecule may inhibit tumor angiogenesis (Iseki et al., Citation1999). These findings suggest that alfacalcidol might be of therapeutic value as a chemopreventive agent in the clinical management of cancer patients. Consistent with these results, clinical studies by Tałalaj et al. (Citation2005) reported that, in patients with advanced prostatic carcinoma treated with complete androgenic blockade, the administration of alfacalcidol and calcium supplement (CaCO3) prevented both trabecular and compact bone loss. On one hand, more recent clinical observations by Obara et al. (Citation2008) have highlighted the fact that in Japanese patients with metastatic renal carcinoma (RCC), the combination of alfacalcidol and interferon-α may prolong the survival of these patients without causing severe adverse events. On the other hand, Trouillas et al. (Citation2001) showed that alfacalcidol may induce in some patients, in synergy with classical surgery–radiotherapy–chemotherapy treatment, a particular progressive and durable regression of the tumor. Cunningham et al. (Citation1985) reported that the administration of alfacalcidol induced a partial remission in patients with lymphoma. On one hand, more recently Akiyama et al. (Citation2010) showed that the addition of alfacalcidol to Vitamin K appears to improve anemia and thrombocytopenia in about one-third of patients with low/intermediate-1 MDS who had not responded to Vitamin K2 monotherapy for 16 weeks. On the other hand, recent findings by van Ginkel et al. (Citation2007) show that doxercalciferol can inhibit human neuroblastoma growth in vivo with relatively low toxicity. However, recent clinical investigation by Gee et al. (Citation2013) reported no obvious beneficial effects in men undergoing prostatectomy for early-stage prostatic neoplasia, while Petrich et al. (2008) showed that a short-term treatment with doxercalciferol has limited activity in patients with MDS. These conflicting results suggest that further clinical studies are needed to better define the clinical effectiveness of this molecule in cancer chemoprevention.
Calcipotriol
Calcipotriol, or calcipotriene (), is a synthetic Vit D analogue that exhibits a vitamin D-like effect by competing for VDR displaying minimal effects on calcium homeostasis (Binderup & Bramm, Citation1988). The intraperitoneal or oral administration of calcipotriol to rats showed that the compound was at least 100 times less active than 1,25(OH)2D3 in determining hypercalcemia and hypercalciuria (Binderup & Bramm, Citation1988; Kissmeyer & Binderup, Citation1991). Experimental observations show that this molecule exerts important effects on cellular differentiation and proliferation in vitro while its effect on calcium metabolism in vivo is negligible (Binderup & Bramm, Citation1988; Cho et al., Citation1996). In vitro studies showed that this molecule may regulate cell differentiation and proliferation and exhibits growth-inhibiting effects against several human cancer cell lines including HL-60 and HL60/MX2 promyelocytic leukemia, U937 histiocytic lymphoma, MCF-7, T47D human breast cancer, HT-29 human colon cancer, and human SCC (Colston et al., Citation1992; Meephansan et al., Citation2012; Milczarek et al., Citation2013a, Citation2014). Regarding the possible mechanisms underlying the growth inhibiting and pro-differentiating effects of calcipotriol on tumor cells, in vitro studies highlighted the fact that a 20-h exposure of LNCaP prostate cancer cells and MCF-7 breast cancer cells to this analogue or to BGP-15, a new calcipotriene-derived vitamin D3 analogue, generated procaspase-3 cleavage and ultimately, apoptosis (Berkovic et al., Citation2010). In addition, Wang et al. (Citation2010b) recently reported that calcipotriol significantly suppressed in vitro colon carcinoma cell invasion and enhanced the cytotoxicity of the anticancer regimen FOLFIRI (folinic acid, 5-FU, irinotecan) to cells in culture or in anchorage-independent growth. These effects appeared to be the consequence of a suppression of gene transcriptional activities and protein expression of survivin and thymidylate synthase, to the enhanced E-cadherin localization in cell membranes and the complex formation of E-cadherin and β-catenin, and repression TCF4 transcriptional activation. Consistent with these observations, on one hand, recent findings by Milczarek et al. (Citation2014) showed that calcipotriol may potentiate the antitumor activity of 5-FU in HT-29 colon tumor-bearing mice. These studies also showed that the mechanism of potentiation of 5-FU antitumor was related to the increased expression of p21 and decreased expression of pERK1/2 level which may lead to a decreased expression of thymidylate synthase. On the other hand, Meephansan et al. (Citation2012) highlighted the fact that calcipotriol affects in vitro the invasive potential of DJM human squamous carcinoma cells by reducing the production of matrix-metalloproteinases MMP-9 and MMP-13 through inhibition of the ERK and p38 phosphorylation. The antitumor activity of calcipotriol was also investigated in vivo using rats transplanted with breast cancer induced by N-methyl-nitrosourea (Colston et al., Citation1992a). These studies showed how the administration of calcipotriol (50 µg/kg) resulted in an inhibition of tumor progression without development of severe hypercalcemia. On one hand, more recent in vivo investigation by Pommergaard et al. (Citation2013) demonstrated that in mice with UVB-induced non-melanoma skin cancer (NMSC), the topical treatment with calcipotriol combined with diclofenac and difluoromethylornithine for 17 weeks significantly reduced the number of mice with tumors as well as tumor area size compared with placebo. On the other hand, early clinical studies reported that in 15 out of 19 patients with localized breast cancer or skin metastases, the topical treatment with calcipotriol (100 mg/d for 6 weeks) caused a reduction of 65% of the diameter of the lesions, a reduction of 50%, in 3/19 patients while one patient showed a minimal response (Bower et al., Citation1991). It is worth noting that only two patients developed hypercalcemia during treatment. Interestingly, recent observations by Al-Jaderi and Maghazachi (Citation2013) suggested that calcipotriol may influence the activity of cells of the innate immune system. In fact, these authors showed that calcipotriol may increase IL-2-activated NK cell lysis of K562 and RAJI tumor cell lines as well as immature and mature dendritic cells and may down-regulate the expression of the killer inhibitory receptor CD158. These findings make calcipotriol of potential interest as another novel chemopreventive agent in cancer treatment.
Maxacalcitol (22-oxa-1α,25-D3)
Maxacalcitol (1α,25-dihydroxy-22-oxacalcitriol) (OCT) () is another non-calcemic Vit D analogue and VDR ligand that has been shown to promote cell differentiation and to inhibit cell proliferation, without inducing hypercalcemia (Abe et al., Citation1991; Nishii & Okano, Citation2001; Funato et al., Citation2002). This molecule contains an oxygen in place of a carbon in position 22 of the side chain () and is less calcemic than 1,25(OH)2D3. However, it retains considerable potency to suppress PTH in vitro and in vivo (Nishii & Okano, Citation2001). In vitro studies show that maxacalcitol could block the G1/S transition and induce tumor cell growth inhibition in responsive pancreatic tumor cells (Kawa et al., Citation2005). The antiproliferative effect of maxacalcitol on these cells appears to be due to the up-regulation of p21 and p27 induced by this molecule (Kawa et al., Citation2005). Interestingly, Funato et al. (Citation2002) also report that the co-administration of OTC with Vitamin K2 induces an accumulation of the cells in the G0/G1 phase but suppresses apoptosis. The growth inhibitory effects of 22-oxa-calcitriol were also reported in breast cancer cells. In fact, in vitro studies showed that this molecule inhibited, in a dose- and time-dependent fashion, the proliferation of ER(+) breast cancer cells (MCF-7, T-47D, and ZR-75-1) and ER(−) breast cancer cells (MDA-MB-231 and BT-20) (Abe et al., Citation1991). In line with these findings, in vivo studies on athymic mice transplanted with MCF-7/ER(+) or MX-1/ER(−) breast cancer cells showed that the administration of OCT elicited a potent antitumor effect in both ER(+) and ER(−) tumor cells (Abe-Hashimoto et al., Citation1993). These studies also demonstrated that the degree of therapeutic effectiveness of OCT was comparable with that of tamoxifen in ER(+) tumors or to that of adriamycin in ER(−) tumors. Furthermore, in MCF7/ER(+) tumors, the co-administration of OCT and tamoxifen resulted in a synergistic antitumor effect (Abe et al., Citation1991; Abe-Hashimoto et al., Citation1993). Interestingly, on one hand, other in vivo studies reported that in mice transplanted intravenously with Lewis Lung Carcinoma cells (LLC), the administration of OTC was highly effective in reducing lung metastasis formation (Nakagawa et al., Citation2005). These authors suggested that this effect was likely related to ability of this compound to affect tumor-induced angiogenesis in vitro and in vivo (Nakagawa et al., Citation2005). On the other hand, the results of these studies are consistent with those of Matsumoto et al. (Citation1999) reporting that the administration of OTC to mice transplanted with MDA-MB-231 ER(−) human breast cancer cells partially suppresses tumor growth by inhibiting the expression levels of the vascular endothelial growth factor (VEGF) and, ultimately, tumor neovascularization. More recent observations by Seubwai et al. (Citation2010) showed that OTC effectively suppressed the growth of cholangiocarcinoma (CCA) cell lines in a time-dependent and dose-dependent manner by blocking tumor cells in the G1 phase of the cell cycle and the transition of CCA cells from G1 to S phase by suppressing or up-regulating the expression of genes, i.e., cyclin D1 and p21, respectively, which regulate this transition. Moreover, supplementation of OTC to CCA-inoculated NOD/Scid/Jak3-deficient mice (NOJ) significantly inhibited tumor growth without hypercalcemia or other serious side effects. This treatment also induced cellular apoptosis in tissue samples from patients with CCA. The effects of maxacalcitol were also investigated in rats treated with five carcinogens (Otoshi et al., Citation1995). In this study, 25 rats were administered intraperitoneally with maxacalcitol (30 µg/kg), three times a week for 24 weeks from the initial exposure to carcinogens. At the end of a 30-week observation period, none of the rats that had received maxacalcitol after the carcinogens developed cancerous lesions in the small intestine while the incidence was higher in control animals. The incidence of colon carcinoma in the group that received only maxacalcitol showed a great tendency to decrease (Otoshi et al., Citation1995). These studies, although not clarifying the mechanisms of action, suggest that the maxacalcitol may be of potential clinical value in the prevention of breast cancer and colorectal cancer.
EB1089 (seocalcitol)
EB1089, also known as seocalcitol (), is a Vit D analogue which has been shown to inhibit either in vitro or in vivo the growth of several types of tumor such as breast cancer (Colston et al., Citation1992a; Macejová et al., Citation2011; Valrance et al., Citation2007; VanWeelden et al., Citation1998), prostate cancer (Bhatia et al., Citation2009; Chen et al., Citation2003; Oades et al., Citation2002), colon cancer (Akhter et al., Citation1997; Oh et al., Citation2001), and hepatocellular carcinoma (Ghous et al., Citation2008; Zhang et al., Citation2013). In vitro observations showed that EB1089 was more effective than 1,25(OH)2D3 in inhibiting the cell proliferation of MCF-7 breast cancer cells (VanWeelden et al., Citation1998). In vivo, the antitumor effects of EB1089 were investigated in rats with mammary cancer induced by N-methyl-nitrosourea (Colston et al., Citation1992) orally administered with two different dose levels. The administration of the lower dose resulted in a significant inhibition of tumor growth while, an equivalent dose of 1,25(OH)2D3 had no effect on tumor growth but induced hypercalcemia. Conversely, at the higher dose, EB1089 determined tumor regression. In addition, on one hand, experimental evidence showed that the combination of EB1089 with cytotoxic agents and/or ionizing radiation resulted in additive antitumor effects on breast cancer tumor cells either in vitro or in vivo (Demasters et al., Citation2006; Koshizuka et al., Citation1999; Sundaram et al., Citation2003; Valrance et al., Citation2007). On the other hand, Oades et al. (Citation2002) showed that EB1089 also inhibited the growth of prostate adenocarcinoma in the Dunning prostate model and in athymic nude mice transplanted with LNCaP. Similar effects were recently reported in an in vivo study by Bhatia et al. (Citation2009). These authors showed that EB1089 inhibited prostate cancer cell proliferation and reduced tumorigenesis as well as tumor metastases. Furthermore, in vitro studies on U937 histiocytic lymphoma cells showed that EB1089 was 50–100 times more effective in inhibiting cell proliferation and in inducing cell differentiation than 1,25(OH)2D3 but less effective than calcitriol in affecting in vivo calcium metabolism in rats (Mathiasen et al., Citation1993). These data match those reported by Gulliford et al. (Citation1998) showing that EB1089 was significantly less calcemic than 1,25-dihydroxyvitamin D3 when administered at a maximum-tolerated dose (7 µg/m2) to patients with breast cancer or colorectal cancer. Other in vitro observations on HT-29 colon adenocarcinoma cells showed that EB1089 was more effective than Vit D in inhibiting HT-29 proliferation (Oh et al., Citation2001). In particular, immunoblot analysis showed that EB1089 inhibited the secretion of IGF-2 and stimulated the production IGFBP-6. More recently Lu et al. (Citation2008) reported that EB1089 inhibits the proliferation of Hep-2 human laryngeal squamous carcinoma cells. The growth-inhibiting activity of this molecule appeared to be due to an increase of p57 cyclin-dependent kinase inhibitor at mRNA and protein levels induced by this molecule. EB1089 brought about tumor cell death by a mechanism, not related to caspase activation, which consisted in the induction of chromatin condensation and DNA fragmentation (Høyer-Hansen et al., Citation2005). Furthermore, recent findings by Ghous et al. (Citation2008) showed that EB1089 may inhibit either in vitro or in vivo the growth of hepatocellular carcinoma (HCC). Interestingly, Zhang et al. (Citation2013) recently reported that a combination of retinoic acid (RA) and EB1089 exerted a synergistic growth inhibition and apoptosis induction in HCC cells. Furthermore, in vivo studies carried out in rats with prostate cancer showed that the administration of EB1089 decreased the tumor size and the number of lung metastases (Chen et al., Citation2003). Although these experimental observations are encouraging, clinical studies aimed at evaluating the therapeutic effectiveness of this molecule in cancer patients have generated controversial results. In particular, phase I and II studies in patients with breast cancer, colorectal cancer, hepatocellular carcinoma, or pancreatic cancer failed to demonstrate any therapeutic response of patients to EB1089 treatment (Dalhoff et al., Citation2003; Evans et al., Citation2002; Gulliford et al., Citation1998). However, it cannot be ruled out that patients’ characteristics (i.e., presence/absence of VDR in tumor tissue, previous treatments, low number of enrolled patients, dose-limiting hypercalcemia, etc.) might, in part, account for this phenomenon. Therefore, further studies are needed to better define the potential clinical effectiveness and toxicity of EB1089.
Analogues of 20-epi vitamin D
The 20-epi-vitamin D3 analogues including CB-1093, KH-1060 (lexicalcitol), BXL-628 (elocalcitol), and 2MD are molecules characterized by an altered stereochemistry at carbon 20 in the side-chain (Binderup et al., Citation1991) (). These molecules except 2MD (2-methylene-19-nor-(20S)-1-,25(OH)2D3) have been shown to possess chemopreventive activity. 2MD has been shown to stimulate bone formation in vivo and in vitro (Ke et al., Citation2005; Mäenpää et al., Citation2001) but it appears to be devoid of antitumor activity (Ke et al., Citation2005). Therefore, this compound has been proposed for the treatment of osteoporosis (Plum et al., Citation2006). In vitro observation showed that KH1060 and CB1093 may inhibit cell proliferation, at lower concentrations and earlier points in time than calcitriol, by blocking the cell cycle in the G0/G1 phase (Mäenpää et al., Citation2001; Ryhänen et al., Citation2003). This phenomenon appeared to be the consequence of an increase in the p27 level and a marked decrease of Cdk2. These phenomena ultimately contribute to keeping retinoblastoma (Rb) protein in its hypophosphorylated, i.e., growth suppressing, form thus preventing cell-cycle progression through the restriction point (Elstner et al., Citation1999; Mäenpää et al., Citation2001; Ryhänen et al., Citation2003). These data are consistent with other in vitro studies showing that CB1093 and KH1060, alone or in combination with 9-cis-retinoic acid, inhibited the growth of LNCaP human prostate cancer cells (Elstner et al., Citation1999) and human neuroblastoma cells (Gumireddy et al., Citation2003). It was also demonstrated that these effects were associated with increased levels of p21(waf-1) and p27(kip1) protein. The inhibiting effects of CB1093 on the growth of prostate adenocarcinoma was also confirmed in vivo by some experimental studies showing that this analogue inhibited tumor growth in the Dunning prostate model and in athymic nude mice transplanted with LNCaP tumor cells (Oades et al., Citation2002). Other studies, performed on MCF-7, T47D, and Hs578T breast cancer cell lines, showed that CB1093 enhanced the response of breast cancer cells to TNF-α and the intracellular production of ceramide which appeared to act as downstream effectors in Vit D-mediated caspase-independent cell death (Mathiasen et al., 1993; Pirianov & Colston, Citation2001). Interestingly, Danielsson et al. (Citation1998) reported that CB1093 appeared to be very effective in inducing apoptosis in the early stage in the WM1341 melanoma cell line, but not in the advanced stage in MeWo melanoma cell line. Other studies examined the effects induced by BXL-628 analogue or Elocalcitol in benign prostatic hyperplasia and in prostate cancer cells (Adorini et al., Citation2007; Penna et al., Citation2009; Tiwari, Citation2009). The results of these studies indicated that the main mechanism appears to be related to the inhibition of growth factors and to the interleukin-8 (IL-8) production via a decrease in COX-2 and PGE2 synthesis (Adorini et al., Citation2007; Penna et al., Citation2009). Elocalcitol was also able to inhibit the proliferation and invasiveness of prostate cancer cell line, DU145 by interfering with keratinocytes growth factor (KGF)-induced proliferation (Marchiani et al., Citation2006). Based on these results, these molecules may be of potential clinical interest as novel chemopreventive agents. Further clinical studies may better assess their clinical effectiveness in cancer prevention and treatment.
Tacalcitol and eldecalcitol
Tacalcitol (1,24-dihydroxyvitamin D3, PRI-2191) is an active metabolite of 1α,25(OH)2D3 () that does not exhibit the high calcemic activity of the original compound (Wietrzyk et al., Citation2004). Studies aimed at assessing the antitumor activity and toxicity of tacalcitol showed a lower toxicity of this molecule after its subcutaneous administration compared with that of calcitriol (Wietrzyk et al., Citation2004). Furthermore, the oral administration of tacalcitol increased calcium serum levels by 47%, while calcitriol increased these levels by 78%. Interestingly, the treatment of mice with breast cancer with tacalcitol caused a reduction of the tumor volume by 41% (Wietrzyk et al., Citation2004). Moreover, the co-administration of tacalcitol with antitumor drugs such as 5-FU and cisplatin or oxaliplatin and irinotecan to mice transplanted with MC38 (mouse) or HT-29 human colon cancer induced a tumor growth inhibition significantly greater than tacalcitol alone and a significant prolongation of the survival time of mice (Milczarek et al., Citation2013b,Citationc). Another study carried out on different cell lines, namely A549 (lung cancer), B16 (murine melanoma), HL-60 (human leukemia), SW707 (human colon cancer), MCF-7 and T47D (breast cancer), WEHI-3 (murine leukemia), and on normal murine fibroblasts BALB-3T3 cells, demonstrated that the administration of tacalcitol in association with cisplatin or doxorubicin resulted in a significant decrease in the IC50 values of these antitumor agents (Pelczynska et al., Citation2006). Furthermore, Switalska et al. (Citation2012) recently reported that the administration of tacalcitol enhanced the antiproliferative activity of imatinib demesilate on HL-60 leukemia cells. Eldecalcitol (1α, 25-dihydroxy-2β-[3-hydroxypropyloxy] vitamin D3), (ED-71), is an orally administered analogue of active Vit D that is mainly used in the clinical treatment of osteoporosis (Sanford & McCormack, Citation2011) (). Further experimental studies showed that ED-71 is endowed with chemopreventive activity against tumors and with reduced hypercalcemic effects (Hatakeyama et al., Citation2010). Studies on myeloid leukemia demonstrated the ability of this molecule to induce tumor cells to differentiate into normal monocyte-macrophages (Gocek & Studzinski, Citation2009; Hatakeyama et al., Citation2010; Nishii & Okano, Citation2001). This molecule also inhibited the proliferation of U937 human, histiocytic lymphoma cells, and increased osteocalcin concentrations in the human osteosarcoma cells (MG-63) (Hatakeyama et al., Citation2010). Eldecalcitol is a CYP24A1-resistant analogue (Ritter & Brown, Citation2011). CYP24A1 is expressed in many tissues and cells, including the prostate, and appear to be implicated in the resistance of prostate cancer to Vit D (Tannour-Louet, Citation2014). Therefore, ED-71 may be of clinical usefulness in the prevention and treatment of prostate cancer (Chen et al., Citation2012; Sakaki et al., Citation2014).
BXL 0124
BXL0124, a new analogue of Vit D, belongs to the deuterated Gemini Vit D compounds (). These compounds have a C-20 methyl group, a deuterium-substituted side chain, and a second side chain that has a double or triple bond and a fluorine. BXL0124 has been shown to possess a chemo-preventive effect on breast cancer and prostate cancer (Lee et al., Citation2008; Spina et al., Citation2007; Wahler et al., Citation2014). Interestingly, in vitro studies on MCF10DCIS cells showed that this molecule had antiproliferative effects on breast cancer and markedly decreased the expression of CD44 (So et al., Citation2011). Intriguingly, recent observations showed that BXL0124 inhibited breast cancer cell invasion by targeting CD44-STAT3 signaling (So et al., Citation2013a). These findings are consistent with the observations that the JAK2/STAT3 signaling pathway is essential for growth of the CD44+/CD24− stem cell-like breast (Marotta et al., Citation2011). In contrast, the inhibiting activity of this molecule on tumor growth observed in vitro was further confirmed by in vivo studies showing that either the oral (0.03 or 0.1 µg/kg) or intraperitoneal (0.1 µg/kg) administration of Gemini 6 d a week for 5 weeks to mice-bearing tumor caused a reduction in the growth of breast cancer and a consistent decrease in the expression of CD44 protein, without causing hypercalcemia (So et al., Citation2011). Consistent with these findings, Wahler et al. (Citation2014) showed that, in mice inoculated with ductal carcinoma in situ (DCIS) MCF10DCIS com. cells, the administration of BXL0124 resulted in a 43% reduction in tumor volume. Moreover, BXL0124 treatment also decreased the mRNA levels of MMPs starting at week 3, thus contributing to the inhibition of invasive transition. These findings indicate that this molecule may be an important target for the chemoprevention and treatment of breast cancer. Consistent with these findings, a more recent study by So et al. (Citation2013b) reported that BXL0124 may be effective, in combination with other molecules, as potential chemopreventive agent, but not for the treatment, against the tumorigenesis of ErbB2-overexpressing breast cancer. The same authors highlighted the fact that BXL0124 (10 nM) induced expression of mRNA coding for osteopontin, one of the genes regulated by Vit D, thus contributing to the regulation of cell proliferation (So et al., Citation2011). BXL0124 has also been shown to reduce tumor growth by 50% and to prevent metastasis formation in the MC-26 colon cancer xenograft model while no effect was noted on calcium homeostasis (Spina et al., Citation2007). These data warrant future clinical studies to assess the pharmacological profile of this analog in humans.
19-nor-1α,25(OH)2D3 analogs: MART-10, MART-11, paracalcitol, and inecalcitol
MART-10 MART-11
19-nor-Vit D compounds are Vit D analogs in which the ring A methylene group on C-19 is replaced with two hydrogen atoms (Chen & Kittaka, Citation2011) (). MART-10 (19-nor-2α-(3-hydroxypropyl)-1α,25(OH)2D3) and MART-11 (19-nor-2β-3-hydroxypropyl-1α,25(OH)2D3) are two new synthetic C2-substituted 19-nor-1α,25(OH)2D3 analogs of Vit D that have negligible effects on calcium plasma levels and that appear to be effective in the prevention and treatment of prostate cancer (Chen & Kittaka, Citation2011; Kittaka et al., Citation2012). In vitro studies on the HL-60 cell line showed that compared with 1,25(OH)2D3, MART-10, and MART-11 are endowed with a different degree of binding affinity for VDR (100 and 3%). In addition, both analogues were more effective in inducing cell differentiation, compared with 1,25(OH)2D3 (Arai & Kittaka, Citation2006; Ono et al., Citation2003). The discrepancy between the rate of VDR binding and differentiation activity was explained, at least in part, by their remarkable ability to recruit co-activators, such as hTIF-2 and HSRC-1 (Arai et al., Citation2007). The antiproliferative activity of MART-10 and MART-11 was investigated in LNCaP and PC3 human prostate cancer cells (Chen et al., Citation2003; Flanagan et al., Citation2009). These investigations have shown that both analogues possess antiproliferative activity comparable with that of 1,25(OH)2D3. However, MART-10 proves about 1000-fold more active than 1α,25(OH)2D3 in inhibiting LNCaP and PC-3 prostate cancer cell proliferation (Chen & Kittaka, Citation2011; Iglesias-Gato et al., Citation2011). Furthermore, in vitro studies which compared the expression level of the enzyme CYP24A1 in LNCaP and PC-3 in response to treatment with 1,25(OH)2D3 and MART-10 showed that this latter molecule was able to induce the expression of CYP24A1, one of the three major enzymes involved in the metabolism of Vit D (Jones et al., Citation2012), to a lower concentration than calcitriol (Flanagan et al., Citation2009). In vivo, the subcutaneous administration of MART-10 was reported to up-regulate CYP24A1 mRNA expression in rat kidneys without affecting their plasma calcium levels (Iglesias-Gato et al., Citation2011). These findings demonstrated that MART-10 is biologically active in vivo and may be of clinical usefulness in treating prostate cancer. Therefore, the induction of the CYP24A1 gene was used as an index for evaluating the biological potency of new Vit D analogs (Chen & Kittaka, Citation2011). These studies additionally highlighted the fact that MART-10 induced CYP24A1 gene expression at a lower concentration with a longer duration compared with 1α,25(OH)2D3, suggesting that MART-10 is less susceptible to CYP24A1 degradation (Flanagan et al., Citation2009; Iglesias-Gato et al., Citation2011). Furthermore, the effects induced by MART-10 lasted for a longer period of time. This phenomenon indicated a low susceptibility of this molecule to being degraded by CYP24A1 (Flanagan et al., Citation2009). Interestingly, MART-10 has been shown to be a potent inhibitor of cancer cell invasiveness (Chen & Kittaka, Citation2011). In fact, in vitro studies on PC-3 prostate cancer cells showed that MART-10 determined down-regulation of matrix metalloproteinase-9 (MMP-9), an enzyme which fosters tumor invasion, angiogenesis, and metastasis (Chen & Kittaka, Citation2011). More recent observations by Chiang et al. (Citation2014) show that MART-10 is more active than 1α,25(OH)2D3 in preventing MCF-7 cell invasion and migration, probably mediated through the up-regulation of E-cadherin, and that of the transcription factors implicated in epithelial–mesenchymal transition (EMT) down-regulation, e.g., Snail, Slug, and Twist, as well as MMP-13. These data further confirm and extend previous finding of the same authors showing that MART-10 is also able to inhibit cell proliferation and to induce apoptosis in MCF-7/ER(+) breast cancer cells (Chiang et al., Citation2012). These findings suggest that these analogues and their structurally related analogues may be good candidates for the treatment of different human tumors including breast, prostate, and liver cancers (Kittaka et al., Citation2012). These observations warrant further in vivo animal study to better assess the pharmacological profile and the therapeutic effectiveness of these molecules in humans.
Paricalcitol
Paricalcitol (19-nor-1α,25-dihydroxyvitamin D2) is a synthetic analog of calcitriol, the active form of Vit D (). Experimental in vitro and in vivo studies have reported that paricalcitol presents anticancer activity against several hematological and solid tumors including myeloid leukemia, myeloma, gastric cancer, colon cancer, and pancreatic cancer and that these effects may be mediated through the VDR (Kumagai et al., Citation2003, Citation2005; Park et al., Citation2012; Schwartz et al., Citation2005, Citation2008). Interestingly, recent clinical observation by Lawrence et al. (Citation2013) has shown that paricalcitol, in combination with taxane-based chemotherapy, appears to be safe and feasible and may have a clinical benefit for women with metastatic breast cancer. Unlike these findings, Schwartz et al. (Citation2005) did not observe any clear response in patients with advanced prostate cancer. On the other hand, in HL-60 and NB4 myeloid leukemia cell lines, paricalcitol was noted to suppress proliferation and induce differentiation (Kumagai et al., Citation2005; Molnar et al., Citation2004). In human NCI-H929 myeloma cells, this molecule inhibited cell growth by causing cell-cycle block and apoptosis indicating its potential as an antileukemic agent (Molnar et al., Citation2004). Furthermore, in patients with all-trans-retinoic acid (ATRA)-resistant myeloid leukemia a combination therapy consisting of paricalcitol and arsenic trioxide also appears to be promising (Kumagai et al., Citation2005). These findings together with its low calcemic activity and achievable therapeutic doses strongly support its use in clinical trials regarding hematological diseases such as MDS and acute myeloid leukemia.
Inecalcitol
Inecalcitol is a novel Vit D 19-nor analogue (19-nor-14-epi-23-yne-1,25 dihydroxyvitamin D3) that differs from 1,25(OH)2D3 through epimerization of C14, deletion of C19, and 23-yne modification in the side chain () (Verlinden et al., Citation2000). This analogue appears to be less inclined to induce hypercalcemia while it remains a potent stimulant of VDR (Verlinden et al., Citation2000). Inecalcitol has been shown to suppress both in vitro and in vivo the growth of human LNCaP prostate cancer and that of SCC (Ma et al., Citation2013a,Citationb; Okamoto et al., Citation2012). The antitumor activity of inecalcitol, at least in SCC, appears to be the consequence of (a) the arrest of tumor cells in G0/G1 transition phase of the cell cycle, (b) the triggering of the apoptosis cascade through the activation of caspase 8/10–caspase 3 pathway, and (c) the inhibition of expression of c-IAP1 and XIAP. Inecalcitol has also been shown to repress cyclin D1 and cyclin C gene expression and to induce p21 and p27 gene expression more efficiently than calcitriol (Ma et al., Citation2013a). Inecalcitol has been utilized in clinical studies in association with the classical antitumor agent docetaxel (Medioni et al., Citation2014). The results of the phase II trial in castration-resistant prostate cancer showed that this drug combination had a better PSA response than docetaxel alone. These data provide support for further evaluation of inecalcitol in cancer treatment.
Conclusions
On one hand, epidemiological observations highlight the fact that high levels of vitamin D may offer protection against many types of cancer (Leyssens et al., Citation2013; Pilz et al., Citation2013). On the other hand, many experimental studies provide evidence for the growth inhibiting, anti-inflammatory, and pro-differentiation effects of Vit D in vitro on human cancer cell lines and in vivo on tumor-bearing animals (Krishnan & Feldman, Citation2011; Meeker et al., Citation2014; Trump et al., Citation2010; Vanoirbeek et al., Citation2011). Therefore, the use of vitamin D or its semisynthetic analogues in cancer therapy could provide effective chemopreventive effects against tumor progression (Byers et al., Citation2012; Krishnan & Feldman, Citation2011; Meeker et al., Citation2014; Trump et al., Citation2010; Vanoirbeek et al., Citation2011). The possible mechanisms by which vitamin D mediates these effects have been, only in part, identified. Although the preclinical data are striking and the epidemiologic data are encouraging, no well-designed clinical trial on the optimal administration of vitamin D as a cancer therapy has ever been undertaken (Krishnan & Feldman, Citation2010; Leyssens et al., Citation2014). Future clinical investigations may better define the clinical role of Vit D or its analogues in cancer prevention and treatment. Another relevant feature of the paradigm Vit D/cancer needing further investigation is related to tumor resistance as this phenomenon may affect the synthesis of the active metabolite of Vit D and, consequently, its potential therapeutic activity (Ajibade et al., Citation2014; Giardina et al., Citation2012; Larriba & Muñoz, Citation2010; Tannour-Louet, Citation2014). Therefore, many studies are currently directed to find new molecules which may circumvent tumor resistance to Vit D analogues (Deeb et al., 2007; Fischer et al., Citation2012; Ritter & Brown, Citation2011; Solomon et al., Citation2014).
Declaration of interest
The authors report that there are no declarations of interest.
References
- Abe E, Miyaura C, Sakagami H, et al. (2004). Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 78:4990–4
- Abe-Hashimoto J, Kikuchi T, Matsumoto T, et al. (1993). Antitumor effect of 22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic mice implanted with human breast carcinoma and its synergism with tamoxifen. Cancer Res 53:2534–7
- Abe J, Nakano T, Nishii Y, et al. (1991). A novel vitamin D3 analog, 22-oxa-1,25-dihydroxyvitamin D3, inhibits the growth of human breast cancer in vitro and in vivo without causing hypercalcemia. Endocrinology 129:832–7
- Adorini L, Penna G, Amuchastegui S, et al. (2007). Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol). J Steroid Biochem 103:689–93
- Aggarwal BB. (2004). Nuclear factor-kappaB: The enemy within. Cancer Cell 6:203–8
- Agoston ES, Hatcher MA, Kensler TW, Posner GH. (2006). Vitamin D analogs as anti-carcinogenic agents. Anticancer Agents Med Chem 6:53–71
- Ajibade AA, Kirk JS, Karasik E, et al. (2014). Early growth inhibition is followed by increased metastatic disease with vitamin D (calcitriol) treatment in the TRAMP model of prostate cancer. PLoS One 9:e89555
- Akhter J, Chen X, Bowrey P, et al. (1997). Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis Colon Rectum 40:317–21
- Akiyama N, Miyazawa K, Kanda Y, et al. (2010). Multicenter phase II trial of vitamin K(2) monotherapy and vitamin K(2) plus 1alpha-hydroxyvitamin D(3) combination therapy for low-risk myelodysplastic syndromes. Leuk Res 34:1151–7
- Al-Jaderi Z, Maghazachi AA. (2013). Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel) 5:1932–47
- An BS, Tavera-Mendoza LE, Dimitrov V, et al. (2010). Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol 30:4890–900
- Anderson LN, Cotterchio M, Vieth R, Knight JA. (2010). Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr 91:1699–707
- Anderson MG, Nakane M, Ruan X, et al. (2006). Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemoth Pharm 57:234–40
- Aparna R, Subhashini J, Roy KR, et al. (2008). Selective inhibition of cyclooxygenase-2 (COX-2) by 1alpha,25-dihydroxy-16-ene-23-yne vitamin D3, a less calcemic vitamin D analog. J Cell Biochem 104:1832–42
- Arai MA, Kittaka A. (2006). Novel 2-alkyl-1alpha,25-dihydroxy-19-norvitamin D3: Efficient synthesis with Julia olefination, evaluation of biological activity and development of new analyzing system for co-activator recruitment. Anticancer Res 26:2621–31
- Arai MA, Takeyama K, Ito S, et al. (2007). High-throughput system for analyzing ligand-induced cofactor recruitment by vitamin D receptor. Bioconjug Chem 18:614–20
- Armas LA, Dowell S, Akhter M, et al. (2007). Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: The effect of UVB dose and skin color. J Am Acad Dermatol 57:588–93
- Baldwin AS. (2012). Regulation of cell death and autophagy by IKK and NF-κB: Critical mechanisms in immune function and cancer. Immunol Rev 246:327–45
- Banakar MC, Paramasivan SK, Chattopadhyay MB, et al. (2004). 1Alpha,25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J Gastroenterol 10:1268–75
- Bao BY, Ting HJ, Hsu JW, Lee YF. (2008). Protective role of 1 alpha,25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int J Cancer 122:2699–706
- Bao BY, Ting HJ, Hsu JW, et al. (2010). Down-regulation of NF-kappaB signals is involved in loss of 1alpha,25-dihydroxyvitamin D3 responsiveness. J Steroid Biochem Mol Biol 120:11–21
- Bao BY, Yao J, Lee YF. (2006). 1Alpha,25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis 27:1883–93
- Beer TM, Ryan CW, Venner PM; ASCENT (AIPC Study of Calcitriol ENhancing Taxotere) Investigators, et al. (2008). Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: Results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer 112:326–30
- Beildeck ME, Islam M, Shah S, et al. (2009). Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS One 4:e7872
- Ben-Shoshan M, Amir S, Dang DT, et al. (2007). 1 Alpha,25 dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther 6:1433–9
- Berkovic L, Ben-Shabat S, Sintov AC. (2010). Induction of apoptosis and inhibition of prostate and breast cancer growth by BGP-15, a new calcipotriene-derived vitamin D3 analog. Anticancer Drugs 21:609–18
- Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. (2002). Antiproliferative effects of 1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology 143:2508–14
- Bertone-Johnson ER, Chen WY, Holick MF, et al. (2005). Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidem Biomarkers Prev 14:1991–7
- Bessler H, Djaldetti M. (2012). 1α,25-Dihydroxyvitamin D3 modulates the interaction between immune and colon cancer cells. Biomed Pharmacother 66:428–32
- Bettoun DJ, Buck DW, Lu J, et al. (2002). A vitamin D receptor-Ser/Thr phosphatase-p70 S6 kinase complex and modulation of its enzymatic activities by the ligand. J Biol Chem 277:24847–50
- Bhatia M, Kirkland JB, Meckling-Gill KA. (1995). Monocytic differentiation of acute promyelocytic leukemia cells in response to 1,25-dihydroxyvitamin D3 is independent of nuclear receptor binding. J Biol Chem 270:15962–5
- Bhatia M, Kirkland JB, Meckling-Gill KA. (1996). 1,25-dihydroxyvitamin D3 primes acute promyelocytic cells for TPA-induced monocytic differentiation through both PKC and tyrosine phosphorylation cascades. Exp Cell Res 222:61–9
- Bhatia V, Saini MK, Shen X, et al. (2009). EB1089 inhibits the parathyroid hormone-related protein enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther 8:1787–98
- Bikle DD. (2012). Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord 13:3–19
- Binderup L, Bramm E. (1988). Effects of a novel vitamin D analogue MC903 on cell proliferation and differentiation in vitro and on calcium metabolism in vivo. Biochem Pharmacol 37:889–95
- Binderup L, Latini S, Binderup E, et al. (1991). 20-Epi-vitamin D3 analogues: A novel class of potent regulators of cell growth and immune responses. Biochem Pharmacol 42:1569–75
- Bizzarri M, Cucina A, Valente MG, et al. (2003). Melatonin and vitamin D3 increase TGF-beta1 release and induce growth inhibition in breast cancer cell cultures. J Surg Res 110:332–7
- Blomberg Jensen M, Andersen CB, Nielsen JE, et al. (2010). Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J Steroid Biochem Mol Biol 121:376–82
- Blutt SE, McDonnell TJ, Polek TC, Weigel NL. (2000). Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology 141:10–17
- Bogh MK, Schmedes AV, Philipsen PA, et al. (2010). Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 130:546–53
- Bouillon R, Carmeliet G, Verlinden L, et al. (2008). Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev 29:726–76
- Bower M, Colston KW, Stein RC, et al. (1991). Topical calcipotriol treatment in advanced breast cancer. Lancet 337:701–2
- Boyle BJ, Zhao XY, Cohen P, Feldman D. (2001). Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin D(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol 165:1319–24
- Brown AJ, Slatopolsky E. (2008). Vitamin D analogs: Therapeutic applications and mechanisms for selectivity. Mol Aspects Med 29:433–52
- Brozek W, Manhardt T, Kállay E, et al. (2012). Relative expression of vitamin D hydroxylases, CYP27B1 and CYP24A1, and of cyclooxygenase-2 and heterogeneity of human colorectal cancer in relation to age, gender, tumor location, and malignancy: Results from factor and cluster analysis. Cancers (Basel) 4:763–76
- Brożyna AA, Jozwicki W, Janjetovic Z, Slominski AT. (2011). Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol 42:618–31
- Byers SW, Rowlands T, Beildeck M, Bong YS. (2012). Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev Endocr Metab Disord 13:31–8
- Byrne B, Welsh J. (2007). Identification of novel mediators of Vitamin D signaling and 1,25(OH)2D3 resistance in mammary cells. J Steroid Biochem Mol Biol 103:703–7
- Campbell MJ, Gombart AF, Kwok SH, et al. (2000). The anti-proliferative effects of 1alpha,25(OH)2D3 on breast and prostate cancer cells are associated with induction of BRCA1 gene expression. Oncogene 19:5091–7
- Cantorna MT. (2012). Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys 523:103–6
- Cao X, Ross FP, Zhang L, et al. (1993). Cloning of the promoter for the avian integrin beta 3 subunit gene and its regulation by 1,25-dihydroxyvitamin D3. J Biol Chem 268:27371–80
- Cardús A, Parisi E, Gallego C, et al. (2006). 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 69:1377–84
- Chan AT, Giovannucci EL. (2010). Primary prevention of colorectal cancer. Gastroenterology 138:2029–43
- Chaudhry M, Sundaram S, Gennings C, et al. (2001). The vitamin D3 analog, ILX-23-7553, enhances the response to adriamycin and irradiation in MCF-7 breast tumor cells. Cancer Chemother Pharmacol 47:429–36
- Chen A, Davis BH, Sitrin MD, et al. (2002). Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)2D3. Am J Physiol Gastrointest Liver Physiol 283:864–74
- Chen TC, Holick MF. (2003). Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab 14:423–30
- Chen TC, Holick MF, Lokeshwar BL, et al. (2003). Evaluation of vitamin D analogs as therapeutic agents for prostate cancer. Recent Res Cancer 164:273–88
- Chen TC, Kittaka A. (2011). Novel vitamin D analogs for prostate cancer therapy. ISRN Urolol 2011:301490. doi:10.5402/2011/301490
- Chen TC, Sakaki T, Yamamoto K, Kittaka A. (2012). The roles of cytochrome P450 enzymes in prostate cancer development and treatment. Anticancer Res 32:291–8
- Chiang KC, Yeh CN, Chen SC, et al. (2012). MART-10, a new generation of vitamin D analog, is more potent than 1α,25-dihydroxyvitamin D(3) in inhibiting cell proliferation and inducing apoptosis in ER+ MCF-7 breast cancer cells. Evid Based Complement Alternat Med, 2012:310872. doi: 10.1155/2012/310872
- Chiang KC, Chen SC, Yeh CN, et al. (2014). MART-10, a less calcemic vitamin D analog, is more potent than 1α,25-dihydroxyvitamin D3 in inhibiting the metastatic potential of MCF-7 breast cancer cells in vitro. Steroid Biochem Mol Biol 139:54–60
- Cho KH, Son YS, Lee DY, et al. (1996). Calcipotriol (MC 903), a synthetic derivative of vitamin D3 stimulates differentiation of squamous carcinoma cell line in the raft culture. Anticancer Res 16:337–47
- Choi M, Makishima M. (2009). Therapeutic applications for novel non-hypercalcemic vitamin D receptor ligands. Expert Opin Ther Pathol 19:593–606
- Chung I, Han G, Seshadri M, et al. (2009). Role of vitamin D receptor in the antiproliferative effects of calcitriol intumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res 69:967–75
- Chung I, Wong MK, Flynn G, et al. (2006). Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res 66:8565–73
- Cianferotti L, Cox M, Skorija K, Demay MB. (2007). Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA 104:9428–33
- Circu ML, Aw TY. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–62
- Clinckspoor I, Hauben E, Verlinden L, et al. (2012). Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J Histochem Cytochem 60:502–11
- Colston K, Colston MJ, Feldman D. (1981). 1,25-Dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 108:1083–6
- Colston KW, Chander SK, Mackay AG, Coombes RC. (1992). Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem Pharmacol 44:693–702
- Colston KW, Mackay AG, James SY, et al. (1992a). EB1089: A new vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochem Pharmacol 44:2273–80
- Colston KW, Perks CM, Xie SP, Holly JM. (1998). Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J Mol Endocrinol 20:157–62
- Colston KW, Hansen CM. (2002). Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer 9:45–59
- Cordes T, Hoellen, Dittmer C, et al. (2012). Correlation of prostaglandin metabolizing enzymes and serum PGE2 levels with vitamin D receptor and serum 25 (OH) 2D3 levels in breast and ovarian cancer. Anticancer Res 32:351–7
- Crescioli C, Villari D, Forti G, et al. (2002). Des (1-3) IGF-I-stimulated growth of human stromal BPH cells is inhibited by a vitamin D3 analogue. Mol Cell Endocrinol 198:69–75
- Cross HS, Bises G, Lechner D, et al. (2005). The vitamin D endocrine system of the gut – Its possible role in colorectal cancer prevention. J Steroid Biochem Mol Biol 97:121–8
- Cross HS, Nittke T, Kallay E. (2011). Colonic vitamin D metabolism: Implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol 347:70–9
- Cross HS, Nittke T, Peterlik M. (2009). Modulation of vitamin D synthesis and catabolism in colorectal mucosa: A new target for cancer prevention. Anticancer Res 29:3705–12
- Cunningham D, Gilchrist NL, Cowan RA, et al. (1985). Alfacalcidol as a modulator of growth of low grade non-Hodgkin's lymphomas. Br Med J (Clin Res Ed) 291:1153–5
- Dalhoff K, Dancey J, Astrup L, et al. (2003). A phase II study of the vitamin D analogue Seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer 89:252–7
- Dalirsani Z, Farajnia S, Javadzadeh Y, et al. (2012). The effects of 5-fluorouracil alone and in combination with 13-cis retinoic acid and vitamin D3 on human oral squamous cell carcinoma lines. J Contemp Dent Pract 13:345–50
- Daniel C, Schroder O, Zahn N, et al. (2007). The TGF beta/Smad 3-signaling pathway is involved in butyrate-mediated vitamin D receptor (VDR)-expression. J Cell Biochem 102:1420–31
- Danielsson C, Fehsel K, Polly P, Carlberg C. (1998). Differential apoptotic response of human melanoma cells to 1 alpha,25-dihydroxyvitamin D3 and its analogues. Cell Death Differ 5:946–52
- Davis JS, Wu X. (2012). Current state and future challenges of chemoprevention. Discov Med 13:385–90
- Deeb KK, Trump DL, Johnson CS. (2007). Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer 7:684–700
- Demasters G, Di X, Newsham I, et al. (2006). Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther 5:2786–97
- Díaz GD, Paraskeva C, Thomas MG, et al. (2000). Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: Possible implications for prevention and therapy. Cancer Res 60:2304–12
- Di Cunto F, Topley G, Calautti E, et al. (1998). Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 280:1069–72
- Drivdahl RH, Loop SM, Andress DL, Ostenson RC. (1995). IGF-binding proteins in human prostate tumor cells: Expression and regulation by 1,25-dihydroxyvitamin D3. Prostate 26:72–9
- Dröge W. (2003). Autophagy and aging – Importance of amino acid levels. Mech Ageing Dev 225:161–8
- Edfeldt K, Liu PT, Chun R, et al. (2010). T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 107:22593–8
- Eide MJ, Johnson DA, Jacobsen GR, et al. (2011). Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol 147:1379–84
- Elstner E, Campbell MJ, Munker R, et al. (1999). Novel 20-epi-vitamin D3 analog combined with 9-cis-retinoic acid markedly inhibits colony growth of prostate cancer cells. Prostate 40:141–9
- Engel P, Fagherazzi G, Boutten A, et al. (2010). Serum 25(OH) vitamin D and risk of breast cancer: A nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 19:2341–50
- Engeset D, Alsaker E, Lund E, et al. (2006). Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 119:175–82
- Escaleira MT, Brentani MM. (1999). Vitamin D3 receptor (VDR) expression in HC-11 mammary cells: Regulation by growth-modulatory agents, differentiation, and Ha-ras transformation. Breast Cancer Res Treat 54:123–33
- Evans TR, Colston KW, Lofts FJ, et al. (2002). A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer 86:680–5
- Fedirko V, Bostick RM, Flanders WD, et al. (2009). Effects of vitamin D and calcium on proliferation and differentiation in normal colon mucosa: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev 18:2933–41
- Fedirko V, Bostick RM, Long Q, et al. (2010). Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev 19:280–91
- Feldman D, Krishnan AV, Swami S, et al. (2014). The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 14:342–57
- Fernandez-Garcia NI, Pálmer HG, Garcia M, et al. (2005). 1,25- Dihydroxyvitamin D3 regulates the expression of ld1 and ld2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene 24:6533–44
- Fischer J, Wang TT, Kaldre D, et al. (2012). Synthetically accessible non-secosteroidal hybrid molecules combining vitamin D receptor agonism and histone deacetylase inhibition. Chem Biol 19:963–71
- Flanagan JN, Zheng S, Chiang KC, et al. (2009). Evaluation of 19-nor-2alpha-(3-hydroxypropyl)-1alpha,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Res 29:3547–53
- Flanagan L, Packman K, Juba B, et al. (2003). Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol 84:181–92
- Fleet JC, DeSmet M, Johnson R, Li Y. (2012). Vitamin D and cancer: A review of molecular mechanisms. Biochem J 441:61–76
- Flores O, Wang Z, Knudsen KE, Burnstein KL. (2010). Nuclear targeting of cyclin-dependent kinase 2 reveals essential roles of cyclin-dependent kinase 2 localization and cyclin E in vitamin D-mediated growth inhibition. Endocrinology 151:896–908
- Flynn G, Chung I, Yu WD, et al. (2006). Calcitriol (1,25-dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology 70:447–57
- Freedman DM, Looker AC, Chang SC, Graubard BI. (2007). Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 99:1594–602
- Friedrich M, Diesing D, Cordes T, et al. (2006). Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in normal and malignant breast tissue. Anticancer Res 26:2615–20
- Fukumoto S. (2014). Phosphate metabolism and vitamin D. Bonekey Rep 3:497. doi:10.1038/bonekey.2013.231
- Funato K, Miyazawa K, Yaguchi M, et al. (2002). Combination of 22-oxa-1,25-dihydroxyvitamin D(3), a vitamin D(3) derivative, with vitamin K(2) (VK2) synergistically enhances cell differentiation but suppresses VK2-inducing apoptosis in HL-60 cells. Leukemia 16:1519–27
- Furigay P, Swamy N. (2004). Anti-endothelial properties of 1,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of calcitriol. J Steroid Biochem Mol Biol 89:427–31
- Gandini S, Boniol M, Haukka J, et al. (2011). Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128:1414–24
- Garcia LA, King KK, Ferrini MG, et al. (2011). 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152:2976–86
- Garland CF, Gorham ED, Mohr SB, et al. (2007). Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol 103:708–11
- Gaschott T, Steinmeyer A, Steinhilber D, Stein J. (2002). ZK156718 a low calcemic, antiproliferative, and prodifferentiating vitamin D analog. Biochem Biophys Res Commun 290:504–9
- Gedlicka C, Hager G, Weissenböck M, et al. (2006). 1,25(OH)2Vitamin D3 induces elevated expression of the cell cycle inhibitor p18 in a squamous cell carcinoma cell line of the head and neck. J Oral Pathol Med 35:472–8
- Gee J, Bailey H, Kim K, et al. (2013). Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1α-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. Prostate 9:970–8
- Geng S, Zhou S, Glowacki J. (2011). Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Miner Res 26:1145–53
- Ghous Z, Akhter J, Pourgholami MH, Morris DL. (2008). Inhibition of hepatocellular cancer by EB1089: In vitro and in vivo study. Anticancer Res 28:3757–61
- Giardina C, Madigan JP, Tierney CA, et al. (2012). Vitamin D resistance and colon cancer prevention. Carcinogenesis 33:475–82
- Gilbert R, Metcalfe C, Fraser WD, et al. (2012). Associations of circulating retinol, vitamin E, and 1,25-dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade. Cancer Causes Control 23:1865–73
- Giovannucci E. (2010). Epidemiology of vitamin D and colorectal cancer: Casual or causal link?. J Steroid Biochem Mol Biol 121:349–54
- Gocek E, Studzinski GP. (2009). Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci 46:190–209
- Gombart AF, Borregaard N, Koeffler HP. (2005). Human cathelicidin antimicrobical peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–77
- González-Sancho JM, Larriba MJ, Ordóñez-Morán P, et al. (2006). Effects of 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Anticancer Res 26:2669–81
- Gonzalo S. (2014). Novel roles of 1α,25(OH)2D3 on DNA repair provide new strategies for breast cancer treatment. J Steroid Biochem Mol Biol 144:59–64
- Goodwin PJ, Ennis M, Pritchard KI, et al. (2009). Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol 27:3757–63
- Gulliford T, English J, Colston KW, et al. (1998). A phase I study of the vitamin D analogue EB1089 in patients with advanced breast and colorectal cancer. Br J Cancer 78:6–13
- Gumireddy K, Reddy GS, Ikegaki N, et al. (2003). Anti-proliferative effects of 20-epi-vitamin-D3 analogue, KH1060 in human neuroblastoma: Induction of RAR-beta and p21(Cip1). Cancer Lett 190:51–60
- Guzey M, Kitada S, Reed JC. (2002). Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther 1:667–77
- Hager G, Formanek M, Gedlicka C, et al. (2001). 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Otolaryngol 121:103–9
- Hansen CM, Frandsen TL, Brünner N, Binderup L. (1994). 1 Alpha,25-dihydroxyvitamin D3 inhibits the invasive potential of human breast cancer cells in vitro. Clin Exp Metastasis 12:195–202
- Hara K, Kusuzaki K, Takeshita H, et al. (2001). Oral administration of 1alpha hydroxyvitamin D3 inhibits tumor growth and metastasis of a murine osteosarcoma model. Anticancer Res 21:321–4
- Harrison JS, Bershadskiy A. (2012). Clinical experience using vitamin D and analogs in the treatment of myelodysplasia and acute myeloid leukemia: A review of the literature. Leuk Res Treatment 2012:125814. doi:10.1155/2012/125814
- Hatakeyama S, Yoshino M, Eto K, et al. (2010). Synthesis and preliminary biological evaluation of 20-epi-eldecalcitol [20-epi-1alpha,25-dihydroxy-2beta-(3 hydroxypropoxy)vitamin D3: 20-epi-ED-71]. J Steroid Biochem Mol Biol 121:25–8
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. (2011). Vitamin D receptor (VDR)-mediated actions of 1α,25(H)2 vitamin D3: Genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab Endocrinol Metab 25:543–59
- Haussler MR, Whitfield GK, Kaneko I, et al. (2013). Molecular mechanisms of vitamin D action. Calcif Tissue Int 92:77–98
- Hedlund TE, Moffatt KA, Miller GJ. (1996). Vitamin D receptor expression is required for growth modulation by 1 alpha,25-dihydroxyvitamin D3 in the human prostatic carcinoma cell line ALVA-31. J Steroid Biochem Mol Biol 58:277–88
- Hendrickson WK, Flavin R, Kasperzyk JL, et al. (2011). Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol 29:2378–85
- Henry HL. (2011). Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab 25:531–41
- Hershberger PA, Yu WD, Modzelewski RA, et al. (2001). Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 7:1043–51
- Hewison M. (2011). Vitamin D and innate and adaptive immunity. Vitam Horm 86:23–62
- Höbaus J, Thiem U, Hummel DM, Kallay E. (2013). Role of calcium, vitamin D, and the extrarenal vitamin D hydroxylases in carcinogenesis. Anticancer Agents Med Chem 13:20–35
- Hofer H, Ho Gm, Peterlik M, et al. (1999). Biological effects of 1alpha-hydroxy- and 1beta-(hydroxymethyl)-vitamin D compounds relevant for potential colorectal cancer therapy. J Pharmacol Exp Ther 291:450–5
- Holick MF. (2013). Vitamin D. Sunlight and cancer connection. Anticancer Agents Med Chem 13:70–82
- Honma Y, Hozumi M, Abe E, et al. (1983). 1 Alpha,25-Dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci USA 80:201–4
- Horváth HC, Lakatos P, Kósa JP, et al. (2010). The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J Histochem Cytochem 58:277–85
- Høyer-Hansen M, Bastholm L, Mathiasen IS, et al. (2005). Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ 12:1297–309
- Høyer-Hansen M, Bastholm L, Szyniarowski P, et al. (2007). Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25:193–205
- Hoyer-Hansen M, Nordbrandt SP, Jäättelä M. (2010). Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol Med 16:295–302
- Hughes PJ, Marcinkowska E, Gocek E, et al. (2010). Vitamin D3-driven signals for myeloid cell differentiation-implications for differentiation therapy. Leuk Res 34:553–65
- Hsu JW, Yasmin-Karim S, King MR, et al. (2011). Suppression of prostate cancer cell rolling and adhesion to endothelium by 1α,25-dihydroxyvitamin D3. Am J Pathol 178:872–80
- Hussain T, Gupta S, Mukhtar H. (2003). Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett 191:125–35
- Huynh H, Pollak M, Zhang JC. (1998). Regulation of insulin-like growth factor (IGF) II and IGF binding protein 3 autocrine loop in human PC-3 prostate cancer cells by vitamin D metabolite 1,25(OH)2D3 and its analog EB1089. Int J Oncol 13:137–43
- Iglesias-Gato D, Zheng S, Flanagan JN, et al. (2011). Substitution at carbon 2 of 19-nor-1α,25-dihydroxyvitamin D3 with 3-hydroxypropyl group generates an analogue with enhanced chemotherapeutic potency in PC-3 prostate cancer cells. J Steroid Biochem Mol Biol 127:269–75
- Ikeda N, Uemura H, Ishiguro H, et al. (2003). Combination treatment with 1alpha,25-dihydroxyvitamin D3 and 9-cis-retinoic acid directly inhibits human telomerase reverse transcriptase transcription in prostate cancer cells. Mol Cancer Ther 2:739–46
- Iino Y, Yoshida M, Sugamata N, et al. (1992). 1-Alpha-hydroxyvitamin D3, hypercalcemia, and growth suppression of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumors. Breast Cancer Res Treat 22:133–40
- Iseki K, Tatsuta M, Uehara H, et al. (1999). Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer 81:730–3
- Ishihara J, Inoue M, Iwasaki M, et al. (2008). Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr 88:1576–83
- Istfan NW, Person KS, Holick MF, Chen TC. (2007). 1Alpha,25-Dihydroxyvitamin D and fish oil synergistically inhibit G1/S-phase transition in prostate cancer cells. J Steroid Biochem Mol Biol 103:726–30
- James SY, Williams MA, Newland AC, Colston KW. (1999). Leukemia cell differentiation: Cellular and molecular interactions of retinoids and vitamin D. Gen Pharmacol 32:143–54
- Janjetovic Z, Brozyna AA, Tuckey RC, et al. (2011). High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer 105:1874–84
- Jäpelt RB, Jakobsen J. (2013). Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front Plant Sci 4:136
- Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. (2010). Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: A nested case-control study. Biochem Med J 340:b5500
- Jensen SS, Madsen MW, Lukas J, et al. (2001). Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol 15:1370–80
- Jiang F, Li P, Fornace AJ Jr, et al. (2003a). G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem 278:48030–40
- Jiang F, Robin AM, Katakowski M, et al. (2003b). Photodynamic therapy with photofrin in combination with buthionine sulfoximine (BSO) of human glioma in the nude rat. Lasers Med Sci 18:128–33
- John EM, Schwartz GG, Koo J, et al. (2007). Sun exposure, vitamin D receptor gene polymorphisms, and breast cancer risk in a multiethnic population. Am J Epidemiol 166:1409–19
- Jones G, Prosser DE, Kaufmann M. (2012). 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys 523:9–18
- Jones G, Prosser DE, Kaufmann M. (2014). Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55:13–31
- Kasiappan R, Shen Z, Tse AK, et al. (2012). 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through MicroRNA-498. J Biol Chem 287:41297–309
- Kawa S, Yoshizawa K, Nikaido T, Kiyosawa K. (2005). Inhibitory effect of 22-oxa-1,25-dihydroxyvitamin D3, maxacalcitol, on the proliferation of pancreatic cancer cell lines. Steroid Biochem Mol Biol 97:173–7
- Ke HZ, Qi H, Crawford DT, et al. (2005). A new vitamin D analog, 2MD, restores trabecular and cortical bone mass and strength in ovariectomized rats with established osteopenia. J Bone Miner Res 20:1742–55
- Kemmis CM, Welsh J. (2008). Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. J Cell Biochem 105:980–8
- Khan QJ, Kimler BF, Fabian CJ. (2010). The relationship between vitamin D and breast cancer incidence and natural history. Curr Oncol Rep 12:136–42
- Kikuchi H, Murakami S, Suzuki S, et al. (2007). Chemopreventive effect of a vitamin D(3) analog, alfacalcidol, on colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Drugs 18:1183–7
- Kim J, Lim SY, Shin A, et al. (2009). Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: A case-control study. BMC Cancer 9:216
- Kim M, Mirandola L, Pandey A, et al. (2012). Application of vitamin D and derivatives in hematological malignancies. Cancer Lett 319:8–22
- Kissmeyer AM, Binderup L. (1991). Calcipotriol (MC 903): Pharmacokinetics in rats and biological activities of metabolites. A comparative study with 1,25(OH)2D3. Biochem Pharmacol 41:1601–6
- Kittaka A, Yoshida A, Chiang KC, et al. (2012). Potent 19-norvitamin D analogs for prostate and liver cancer therapy. Future Med Chem 4:2049–65
- Knight JA, Lesosky M, Barnett H, et al. (2007). Vitamin D and reduced risk of breast cancer: A population-based case control study. Cancer Epidem Biomarkers Prev 16:422–9
- Koli K, Keski-Oja J. (1995). 1,25-Dihydroxyvitamin D3 enhances the expression of transforming growth factor beta 1 and its latent form binding protein in cultured breast carcinoma cells. Cancer Res 55:1540–6
- Komagata S, Nakajima M, Takagi S, et al. (2009). Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol 76:702–9
- Koshizuka K, Koike M, Asou H, et al. (1999). Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res Treat 53:113–20
- Kovalenko PL, Zhang Z, Cui M, et al. (2010). 1,25-Dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics 11:26
- Kriebitzsch C, Verlinden L, Eelen G, et al. (2009). The impact of 1,25(OH)2D3 and its structural analogs on gene expression in cancer cells: A microarray approach. Anticancer Res 29:3471–83
- Krill D, DeFlavia P, Dhir R, et al. (2001). Expression patterns of vitamin D receptor in human prostate. J Cell Biochem 82:566–72
- Krishnan AV, Feldman D. (2010). Molecular pathways mediating the anti-inflammatory effects of calcitriol: Implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer 17:19–38
- Krishnan AV, Feldman D. (2011). Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 10:311–36
- Krishnan AV, Moreno J, Nonn L, et al. (2007). Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol 103:694–702
- Krishnan AV, Shinghal R, Raghavachari N, et al. (2004). Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 59:243–51
- Krishnan AV, Swami S, Feldman D. (2012). The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 77:1107–12
- Kristal AR, Till C, Song X, et al. (2014). Plasma vitamin D and prostate cancer risk: Results from the selenium and vitamin E cancer prevention trial. Cancer Epidemiol Biomarkers Prev 23:1494–504
- Kumagai T, O’Kelly J, Said JW, Koeffler HP. (2003). Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: Antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst 95:896–905
- Kumagai T, Shih LY, Hughes SV, et al. (2005). 19-Nor-1,25(OH)2D2 (a novel, noncalcemic vitamin D analogue), combined with arsenic trioxide, has potent antitumor activity against myeloid leukemia. Cancer Res 65:2488–97
- Kure S, Nosho K, Baba Y, et al. (2009). Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidem Biomarkers Prev 18:2765–72
- Lambert JR, Kelly JA, Shim M, et al. (2006). Prostate derived factor in human prostate cancer cells: Gene induction by vitamin D via a p53-dependent mechanism and inhibition of prostate cancer cell growth. J Cell Physiol 208:566–74
- Lankelma JM, Voorend DM, Barwari T, et al. (2010). Cathepsin L, target in cancer treatment? Life Sci 86:225–33.
- Larriba MJ, Muñoz A. (2010). Mechanisms of resistance to vitamin D action in human cancer cells. In: Holick MF, ed. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. New York, NY, USA: Humana Press, 325–33
- Larriba MJ, González-Sancho JM, Barbáchano A, et al. (2013). Vitamin D is a multilevel repressor of wnt/b-catenin signaling in cancer cells. Cancers (Basel) 5:1242–60
- Lawrence JA, Akman SA, Melin SA, et al. (2013). Oral paricalcitol (19-nor-1,25-dihydroxyvitamin D2) in women receiving chemotherapy for metastatic breast cancer: A feasibility trial. Cancer Biol Ther 14:476–80
- Lee HJ, Liu H, Goodman C, et al. (2006). Gene expression profiling changes induced by a novel Gemini vitamin D derivative during the progression of breast cancer. Biochem Pharmacol 72:332–43
- Lee HJ, Paul S, Atalla N, et al. (2008). Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev Res 1:1476–84
- Lee SJ, Lee YS, Zimmers TA, et al. (2010). Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24:1998–2008
- Leto G, Sepporta MV, Crescimanno M, et al. (2010). Cathepsin L in metastatic bone disease: Therapeutic implications. Biol Chem 91:655–64
- Leyssens C, Verlinden L, Verstuyf A. (2013). Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer 20:R31–47
- Leyssens C, Verlinden L, Verstuyf A. (2014). The future of vitamin D analogs. Front Physiol 5:122. doi:10.3389/fphys.2014.00122
- Li Y, Brown PH. (2009). Prevention of ER-negative breast cancer. Recent Results Cancer Res 181:121–34
- Li F, Ling X, Huang H, et al. (2005). Differential regulation of survivin expression and apoptosis by vitamin D3 compounds in two isogenic MCF-7 breast cancer cell sublines. Oncogene 24:1385–95
- Liang G, Nan H, Qureshi AA, Han J. (2012). Pre-diagnostic plasma 25-hydroxyvitamin D levels and risk of non-melanoma skin cancer in women. PLoS One 7:e35211
- Lin R, Nagai Y, Sladek R, et al. (2002). Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol 16:1243–56
- Liu G, Hu X, Chakrabarty S. (2010). Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: Downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int J Cancer 126:631–9
- Lopes N, Sousa B, Martins D, et al. (2010). Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions Vitamin D pathways unbalanced in breast lesions. BMC Cancer 10:483
- Lopes N, Carvalho J, Durães C, et al. (2012). 1 Alpha,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res 32:249–57
- Lowe L, Hansen CM, Senaratne S, Colston KW. (2003). Mechanisms implicated in the growth regulatory effects of vitamin D compounds in breast cancer cells. Results Cancer Res 164:99–110
- Lowe LC, Guy M, Mansi JL, et al. (2005). Plasma 25-hydroxyvitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer 41:1164–9
- Lu L, Qiu J, Liu S, Luo W. (2008). Vitamin D3 analogue EB1089 inhibits the proliferation of human laryngeal squamous carcinoma cells via p57. Mol Cancer Ther 7:1268–74
- Ma Y, Trump DL, Johnson CS. (2010). Vitamin D in combination cancer treatment. J Cancer 1:101–7
- Ma Y, Zhang P, Wang F, et al. (2011). Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J Clin Oncol 29:3775–82
- Ma Y, Yu WD, Hidalgo AA, et al. (2013a). Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle 12:743–52
- Ma Y, Yu WD, Su B, et al. (2013b). Regulation of motility, invasion, and metastatic potential of squamous cell carcinoma by 1α,25-dihydroxycholecalciferol. Cancer 119:563–74
- Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H. (2014). Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: Systematic review and meta-analysis of prospective cohort studies. Eur J Cancer 50:1510–21
- Macejová D, Ondková S, Jakubíková L, et al. (2011). MNU-induced mammary gland carcinogenesis: Chemopreventive and therapeutic effects of vitamin D and Seocalcitol on selected regulatory vitamin D receptor pathways. Toxicol Lett 207:60–72
- Maier S, Daroqui MC, Scherer S, et al. (2009). Butyrate and vitamin D3 induce transcriptional attenuation at the cyclin D1 locus incolonic carcinoma cells. J Cell Physiol 218:638–42
- Maehr H, Rochel N, Lee HJ, et al. (2013). Diastereotopic and deuterium effects in Gemini. J Med Chem 56:3878–88
- Mäenpää PH, Väisänen S, Jääskeläinen T, et al. (2001). Vitamin D(3) analogs (MC 1288, KH 1060, EB1089, GS1558, and CB1093): Studies on their mechanism of action. Steroids 66:223–5
- Mantell DJ, Owens PE, Bundred NJ, et al. (2000). 1Alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res 87:214–20
- Marchiani S, Bonaccorsi L, Ferruzzi P, et al. (2006). The vitamin D analogue BXL-628 inhibits growth factor-stimulated proliferation and invasion of DU145 prostate cancer cells. J Cancer Res Clin Oncol 132:408–16
- Marotta LL, Almendro V, Marusyk A, et al. (2011). The JAK2/STAT3 signaling pathway is required for growth of CD44–CD24- stem cell-like breast cancer cells in human tumors. J Clin Invest 121:2723–35
- Maruyama R, Aoki F, Toyota M, et al. (2006). Comparative genome analysis identifies the vitamin D receptor gene as a direct target of p53-mediated transcriptional activation. Cancer Res 66:4574–83
- Massagué J. (2008). TGF beta in cancer. Cell 134:215–30
- Massoner P, Colleselli D, Matscheski A, et al. (2009). Novel mechanism of IGF-binding protein-3 action on prostate cancer cells: Inhibition of proliferation, adhesion, and motility. Endocr Relat Cancer 16:795–808
- Mathiasen IS, Colston KW, Binderup L. (1993). EB1089, a novel vitamin D analogue, has strong antiproliferative and differentiation inducing effects on cancer cells. J Steroid Biochem 46:365–71
- Mathiasen IS, Lademann U, Jäättelä M. (1999). Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res 59:4848–56
- Matilainen M, Malinen M, Saavalainen K, Carlberg C. (2005). Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res 33:5521–32
- Matsumoto H, Iino Y, Koibuchi Y, et al. (1999). Antitumor effect of 22-oxacalcitriol on estrogen receptor-negative MDA-MB-231 tumors in athymic mice. Oncol Rep 6:349–52
- Matusiak D, Benya RV. (2007). CYP27A1 and CYP24 expression as a function of malignant transformation in the colon. J Histochem Cytochem 55:1257–64
- Matusiak D, Murillo G, Carroll RE, et al. (2005). Expression of vitamin D receptor and 25-hydroxyvitamin D31{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidem Biomarkers Prev 14:2370–6
- Maund SL, Barclay WW, Hover LD, et al. (2011). Interleukin-1α mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res 71:5276–86
- McCullough ML, Bostick RM, Mayo TL. (2009). Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr 29:111–32
- Medioni J, Deplanque G, Ferrero JM, et al. (2014). Phase I safety and pharmacodynamic of inecalcitol, a novel VDR agonist with docetaxel in metastatic castration-resistant prostate cancer patients. Clin Cancer Res 20:4471–7
- Meeker S, Seamons A, Paik J, et al. (2014). Increased dietary Vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res 74:4398–408
- Meephansan J, Komine M, Tsuda H, Ohtsuki M. (2012). Suppressive effect of calcipotriol on the induction of matrix metalloproteinase (MMP)-9 and MMP-13 in a human squamous cell carcinoma cell line. Clin Exp Dermatol 37:889–96
- Mellibovsky L, Díez A, Pérez-Vila E, et al. (1998). Vitamin D treatment in myelodysplastic syndromes. Br J Haematol 100:516–20
- Merke J, Milde P, Lewicka S, et al. (1989). Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest 83:1903–15
- Mehta RG, Peng X, Alimirah F, et al. (2012). Vitamin D and breast cancer: Emerging concepts. Cancer Lett 334:95–100
- Milczarek M, Chodyński M, Filip-Psurska B, et al. (2013a). Synthesis and biological activity of diastereomeric and geometric analogs of calcipotriol, PRI-2202 and PRI-2205, against human HL-60 leukemia and MCF-7 breast cancer cells. Cancers (Basel) 5:1355–78
- Milczarek M, Psurski M, Kutner A, Wietrzyk J. (2013b). Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 13:294
- Milczarek M, Rosinska S, Psurski M, et al. (2013c). Combined colonic cancer treatment with vitamin D analogs and irinotecan or oxaliplatin. Anticancer Res 13:433–44
- Milczarek M, Filip-Psurska B, Swiętnicki W, et al. (2014). Vitamin D analogs combined with 5-fluorouracil in human HT-29 colon cancer treatment. Oncol Rep 32:491–504
- Mimori K, Tanaka Y, Yoshinaga K, et al. (2004). Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol 15:236–41
- Mitschele T, Diesel B, Friedrich M, et al. (2004). Analysis of the vitamin D system in basal cell carcinomas (BCCs). Lab Invest 84:693–702
- Mittal MK, Myers JN, Misra S, et al. (2008). In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem Biophys Res Commun 372:30–4
- Mohr SB, Gorham ED, Alcaraz JE, et al. (2011). Serum 25-hydroxyvitamin D and prevention of breast cancer: Pooled analysis. Anticancer Res 31:2939–48
- Molnar I, Kute T, Willingham MC, Schwartz GG. (2004). 19-Nor-1alpha,25-dihydroxyvitamin D2 (paricalcitol) exerts anticancer activity against HL-60 cells in vitro at clinically achievable concentrations. J Steroid Biochem Mol Biol 89–90:539–43
- Moreno J, Krishnan AV, Swami S, et al. (2005). Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 65:7917–25
- Moreno J, Krishnan AV, Peehl DM, Feldman D. (2006). Mechanisms of vitamin D-mediated growth inhibition in prostate cancer cells: Inhibition of the prostaglandin pathway. Anticancer Res 26:2525–30
- Mori R, Xiong S, Wang Q, et al. (2009). Gene profiling and pathway analysis of neuroendocrine transdifferentiated prostate cancer cells. Prostate 69:12–23
- Morselli E, Galluzzi L, Kepp O, et al. (2009). Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta 1793:1524–32
- Motomura S, Kanamori H, Maruta A, et al. (1991). The effect of 1-hydroxyvitamin D3 for prolongation of leukemic transformation-free survival in myelodysplastic syndromes. Am J Hematol 38:67–8
- Muindi JR, Nganga A, Engler KL, et al. (2007). CYP24 splicing variants are associated with different patterns of constitutive and calcitriol-inducible CYP24 activity in human prostate cancer cell lines. J Steroid Biochem Mol Biol 103:334–7
- Muindi JR, Yu WD, Ma Y, et al. (2010). CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology 151:4301–12
- Munetsuna E, Nakabayashi S, Kawanami R, et al. (2011). Mechanism of the anti-proliferative action of 25-hydroxy-19-nor-vitamin D3 in human prostate cells. J Mol Endocrinol 47:209–18
- Murthy S, Weigel NL. (2004). 1Alpha,25-dihydroxyvitamin D3 induced growth inhibition of PC-3 prostate cancer cells requires an active transforming growth factor beta signaling pathway. Prostate 59:282–91
- Nagpal S, Na S, Rathnachalam R. (2005). Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–87
- Nair-Shalliker V, Armstrong BK, Fenech M. (2012). Does vitamin D protect against DNA damage? Mutat Res 733:50–7
- Nakagawa K, Sasaki Y, Kato S, et al. (2005). 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis 26:1044–54
- Nickerson T, Huynh H. (1999). Vitamin D analogue EB1089-induced prostate regression is associated with increased gene expression of insulin-like growth factor binding proteins. J Endocrinol 160:223–9
- Nishii Y, Okano T. (2001). History of the development of new vitamin D analogs: Studies on 22-oxacalcitriol (OCT) and 2beta-(3-hydroxypropoxy) calcitriol (ED-71). Steroids 66:137–46
- Oades GM, Dredge K, Kirby RS, Colston KW. (2002). Vitamin D receptor-dependent antitumour effects of 1,25-dihydroxyvitamin D3 and two synthetic analogues in three in vivo models of prostate cancer. BJU Int 90:607–16
- Obara W, Mizutani Y, Oyama C, et al. (2008). Prospective study of combined treatment with interferon-alpha and active vitamin D3 for Japanese patients with metastatic renal cell carcinoma. Int J Urol 15:794–9
- Oh YS, Kim EJ, Schaffer BS, et al. (2001). Synthetic low-calcaemic vitamin D(3) analogues inhibit secretion of insulin-like growth factor II and stimulate production of insulin-like growth factor-binding protein-6 in conjunction with growth suppression of HT-29 colon cancer cells. Mol Cell Endocrinol 183:141–9
- Okamoto R, Gery S, Kuwayama Y, et al. (2014). Novel Gemini vitamin D3 analogs: Large structure/function analysis and ability to induce antimicrobial peptide. Int J Cancer 134:207–17
- Okamoto R, Wakimoto N, Doan NB, et al. (2012). Inecalcitol, an analog of 1α,25(OH)(2) D(3) induces growth arrest of androgen-dependent prostate cancer cells. Int J Cancer 130:2464–73
- Ono K, Yoshida A, Saito N, et al. (2003). Efficient synthesis of 2-modified 1alpha,25-dihydroxy-19-norvitamin D3 with Julia olefination: High potency in induction of differentiation on HL-60 cells. J Org Chem 68:7407–15
- Otoshi T, Iwata H, Kitano M, et al. (1995). Inhibition of intestinal tumor development in rat multi-organ carcinogenesis and aberrant crypt foci in rat colon carcinogenesis by 22-oxa-calcitriol, a synthetic analogue of 1alpha,25-dihydroxyvitamin D3. Carcinogenesis 16:2091–7
- Pala V, Krogh V, Berrino F, et al. (2009). Meat, eggs, dairy products, and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr 90:602–12
- Pálmer HG, González-Sancho JM, Espada J, et al. (2001). Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154:369–87
- Pálmer HG, Larriba MJ, García JM, et al. (2004). The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med 10:917–19
- Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, et al. (2003). Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res 63:7799–806
- Pan L, Matloob AF, Du J, et al. (2010). Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. FEBS J 277:989–99
- Park MR, Lee JH, Park MS, et al. (2012). Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J Korean Med Sci 27:1037–43
- Peehl DM, Shinghal R, Nonn L, et al. (2004). Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol 92:131–41
- Pelczynska M, Switalska M, Maciejewska M, et al. (2006). Antiproliferative activity of vitamin D compounds in combination with cytostatics. Anticancer Res 26:2701–5
- Peña C, García JM, Silva J, et al. (2005). E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum Mol Genet 14:3361–70
- Pendás-Franco N, González-Sancho JM, Suárez Y, et al. (2007). Vitamin D regulates the phenotype of human breast cancer cells. Differentiation 75:193–207
- Pendás-Franco N, Aguilera O, Pereira F, et al. (2008a). Vitamin D and Wnt/beta-catenin pathway in colon cancer: Role and regulation of DICKKOPF genes. Anticancer Res 28:2613–23
- Pendás-Franco N, García JM, Peña C, et al. (2008b). DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene 27:4467–77
- Peng L, Malloy PJ, Feldman D. (2004). Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol 18:1109–19
- Peng L, Wang J, Malloy PJ, Feldman D. (2008). The role of insulin-like growth factor binding protein-3 in the growth inhibitory actions of androgens in LNCaP human prostate cancer cells. Int J Cancer 22:558–66
- Penna G, Fibbi B, Amuchastegui S, et al. (2009). The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate 69:480–93
- Pereira F, Larriba MJ, Muñoz A. (2012). Vitamin D and colon cancer. Endocr Relat Cancer 19:51–71
- Pervin S, Hewison M, Braga M, et al. (2013). Down-regulation of vitamin D receptor in mammospheres: Implications for vitamin D resistance in breast cancer and potential for combination therapy. PLoS One 8:e53287
- Petrich A, Kahl B, Bailey H, et al. (2008). Phase II study of doxercalciferol for the treatment of myelodysplastic syndrome. Leuk Lymphoma 49:57–6
- Pilz S, Kienreich K, Tomaschitz A, et al. (2013). Vitamin D and cancer mortality: Systematic review of prospective epidemiological studies. Anticancer Agents Med Chem 13:107–17
- Pirianov G, Colston KW. (2001). Interactions of vitamin D analogue CB1093, TNFalpha and ceramide on breast cancer cell apoptosis. Mol Cell Endocrinol 172:69–78
- Plum LA, Fitzpatrick LA, Ma X, et al. (2006). 2MD, a new anabolic agent for osteoporosis treatment. Osteoporos Int 17:704–15
- Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. (2013). Combination chemoprevention with diclofenac, calcipotriol and difluoromethylornithine inhibits development of non-melanoma skin cancer in mice. Anticancer Res 33:3033–9
- Proietti S, Cucina A, D’Anselmi F, et al. (2011). Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res 50:150–8
- Reddy KK. (2013). Vitamin D level and basal cell carcinoma, squamous cell carcinoma, and melanoma risk. J Invest Dermatol 133:589–92
- Reichrath J, Rafi L, Rech M, et al. (2004). Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol 31:224–31
- Ritter CS, Brown AJ. (2011). Suppression of PTH by the vitamin D analog eldecalcitol is modulated by its high affinity for the serum vitamin D-binding protein and resistance to metabolism. J Cell Biochem 112:1348–52
- Rollison DE, Cole AL, Tung KH, et al. (2012). Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern U.S. Breast Cancer Res Treat 132:683–91
- Rook GA, Steele J, Fraher L, et al. (1986). Vitamin D3, gamma interferon, and control of proliferation of mycobacterium tuberculosis by human monocytes. Immunology 57:159–63
- Rose AA, Elser C, Ennis M, Goodwin PJ. (2013). Blood levels of vitamin D and early stage breast cancer prognosis: A systematic review and meta-analysis. Breast Cancer Res Treat 141:331–9
- Ross AC, Manson JE, Abrams SA, et al. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 96:53–8
- Rossi M, McLaughlin JK, Lagiou P, et al. (2009). Vitamin D intake and breast cancer risk: A case-control study in Italy. Ann Oncol 20:374–8
- Rots NY, Liu M, Anderson EC, Freedman LP. (1998). A differential screen for ligand-regulated genes: Identification of HoxA10 as a target of vitamin D3 induction in myeloid leukemic cells. Mol Cell Biol 18:1911–18
- Roy R, Chun J, Powell SN. (2011). BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat Rev Cancer 12:68–78
- Rozen F, Yang XF, Huynh H, Pollak M. (1997). Antiproliferative action of vitamin D-related compounds and insulin-like growth factor-binding protein 5 accumulation. J Natl Cancer Inst 89:652–6
- Ryhänen S, Jääskeläinen T, Mahonen A, Mäenpää PH. (2003). Inhibition of MG-63 cell cycle progression by synthetic vitamin D3 analogs mediated by p27, Cdk2, cyclin E, and the retinoblastoma protein. Biochem Pharmacol 66:495–504
- Sahin M, Sahin E, Gumuslu S. (2009). Cyclooxygenase-2 in cancer and angiogenesis. Angiology 60:242–53
- Samuel S, Sitrin MD. (2008). Vitamin D's role in cell proliferation and differentiation. Nutr Rev 66:S116–24
- Sanford M, McCormack PL. (2011). Eldecalcitol: A review of its use in the treatment of osteoporosis. Drugs 10:1755–70
- Sakaki T, Yasuda K, Kittaka A, et al. (2014). CYP24A1 as a potential target for cancer therapy. Anticancer Agents Med Chem 14:97–108
- Saunders DE, Christensen C, Wappler NL, et al. (1993). Inhibition of c-myc in breast and ovarian carcinoma cells by 1,25-dihydroxyvitamin D3, retinoic acid and dexamethasone. Anticancer Drugs 4:201–8
- Schuster I. (2011). Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta 1814:186–99
- Schwab M, Reynders V, Loitsch S, et al. (2007). Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol Immunol 44:3625–32
- Schwartz GG, Hall MC, Stindt D, et al. (2005). Phase I/II study of 19-nor-1a-25-dihydroxyvitamin D2 (paricalcitol) in advanced, androgen-insensitive prostate cancer. Clin Cancer Res 11:8680–5
- Schwartz GG, Eads D, Naczki C, et al. (2008). 19-nor-1 alpha,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther 7:430–6
- Segersten U, Correa P, Hewison M, et al. (2002). 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 87:2967–72
- Sergeev IN. (2012). Vitamin D and cellular Ca2+ signaling in breast cancer. Anticancer Res 32:299–302
- Seubwai W, Wongkham C, Puapairoj A, et al. (2010). 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer 116:5535–43
- Shah S, Islam MN, Dakshanamurthy S, et al. (2006). The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell 21:799–809
- Shanafelt TD, Drake MT, Maurer MJ, et al. (2011). Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood 3:1492–8
- Shin MH, Holmes MD, Hankinson SE, et al. (2002). Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst 94:1301–11
- Singletary K, Milner J. (2008). Diet, autophagy, and cancer: A review. Cancer Epidemiol Biomarkers Prev 17:1596–610
- So JY, Lee HJ, Smolarek AK, et al. (2011). A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol 79:360–7
- So JY, Smolarek AK, Salerno DM, et al. (2013a). Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS One 8:e54020
- So JY, Wahler JE, Yoon T, et al. (2013b). Oral administration of a gemini vitamin D analog, a synthetic triterpenoid and the combination prevents mammary tumorigenesis driven by ErbB2 overexpression. Cancer Prev Res (Phila) 6:959–70
- Solomon C, White JH, Kremer R. (1999). Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J Clin Invest 103:1729–35
- Solomon JD, Heitzer MD, Liu TT, et al. (2014). VDR activity is differentially affected by hic-5 in prostate cancer and stromal cells. Mol Cancer Res 12:1166–80
- Spina CS, Ton L, Yao M, et al. (2007). Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol 103:757–62
- Sprenger CC, Peterson A, Lance R, et al. (2001). Regulation of proliferation of prostate epithelial cells by 1,25-dihydroxyvitamin D3 is accompanied by an increase in insulin-like growth factor binding protein-3. J Endocrinol 170:609–18
- Srinivasan M, Parwani AV, Hershberger PA, et al. (2011). Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J Steroid Biochem 123:30–6
- Stambolsky P, Tabach Y, Fontemaggi G, et al. (2010). Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell 17:273–85
- Stewart LMV, Weigel NL. (2004). Vitamin D and prostate cancer. Exp Biol Med 229:4277–84
- Stio M, Martinesi M, Simoni A, et al. (2011). The novel vitamin D analog ZK191784 inhibits prostate cancer cell invasion. Anticancer Res 31:4091–8
- Stubbs JR, Idiculla A, Slusser J, et al. (2010). Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol 21:353–61
- Sundaram S, Chaudhry M, Reardon D, et al. (2000). The vitamin D3 analog EB 1089 enhances the antiproliferative and apoptotic effects of adriamycin in MCF-7 breast tumor cells. Breast Cancer Res Treat 63:1–10
- Sundaram S, Sea A, Feldman S, et al. (2003). The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res 9:2350–6
- Suzuki Y, Ichiyama T, Ohsaki A, et al. (2009). Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J Steroid Biochem 113:134–8
- Swami S, Raghavachari N, Muller UR, et al. (2003). Vitamin D growth inhibition of breast cancer cells: Gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat 80:49–62
- Swami S, Krishnan AV, Feldman D. (2011). Vitamin D metabolism and action in the prostate: Implications for health and disease. Mol Cell Endocrinol 5:61–9
- Switalska M, Nasulewicz-Goldeman A, Opolska A, et al. (2012). The in-vitro antiproliferative effect of PRI-2191 and imatinib applied in combined treatment with cisplatin, idarubicin, or docetaxel on human leukemia cells. Anticancer Drugs 23:70–80
- Széles L, Kresesztes G, Töröcsik D, et al. (2009). 1,25-Dihydroxyvitamin D3 is an autonomus regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 182:2074–83
- Tałalaj M, Kapitan-Malinowska B, Debski K, et al. (2005). Administration of 1 alpha-OH vitamin D3 and calcium prevents bone mass loss in patients with advanced prostatic carcinoma after orchidectomy treated with complete androgenic blockade. Endokrynol Pol 56:225–32
- Tang JY, Fu T, Lau C, et al. (2012). Vitamin D in cutaneous carcinogenesis: Part II. J Am Acad Dermatol 67:817.e1–11, quiz 827–8
- Tang JY, Parimi N, Wu A, et al. (2010). Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control 21:387–91
- Tannour-Louet M, Lewis SK, Louet JF, et al. (2014). Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB J 28:364–72
- Tavera-Mendoza L, Wang TT, Lallemant B, et al. (2006). Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep 7:180–5
- Teegarden D, Donkin SS. (2009). Vitamin D: Emerging new roles in insulin sensitivity. Nutr Res Rev 22:82–92
- Thill M, Hoellen F, Becker S, et al. (2012). Expression of prostaglandin- and vitamin D-metabolising enzymes in benign and malignant breast cells. Anticancer Res 32:367–72
- Thill M, Fischer D, Kelling K, et al. (2010). Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3) and prostaglandin E2 (PGE2) serum level in ovarian cancer patients. J Steroid Biochem Mol Biol 121:387–90
- Thorne JL, Maguire O, Doig CL. (2011). Epigenetic control of a VDR-governed feed-forward loop that regulates p21(waf1/cip1) expression and function in non-malignant prostate cells. Nucleic Acids Res 39:2045–56
- Ting HJ, Bao BY, Reeder JE, et al. (2007a). Increased expression of corepressors in aggressive androgen-independent prostate cancer cells results in loss of 1α,25-Dihydroxyvitamin D3 responsiveness. Mol Cancer Res 5:967–80
- Ting HJ, Hsu J, Bao BY, Lee YF. (2007b). Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1alpha,25-dihydroxyvitamin D3. Cancer Lett 247:122–9
- Ting HJ, Yasmin-Karim S, Yan SJ, et al. (2012). A positive feedback signaling loop between ATM and the vitamin D receptor is critical for cancer chemoprevention by vitamin D. Cancer Res 72:958–68
- Tiwari A. (2009). Elocalcitol, a vitamin D3 analog for the potential treatment of benign prostatic hyperplasia, overactive bladder and male infertility. Drugs 12:381–93
- Tosetti F, Ferrari N, De Flora S, Albini A. (2002). ‘Angioprevention': Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J 16:2–14
- Tretli S, Hernes E, Berg JP, et al. (2009). Association between serum 25(OH)D and death from prostate cancer. Br J Cancer 100:450–4
- Trouillas P, Honnorat J, Bret P, et al. (2001). Redifferentiation therapy in brain tumors: Long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. J Neurooncol 51:57–66
- Trump DL, Deeb KK, Johnson CS. (2010). Vitamin D: Considerations in the continued development as an agent for cancer prevention and therapy. Cancer J 16:1–9
- Tse AK, Zhu GY, Wan CK, et al. (2010). 1Alpha,25-Dihydroxyvitamin D3 inhibits transcriptional potential of nuclear factor kappa B in breast cancer cells. Mol Immunol 47:1728–38
- Tu H, Flanders WD, Ahearn TU, et al. (2013). Effects of calcium and vitamin D3 on transforming growth factors in rectal mucosa of sporadic colorectal adenoma patients: A randomized controlled trial. Mol Carcinog. [Epub ahead of print]. doi:10.1002/mc.22096
- Urbschat A, Paulus P, von Quernheim QF, et al. (2013). Vitamin D hydroxylases CYP2R1, CYP27B1 and CYP24A1 in renal cell carcinoma. Eur J Clin Invest 43:1282–90
- Unten S, Ishihara M, Sakagami H. (2004). Relationship between differentiation-inducing activity and hypercalcemic activity of hexafluorotrihydroxyvitamin D3 derivatives. Anticancer Res 24:683–9
- Valko M, Rhodes CJ, Moncol J, Izakovic MM. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
- Valrance ME, Brunet AH, Welsh J. (2007). Vitamin D receptor-dependent inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology 148:4887–94
- van den Bemd GJ, Chang GT. (2002). Vitamin D and vitamin D analogs in cancer treatment. Curr Drug Targets 3:85–94
- van Driel M, Koedam M, Buurman CJ, et al. (2006). Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem 99:922–35
- van Ginkel PR, Yang W, Marcet MM, et al. (2007). 1Alpha-hydroxyvitamin D2 inhibits growth of human neuroblastoma. J Neurooncol 85:255–62
- Vanoirbeek E, Krishnan A, Eelen G, et al. (2011). The anti-cancer and anti-inflammatory actions of 1,25(OH)D. Best Pract Res Clin Endocrinol Metab 25:593–604
- VanWeelden K, Flanagan L, Binderup L, et al. (1998). Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology 139:2102–10
- Verlinden L, Verstuyf A, Convents R, et al. (1998). Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol 142:57–65
- Verlinden L, Verstuyf A, Van Camp M, et al. (2000). Two novel 14-epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res 60:2673–9
- Vuolo L, Di Somma C, Faggiano A, Colao A. (2012). Vitamin D and cancer. Front Endocrinol 3:58. doi:10.3389/fendo.2012.00058
- Wactawski-Wende J, Kotchen JM, Anderson GL, et al. (2006). Women's Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of colorectal cancer. New Eng J Med 354:684–96
- Wahler J, So JY, Kim YC, et al. (2014). Inhibition of the transition of ductal carcinoma in situ to invasive ductal carcinoma by a Gemini vitamin D analog. Cancer Prev Res (Phila) 7:617–26
- Wang D, Dubois RN. (2010). Eicosanoids and cancer. Nat Rev Cancer 10:181–93
- Wang J, Lian H, Zhao Y, et al. (2008). Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem 12:25596–605
- Wang QM, Jones JB, Studzinski GP. (1996). Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res 56:264–7
- Wang TT, Nestel FP, Bourdeau V, et al. (2004). Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–12
- Wang TT, Dabbas B, Laperriere D, et al. (2010a). Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem 285:2227–31
- Wang X, Chen W, Singh N, et al. (2010b). Effects of potential calcium sensing receptor inducers on promoting chemosensitivity of human colon carcinoma cells. Int J Oncol 36:1573–80
- Wang Y, Zhu J, DeLuca HF. (2012). Where is the vitamin D receptor? Arch Biochem Biophys 523:123–33.
- Wang X, Studzinski GP. (1997). Antiapoptotic action of 1,25-dihydroxyvitamin D3 is associated with increased mitochondrial MCL-1 and RAF-1 proteins and reduced release of cytochrome c. Exp Cell Res 235:210–17
- Welsh J. (2012). Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys 523:107–14
- Wietrzyk J, Peczyska M, Madej J, et al. (2004). Toxicity and antineoplastic effect of (24R)-1,24-dihydroxyvitamin D3 (PRI2191). Steroids 69:629–35
- Wigington DP, Urben CM, Strugnell SA, Knutson JC. (2004). Combination study of 1,24(S)-dihydroxyvitamin D2 and chemotherapeutic agents on human breast and prostate cancer cell lines. Anticancer Res 24:2905–12
- Woeckel VJ, van der Eerden BC, Schreuders-Koedam M, et al. (2013). 1α,25-Dihydroxyvitamin D3 stimulates activin A production to fine-tune osteoblast-induced mineralization. J Cell Physiol 228:2167–74
- Woolcott CG, Wilkens LR, Nomura AM, et al. (2010). Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: The multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 19:130–4
- Wu G, Fan RS, Li W, et al. (1998). Regulation of transforming growth factor-beta type II receptor expression in human breast cancer MCF-7 cells by vitamin D3 and its analogues. J Biol Chem 273:7749–56
- Wu RY, Zhang Y, Feng XH, Derynck R. (1997a). Homomeric and heteromeric interactions are required for signaling activity and functional cooperativity of Smad-3 and -4. Mol Cell Biol 17:2521–8
- Wu S, Sun J. (2011). Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med 11:325–35
- Wu Y, Haugen JD, Zinsmeister AR, Kumar R. (1997b). 1Alpha,25-dihydroxyvitamin D3 increases transforming growth factor and transforming growth factor receptor type I and II synthesis in human bone cells. Biochem Biophys Res Commun 239:734–9
- Xiao W, Hodge DR, Wang L, et al. (2004). Co-operative functions between nuclear factors NFκB and CCAT/enhancer-binding protein-β (C/EBP-β) regulate the IL-6 promoter in autocrine human prostate cancer cells. Prostate 61:354–70
- Xu H, Posner GH, Stevenson M, Campbell FC. (2010). Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis 31:1434–41
- Xu J, Li W, Ma J, et al. (2013). Vitamin D pivotal nutraceutical in the regulation of cancer metastasis and angiogenesis. Curr Med Chem 20:4109–20
- Yanagisawa J, Yanagi Y, Masuhiro Y, et al. (1999). Convergence of transforming growth factor-beta and vitamin D signaling pathways on SMAD transcriptional coactivators. Science 26:1317–21
- Yang ES, Burnstein KL. (2003). Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem 278:46862–8
- Yang L, Yang J, Venkateswarlu S, et al. (2001). Autocrine TGFbeta signaling mediates vitamin D3 analog-induced growth inhibition in breast cells. J Cell Physiol 188:383–93
- Yao S, Ambrosone CB. (2013). Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol 136:337–41
- Young MR, Day TA. (2013). Immune regulatory activity of vitamin D3 in head and neck cancer. Cancers (Basel) 5:1072–85
- Yuan L, Jiang R, Yang Y, et al. (2012). 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol Rep 28:2131–7
- Zehnder D, Bland R, Chana RS, et al. (2002). Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13:621–9
- Zhang J, Zhang H, Zhang X, Yu Z. (2013). Synergistic effect of retinoic acid and vitamin D analog EB1089-induced apoptosis of hepatocellular cancer cells. Cytotechnology 65:457–65
- Zhang X, Li P, Bao J, et al. (2005). Suppression of death receptor-mediated apoptosis by 1,25-dihydroxyvitamin D3 revealed by microarray analysis. J Biol Chem 280:35458–68
- Zhang Y, Guo Q, Zhang Z, et al. (2014). VDR status arbitrates the prometastatic effects of tumor-associated macrophages. Mol Cancer Res 12:1181–91
- Zhang Z, Kovalenko P, Cui M, et al. (2010). Constitutive activation of the mitogen-activated protein kinase pathway impairs vitamin D signaling in human prostate epithelial cells. J Cell Physiol 224:433–42
- Zhao XY, Ly LH, Peehl DM, Feldman D. (1997). 1Alpha,25-dihydroxyvitamin D3 actions in LNCaP human prostate cancer cells are androgen-dependent. Endocrinology 138:3290–8
- Zhou S, LeBoff MS, Glowacki J. (2010). Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology 151:14–22
- Zhuang SH, Burnstein KL. (1998). Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology 139:1197–207
- Zhuang SH, Schwartz GG, Cameron D, Burnstein KL. (1997). Vitamin D receptor content and transcriptional activity do not fully predict antiproliferative effects of vitamin D in human prostate cancer cell lines. Mol Cell Endocrinol 126:83–90