Abstract
Context: Lippia thymoides Mart. & Schauer (Verbenaceae) is used in folk medicine to treat wounds, fever, bronchitis, rheumatism, headaches, and weakness.
Objective: This study determinates the chemical composition of essential oils from L. thymoides, obtained at during each of the four seasons and correlates with pharmacological properties.
Materials and methods: Essential oils were obtained by hydrodistillation and analyzed by gas chromatography coupled to mass spectroscopy (GC-MS). Antioxidant activity was determined by DPPH free radical scavenging and β-carotene bleaching methods. The antimicrobial assays were performed by minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) methods. Isolated rat aorta and uterus, and guinea-pig trachea were utilized to evaluate relaxant potential in pre-contracted smooth muscle.
Results and discussion: Essential oils from leaves of L. thymoides had the sesquiterpene β-caryophyllene (17.22–26.27%) as the major constituent followed by borneol (4.45–7.36%), camphor (3.22–8.61%), camphene (2.64–5.66%), and germacrene D (4.72–6.18%). In vitro assays showed that these essential oils do not have antioxidant activity, have antimicrobial selectivity to Gram-positive bacteria Staphylococcus aureus (MIC = 0.004 mg/mL and MMC = 0.26–10.19 mg/mL) and Micrococcus luteus (MIC = 0.03 mg/mL and MMC = 8.43 mg/mL), relax isolated rat aorta (EC50 = 305–544 μg/mL, with endothelium; and EC50 = 150–283 μg/mL, without endothelium), and uterus (EC50 = 74–257 μg/mL), and minor potency, isolated guinea-pig trachea.
Conclusions: Lippia thymoides is a source of natural products of pharmaceutical interest, being necessary additional studies to determine the substances involved in the biological activities.
Introduction
Essential oils or volatile oils are natural, complex, multi-component systems composed mainly of terpenes and phenylpropanoids. Extraction techniques used to obtain essential oils vary according to plant parts, ranging from hydrodistillation to supercritical fluid extraction (Edris, Citation2007; Lahlou, Citation2004). The extracted oils and their isolated constituents have been used as ingredients in perfumes, cosmetics, household cleaning, and insecticide, in addition to their large potential use as pharmaceutical products, pharmacological agents, or adjuvants in formulations to improve transdermal drug delivery (Adorjan & Buchbauer, Citation2010).
Among the plants in which essential oils are abundant, species of the genus Lippia (Verbenaceae) are highlighted. This genus contains about 200 species, varying among herbs, shrubs, and small trees, mainly distributed in the tropical and subtropical territories of the America and Africa (Salimena, Citation2010). The majority of the Lippia species are found in Brazil, with about 150 species present especially in the Cerrado and rocky fields (Salimena, Citation2002). Economical interest have induced studies on the essential oils of these species (Lampasona & Catalan, Citation2002) that have shown great variation in the chemical composition; the components that were found in the highest frequency were limonene, β-caryophyllene, p-cymene, camphor, linalool, α-pinene, and thymol (Pascual et al., Citation2001), as well as biological activities such antihelmintic (Camurça-Vasconcelos et al., Citation2007, Citation2008), antimicrobial (Albuquerque et al., Citation2006; Botelho et al., Citation2007; Pessoa et al., Citation2005), antioxidant (Arana-Sánchez et al., Citation2010; Rocha-Guzmán et al., Citation2007), antispasmodic (Görnemann et al., Citation2008), vasorelaxant (Maynard et al., Citation2011), analgesic, anti-inflammatory (Abena et al., Citation2003; Mendes et al., Citation2010), and larvicidal against Aedes aegypti (Cavalcanti et al., Citation2004; Carvalho et al., Citation2003). Despite the potential demonstrated by this genus, studies are concentrated in few species, for instance Lippia sidoides Cham., Lippia alba (Mill.) N. E. Brown, Lippia dulcis Trev., and Lippia graveolens H. B. K.
Lippia thymoides Mart. & Schauer is a shrub of 2 m height, very thin, erect, branched, with little and aromatic leaves, and white or lilac flowers, occurring in the Caatinga vegetation from states of Bahia and Minas Gerais, Brazil (Funch et al., Citation2004). It is popularly known as “alecrim-do-mato” or “alecrim-do-campo” and is used in religious rituals and folk medicine to treat wound, fever, bronchitis, rheumatism, headaches, and weakness (Almeida et al., Citation2010; Funch et al., Citation2004). Thus, considering the popular uses and the absence of reports in the literature about this plant, the aim of this study was to determinate the chemical composition of the essential oils from L. thymoides, harvested during each of the four seasons, and to investigate biological properties that can highlight the potential of this species as a source of pharmaceutical products of interest.
Materials and methods
Drugs and reagents
The following reference chemicals were used in the experiments: butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), resazurin, 2,3,5-triphenyltetrazolium chloride (TTC), acetylcholine chloride (ACh), carbamyl choline chloride (carbachol, CCh), diethylstilbestrol, phenylephrine hydrochloride (Phe), chloramphenicol (CHL), and nystatin (NYS), all provide by Sigma-Aldrich (St. Louis, MO); ascorbic acid (AAc) was purchased of Dinâmica (Diadema, São Paulo, Brazil); linoleic acid was obtained from Vetec (Duque de Caxias, Rio de Janeiro, Brazil); 2,2-diphenyl-1-picrylhydrazyl (DPPH) provided by FLUKA (St. Louis, MO). Stock solutions of these chemicals were prepared with suitable solvent and dilutions were made fresh on the day of experiment. Mueller–Hinton Agar and Mueller–Hinton Broth culture mediums were both purchased of HiMedia (Mumbai, India) and prepared with sterile distilled water. All nutritive solutions used in the experiments with isolated organs were made in distilled water and chemicals used were of the purity grade.
Plant material
Lippia thymoides was collected from Feira de Santana, Bahia, Brazil, always at the same horary and location (12°11′45′′ S latitude and 38°58′05′′ W longitude), at the end of the first month of each season: April 2009 (autumn), July 2009 (winter), October 2009 (spring), and January 2010 (summer). Voucher specimens were deposited under the number 77554 at the Universidade Estadual de Feira de Santana Herbarium. The specimen was identified by Tânia Regina dos Santos Silva. Leaves of a pool of individuals were separated from the stem and air-dried at room temperature, protected from light, until constant weight, and powdered in the cutting mill. The residual water was measured using 1 g of the dry leaves on a moisture analyzer (Series ID Version 1.8, Marte, São Paulo, SP, Brazil). The essential oils were obtained by hydrodistillation with a Clevenger-type apparatus for 3 h, dried over sodium sulfate anhydrous, and stored at −20 °C protected from light. The essential oils were codified as OAA (autumn), OJW (winter), OOS (spring), and OJS (summer). In the antimicrobial assay, essential oils were diluted in a solution of Tween 80 in distilled water (10% v/v), resulting in a solution at 100 μL/mL which was homogenized on a vortex mixer, and then sterilized by filtration through a cellulose acetate membrane (0.2 μm). In all other experiments, the dilutions were made in Chremophor EL (Sigma-Aldrich, St. Louis, MO) 3% v/v in distilled water.
Animals
Male (250–300 g) and female (200–250 g) Wistar rats and guinea-pigs (400–500 g) of either sex used in the assays were bred and housed at the animal house of Universidade Federal do Vale do São Francisco. All experiments were approved by the Animal and Human Ethic Commission of the Universidade Federal do Vale do São Francisco (protocol no. 18061031). Animals were kept under conditions of controlled temperature (23–25 °C), 12 h light/dark cycle with water and food ad libitum. At the moment of experiment, all animals were sacrificed by cervical dislocation.
Chemical analysis
Analyses of the essential oils were performed using a Varian CP-3380 (Palo Alto, CA) gas chromatograph (GC) equipped with a flame ionization detector (FID) and capillary column, Chrompack CP-SIL 5, (30 m × 0.5 mm× 0.25 μm film thickness). The analyses were carried out in the following conditions: injector and detector temperatures, 220 °C and 240 °C, respectively; a carrier gas helium flowing at 1 mL/min; split ratio 1:100; injection volume 0.20 μL; the column temperature was initially 60 °C and was gradually increased in 3 °C/min until 240 °C, and finally held isothermally for 20 min.
Also, essential oils were analyzed by Shimadzu CG-2010 chromatograph (Shimadzu, Tokyo, Japan) coupled to mass spectrometer (MS) CG/MS-QP 2010 equipped with a capillary column, DB-5 ms, (30 m × 0.25 mm × 0.25 μm film thickness). The analysis conditions were the following: injector temperature 220 °C, interface and ionization source temperature 240 °C; the ionization mode was electronic impact at 70 eV; ionization current 0.70 kV; carrier gas helium at a flow 1 mL/min; split ratio 1:30; injection volume 0.20 μL; column temperature was initially 60 °C and was gradually increased at a 3 °C/min until 240 °C, and finally held isothermally for 20 min.
Constituents were identified by comparing its mass spectra with those of the GC/MS and confirmed by comparing the retention indexes relative to C8–C24 n-alkanes, with calculation of the Kovats Index (KI) to each peak, and values from the literature. The quantification of relative percentage of the identified components was obtained by the normalization method. The data are reported as mean value of two oils’ injections.
Antioxidant assays
The antioxidant activity was evaluated by DPPH free radical scavenging and β-carotene bleaching methods (Mensor et al., Citation2001; Wannes et al., Citation2010), each in triplicate and three independent experiments. In the DPPH assay, sample stock solutions (1.0 mg/mL) of the essential oils (OAA, OJW, OOS, and OJS) and positive controls (AAc, BHA, and BHT) were diluted to final concentrations of 243, 81, 27, 9, 3, and 1.0 μg/mL. One mL of a 50 μg/mL DPPH ethanol solution was added to 2.5 mL of sample solutions previously prepared and allowed to react at room temperature. After 30 min, the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity. The essential oils plus diluents were used as blanks and DPPH solution without sample solutions was used as a negative control.
In the bleaching method, β-carotene (2.0 mg) was dissolved in 10 mL chloroform and to 2 mL of this solution, linoleic acid (40 mg) and Tween 40 (400 mg) were added. Chloroform was evaporated under vacuum at 40 °C and 100 mL of distilled water was added, then the emulsion was vigorously shaken during 2 min. The emulsion (3.0 mL) was added to a tube containing 0.12 mL of solutions 1 mg/mL of positive controls (AAc, BHA, and BHT) and sample essential oils (OAA, OJW, OOS, and OJS). The absorbance was immediately measured at 470 nm and the test emulsion was incubated in water bath at 50 °C for 2 h, when the absorbance was measured again. In the negative control, the essential oils were substituted with an equal volume of diluent. The percentage antioxidant activity was evaluated in terms of the bleaching of the β-carotene.
Antimicrobial assays
The antimicrobial assays were performed by minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) methods, according to approved by Clinical and Laboratory Standards Institute (CLSI, Citation2006, Citation2008). The microorganism-tested Escherichia coli CCMB261 resistant to sulfonamide and sensible to trimethoprim, Staphylococcus aureus CCMB262 resistant to streptomycin and dihydrostreptomycin, Pseudomonas aeruginosa CCMB268, Micrococcus luteus CCMB283, Salmonella choleraesuis CCMB281, Bacillus cereus CCMB282, and Candida albicans CCMB286 resistant to fluconazole and amphotericin B were obtained from Culture Collection of Microorganisms of the Bahia (CCMB). In 96-well plates, 90 μL of the essential oil solution (OAA, OJW, OOS, and OJS) and 90 μL of Müeller–Hinton broth (2 × concentrated) were conditioned in the first well and the serial dilutions were carried out in all subsequent wells. The range of evaluated extract concentration was 22.20–0.005 mg/mL. Cultures of 18 h (bacteria) and 36 h (yeast) were collected to saline solution 0.45% and 10 μL of microorganism suspension at 1.5 × 106 CFU/ml (bacteria) and 5 × 105 CFU/ml (yeast) were added in each well. The MIC of the 10% Tween/water solution and positives controls chloramphenicol (20 mg/mL) and nystatin (10 mg/mL) was also determinate. Controls of the microbial strains viability, sample sterility, and water were also performed. After incubation (24 h at 37 °C to bacteria and 48 h at 28 °C to yeast), 30 µL of resazurin (0.01% w/v) were added in each well. The MIC was defined as the lowest concentrations which did not show any growth of tested organism.
To confirm the activity, all wells of MIC test which not showed any growth of the bacteria fungus after the incubation period were subcultured onto the surface of the freshly prepared Mueller–Hinton Agar plates and incubated at 37 °C for 24 h (bacteria) or 48 h at 28 °C (fungus). The MBC was recorded as the lowest concentration of the essential oil that did not permit any visible bacteria colony growth on appropriate agar plate after the period of incubation.
Preparation of isolated rat thoracic aorta
After sacrifice, the thoracic artery aorta was removed from male rats (n = 5) and cleaned from connective tissue and fat. Rings (4–5 mm) were obtained and suspended individually by stainless steel rods knotted to cotton threads in a 10 mL organ bath at 37 °C containing the Krebs solution (NaCl 118.0 mM, KCl 4.55 mM, MgSO4ċ7H2O 5.70 mM, KH2PO4ċH2O 1.10 mM, CaCl2ċ2H2O 2.52 mM, NaHCO3 25.0 mM, and glucose 11.00 mM) and aerated with carbogenic mixture (95% O2 and 5% CO2). Rings were stabilized with a resting tension of 1.0 g for 1 h, with solution being changed every 15 min. The isometric tension was recorded by a force transducer coupled to a data acquisition system (WinDaq, DATAQ Instruments, Inc., Akron, OH). The preparations were made in the presence or absence of the endothelium, being this removed by gently rubbing the intimal layer with stainless steel rods. The endothelium integrity was verified by relaxation to Ach (1.0 μM) in rings pre-contracted by Phe (1.0 μM). The vascular endothelium was considered intact when aortic rings were equally relaxed or more than 50% of Phe-induced (1.0 μM) pre-contractions (Silva-Filho et al., Citation2011). After stabilization period, a Phe-induced pre-contraction was elicited in endothelium-intact and endothelium-denuded rings to promote similar magnitude contractions, and essential oils (OAA, OJW, OOS, and OJS) was added cumulatively (1–729 μg/mL), after response to Phe had stabilized, to obtain a concentration–response curve.
Preparation of isolated rat uterus
Virgin female rats (n = 5) were pre-treated with diethylstilbestrol (100 μg/kg, subcutaneously) 18–24 h prior to performing the experiments to induce estrous stage. After sacrifice, myometrial tissue was removed and trimmed from surrounding connective tissue. The segment of uterus was cut longitudinally into strips that had about 1 cm in length and 1 mm wide, and were suspended individually in organ bath by cotton threads in a 10 mL organ bath at 32 °C containing Locke Ringer solution (NaCl 154.0 mM, KCl 5.63 mM, CaCl2 2.16 mM, NaHCO3 5.95 mM, and glucose 11.00 mM) and aerated with carbogenic mixture. Strips were stabilized with a resting tension of 1.0 g for 45 min, with solution being changed every 15 min (Macêdo et al., Citation2011). The isometric tension was recorded by a force transducer coupled to a data acquisition system (WinDaq, DATAQ Instruments, Inc., Akron, OH). After the stabilization period, tissues were stimulated with KCl (60 mM) to obtain two similar contractile responses and essential oils (OAA, OJW, OOS, and OJS) was added cumulatively (1–729 μg/mL), after response to KCl had stabilized, to obtain a concentration–response curve.
Preparation of isolated guinea-pig trachea
The trachea from guinea-pigs (n = 5) sacrificed was cleaned and segmented to each 4–5 cartilaginous rings, being suspended individually by stainless steel rods knotted to cotton threads in a 10 mL organ bath at 37 °C containing the Krebs solution and aerated with carbogenic mixture. Rings were stabilized with a resting tension of 1.0 g for 1 h, with solution being changed every 15 min (Ribeiro et al., Citation2007). The isometric tension was recorded by a force transducer coupled to a data acquisition system (WinDaq, DATAQ Instruments, Inc., Akron, OH). The rings used in this experiment not were submitted to any mechanical event to retire epithelial layer, being considered as intact; however, the integrity of these epithelium not was experimentally determined. After stabilization period, two similar contractile responses were elicited with CCh (1.0 μM) and essential oils (OAA, OJW, OOS, and OJS) was added cumulatively (1–729 μg/mL), after second response to CCh had stabilized, to obtain a concentration–response curve.
Data analysis and statistics
The essential oil yield was calculated by the formula:
where Y is the final yield (%), Vo is the essential oil volume collected, Lm is the vegetal mass of the L. thymoides leaves, H is the humidity present in the Lm.
KI was calculated according to the formula:
where N is the number of carbon atoms of the alkane’s pattern (C8–C24);
is the retention time calculated of the peak;
is the retention time of the alkane corresponding to the peak calculated;
is the retention time of the alkane that eluted afterwards to calculated peak.
The antioxidant activity of DPPH assay was calculated using the formula % of activity = [(absorbance of the control − absorbance of the sample)/absorbance of the control] × 100, and concentration that caused 50% of the scavenging (EC50) was calculated by the non-linear curve fitting.
The antioxidant activity of bleaching assay was calculated using the following formula:
where A0 is the initial absorbance, At is the final absorbance measured for the test sample,
is the initial absorbance, and
is the final absorbance measured for the negative control (blank).
All numeric data were expressed as mean ± standard error of the mean (SEM); concentration that caused 50% of the relaxation (EC50) in the assays involving smooth muscle was calculated by the non-linear curve fitting. The Student t-test and analysis of variance (ANOVA) were statistical parameters applied following the Tukey post test, being considered as significantly different at p < 0.05.
Results
Chemical analysis
To observe if there is variation in the chemical composition of the essential oil from the leaves of L. thymoides during the year, the plant was collected in the last week of the first month of each season. Climatological data from the city of Feira de Santana, Bahia, Brazil, where the plant was collected, exhibited characteristics typical of semi-arid climate (), with higher average temperatures, high insolation, and low rainfall, with irregular rainfall concentrated in a few months. According to the data presented in , it is observed that there was variation in the content of the oils, with OJS having the lowest value with 2.14% in the period of greatest insolation (420.7 h) and average temperature (28.9 °C), in contrast to OOS that had the highest level with 2.93%, which was obtained in a period that heat stroke was the second highest (382.4 h) and lower rainfall (74.1 mm). With this, it can be noted that rainfall and sunshine, which varied in the months that collections were made, did not influence the oil content of L. thymoides.
Table 1. Monthly climatological data and content of essential oils from leaves of L. thymoides obtained in the four seasons.
Chemical analysis of essential oils from L. thymoides, of Brazilian Northeast, revealed the presence of 45 terpenoids compounds (), with predominance of sesquiterpenes. Essential oil obtained in the four seasons had as a major constituent the sesquiterpene β-caryophyllene with concentrations between 17.22% and 26.27%. The highest percentage of β-caryophyllene was found in essential oil collected in the winter season. Other main terpenes that appeared after β-caryophyllene were camphene, camphor, borneol, α-caryophyllene, and germacrene D.
Table 2. Chemical composition of the essential oils from the leaves of L. thymoides.
Antioxidant activity
Antioxidant potential of essential oils from L. thymoides was determined by scavenging of the DPPH free radical and β-carotene bleaching test, being data presented in . In both assays, essential oils do not presented efficacy, with values of EC50 values more than 236 μg/mL in the DPPH assay and percentual of inhibition minor than 11% in the β-carotene bleaching test.
Table 3. Antioxidant activity of the essential oils from the leaves of L. thymoides.
Antimicrobial activity
Antimicrobial activity of essential oils from L. thymoides was tested against Gram-negative and Gram-positive bacteria and fungus, as presented in . The microorganisms E. coli, P. aeruginosa, B. cereus, and S. choleraesuis were resistant to all essential oils. Staphylococcus aureus and C. albicans were the most sensitive microorganisms (MIC <0.004 mg/mL) being inhibited by OAA, OOS, and OJS samples, as well as M. luteus. The essential oil from L. thymoides collected in winter (OJW) showed action only against S. aureus CCMB263 with lower activity (MIC = 10.19 mg/mL). For most of these essential oils, the values of MIC were not equivalent to MMC (MMC = 0.26 to >8.43 mg/mL).
Table 4. Antimicrobial activity of the essential oils from leaves of L. thymoides.
Vasorelaxant activity in aorta smooth muscle
The essential oils relaxed isolated rat rings aorta with functional endothelium, pre-contracted with Phe 1 μM, in a concentration-dependent manner (1–729 μg/mL), as shown in . CE50 values obtained of OAA (462.8 ± 8.9 μg/mL), OJW (511.1 ± 95.7 μg/mL), OOS (543.9 ± 73.9 μg/mL), and OJS (305.2 ± 126.0 μg/mL) do not differ significantly. None of the essential oils relaxed rings aorta in the endothelium presence to 100%, Emax value being obtained by OAA, OJW, OOS, and OJS, in the concentration of 729 μg/mL, 86.8 ± 5.5%, 62.2 ± 7.0%, 64.2 ± 7.1%, and 84.4 ± 10.5%, respectively.
Figure 1. Vasorelaxant effect of essential oils from leaves of L. thymoides on tonic contractions induced by phenylephrine (Phe) 1.0 µM in isolated rat rings aorta. (A) Contractions induced in the presence of functional endothelium; (B) contractions induced in the absence of functional endothelium; (C) EC50 values in the presence (E+) or absence (E−) of endothelium; (D) Emax values obtained in the presence (E+) or absence (E−) of endothelium. Data are presented as mean ± SEM (n = 5); *EC50 or Emax values that were statistically different (t-test, p < 0.05) when compared with values of the same oil in the presence (E+) or absence (E−) of functional endothelium. OAA, essential oil from L. thymoides collected in autumn; OJW, essential oil from L. thymoides collected in winter; OOS, essential oil from L. thymoides collected in spring; OJS, essential oil from L. thymoides collected in summer.
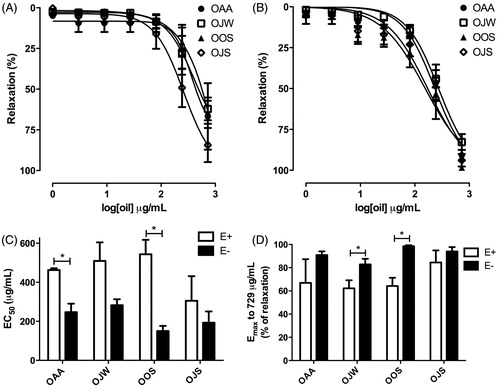
In the endothelium absence, OAA (EC50 = 246.9 ± 43.7 μg/mL), OJW (EC50 = 282.6 ± 30.8 μg/mL), OOS (EC50 = 150.3 ± 26.3 μg/mL), and OJS (EC50 = 193.1 ± 57.4 μg/mL) also relaxed rings aorta pre-contracted with Phe 1.0 μM, in a concentration-dependent manner (1–729 μg/mL), as shown in . There was no difference between CE50 values and the maximum relaxations to 729 μg/mL were OAA 90.9 ± 3.2%, OJW 82.8 ± 4.9%, OOS 98.5 ± 1.1%, and OJS 93.9 ± 3.8%.
Comparing the EC50 values of rings aorta relaxation pre-contracted with Phe in the presence and absence of functional endothelium (), it was observed that OAA and OOS were more potent into relax smooth muscle in the absence than in the presence of the endothelium. Further, Emax values achieved by OJW and OOS to 729 μg/mL were significantly higher in the absence than in the presence of functional endothelium ().
Tocolytic activity in rat uterus
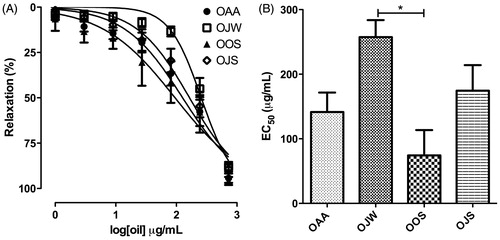
The essential oils relaxed isolated rat uterus pre-contracted with depolarizing KCl 60 mM, in a concentration-dependent manner (1–729 μg/mL), as shown in . On one hand, only EC50 values of OJW (257.4 ± 26.4 μg/mL) and OOS (74.3 ± 39.2 μg/mL) differed significantly, as shown in . On the other hand, Emax values to 729 μg/mL of OAA (94.4 ± 2.6%), OJW (88.0 ± 2.6%), OOS (94.0 ± 2.6%), and OJS (89.2 ± 2.5%) do not differ significantly.
Figure 2. Spasmolytic effect of essential oils from leaves of L. thymoides on the tonic contractions induced with KCl 60 mM in isolated rat uterus. (A) Concentration–response curves of essential oils; (B) EC50 values of essential oils. Data are presented as mean ± SEM (n = 5); *EC50 values that were statistically different (ANOVA, p < 0.05). OAA, essential oil from L. thymoides collected in autumn; OJW, essential oil from L. thymoides collected in winter; OOS, essential oil from L. thymoides collected in spring; OJS, essential oil from L. thymoides collected in summer.
Relaxant effect in trachea smooth muscle
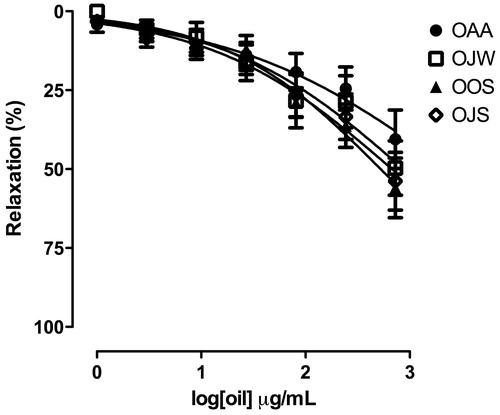
The essential oils relaxed isolated guinea-pig trachea pre-contracted with CCh 1.0 μM, in a concentration-dependent manner (), but Emax values of 729 μg/mL were lower than 60% and it was not possible to determine EC50.
Figure 3. Effect of essential oils from leaves of L. thymoides on the tonic contractions induced with carbachol (CCh) 1.0 µM in isolated guinea-pig rings trachea. Data are presented as mean ± SEM (n = 5). OAA, essential oil from L. thymoides collected in autumn; OJW, essential oil from L. thymoides collected in winter; OOS, essential oil from L. thymoides collected in spring; OJS, essential oil from L. thymoides collected in summer.
Discussion
Lippia thymoides is a native species of the Brazilian Caatinga vegetation, popularly used to treat various illnesses. Thus, due to their ethnopharmacological potential, our group was inspired to study, for the first time, the chemical composition and pharmacological properties of essential oils of this species, attempting to identify a pharmaceutical product of interest.
The essential oils from L. thymoides presented chemical composition similar to other species of the genus Lippia and there are not great variations between the seasons, indicating that climatic factors are not associated with the presence of these constituents. Considering that the sesquiterpene β-caryophyllene is the majority in all essential oils, studies have indicated that interrelationship of the plant with various organisms in their environment induce the synthesis this compound, since β-caryophyllene can attract natural enemies of herbivores, as entomopathogenic nematodes below ground and parasitic wasps (Köllner et al., Citation2008). Furthermore, gene expression of β-caryophyllene synthase, that catalyses the conversion of farnesyl diphosphate to β-caryophyllene, is induced in response to elevated CO2 and may enhance biosynthesis and phytotoxicity of allelochemicals, as β-caryophyllene (Elakovich & Stevens, Citation1985; Wang et al., Citation2010). Pascual et al. (Citation2001) extensively revised the chemistry of Lippia and found that the components with the highest frequency in the essential oils of this genus are limonene, β-caryophyllene, p-cymene, camphor, linalool, α-pinene, and thymol. Furthermore, the sesquiterpene β-caryophyllene present in L. thymoides also appears as the main compound in other species at 11% in Lippia americana2 (Bueno et al., Citation2011), 15–16% in L. graveolens (Rivero-Cruz et al., Citation2011), 13% in Lippia multiflora Moldenke (Bassolé et al., Citation2010), and 18% in L. dulcis (Moreno-Murillo et al., Citation2010). Minor compounds present in the essential oils from L. thymoides are also in other species of the same genus, as camphor at 33% in L. dulcis (Görnemann et al., Citation2008), borneol at 9% in Lippia integrifolia Griseb. Hieron. (Lima et al., Citation2011), 1,8-cineole at 40% in L. multiflora (Avlessi et al., Citation2005), and 17% in L. graveolens (Rivero-Cruz et al., Citation2011). Recently, Cruz et al. (Citation2014) reported that the concentration of major constituent from Lippia gracilis Schauer showed little variation between seasons, demonstrating the stability of the chemical composition of the essential oils even with different climatic conditions, confirming the findings of this work and indicating that this behavior could possibly be a characteristic of the species Lippia.
Despite reports indicate that essential oils also have antioxidant activity (Javanmardi et al., Citation2003; Terpinc et al., Citation2012), in this paper we demonstrated that L. thymoides was not efficient in scavenging DPPH free radical or in inhibiting β-carotene oxidations. The DPPH method involves the use of organic free radical DPPH•, where antioxidants are allowed to react with the stable radical. In its radical form, DPPH• absorbs at 515 nm, but upon reduction by an antioxidant, the absorption disappears, possibly due to the ability of this compound to transfer labile H atoms to radical (Aruoma, Citation2003; Villaño et al., Citation2007). The β-carotene bleaching method (coupled oxidation of β-carotene and linoleic acid) estimates the relative ability of antioxidant compounds in essential oils to scavenge the radical of linoleic acid peroxide (LOO•) that oxidizes β-carotene in the emulsion phase (Miguel, Citation2010). In both the methods, compounds that have the capability of transferring H to DPPH• or LOO• may possibly present antioxidant potential, as observed in essential oils containing phenolic terpenes as a major constituent, as carvacrol, thymol, and eugenol, they are also more responsible for the antioxidant effect than mono- and sesquiterpenes (Adorjan & Buchbauer, Citation2010; Anthony et al., Citation2012). Thus, as there is a small amount of phenolic compounds in the essential oils from L. thymoides, we observed low potencies in such mixtures to act as an antioxidant. Still, various inflammatory disorders are related to oxidative stress and data of this work suggest that popular uses of this plant to treat fever, rheumatism, headache, and others are not related to essential oils, indicating the necessity to study non-volatile constituents.
In contrast, it was demonstrated that essential oils from L. thymoides (OAA, OOS, and OJS) were effective against S. aureus and M. luteus, both Gram-positive bacteria, as well as against C. albicans, but sample collected in winter season (OOW) was not so active. Since sesquiterpene β-caryophyllene is the major constituent in all oils, it is likely that this constituent is not mainly responsible for the antimicrobial effect. Furthermore, essential oils constitute a mixture of various substances and variations on chemical composition can influence the antimicrobial activity, as observed in essential oils containing camphor, borneol, and camphene, which were effective against fungi and Gram-positive and negative bacteria (Alva et al., Citation2012; Cárdenas et al., Citation2012; Wang et al., Citation2006). Studies indicate that monoterpenes and sesquiterpenes induce alteration in the bacterial membrane, promoting disruption and increasing the permeability (Cristani et al., Citation2007; Kuroda et al., Citation2007; Trombetta et al., Citation2005). So the morphology of the microorganisms influences the antimicrobial activity and can explain the selective effect of essential oils from L. thymoides against Gram-positive bacteria in comparison with Gram-negative. These data are according to popular use that this species can be used to treat wound and skin problems, since S. aureus, M. luteus and C. albicans are pathogen colonizing of the skin (Miltiadous & Elisaf, Citation2011; O’Riordan & Lee, Citation2004) indicating a possible antiseptic effect.
The essential oils obtained from Lippia species have demonstrated effective relaxation in different smooth muscles, as L. alba in rat mesenteric artery (Maynard et al., Citation2011) and L. graveolens in guinea-pig ileum (Rivero-Cruz et al., Citation2011). Thus, aiming to identify L. thymoides as a source of natural product of pharmacological interest, essential oils were tested in different isolated organs. Our data showed that OAA, OJW, OOS, and OJS relaxed effectively smooth muscle of rat aorta and uterus, but were not capable to relax guinea-pig trachea to a significant degree.
In rat aorta, the relaxation was endothelium independent, indicating that nitric oxide is probably not involved. On one hand, EC50 values of the essential oils in aorta preparations with functional endothelium can be related to induction, by some constituent of the mixture, of the production of endothelium-derived contracturant factors, as superoxide anions, endoperoxides, tromboxane A2, or endothelin-1, which antagonized the spasmolytic effect of other constituent. On the other hand, the relaxation of rat uterus pre-contracted with KCl 60 mM indicates that relaxation induced by essential oils, probably, is related to blockage of Ca2+ influx or activation of hyperpolarizing component, as Ca2+-activated K+ channels (KCa). The indication of the popularity of L. thymoides to treat bronchitis stimulated the investigation on isolated guinea-pig trachea; however, the essential oils were not effective on relaxing airway smooth muscle, suggesting that folk medicine use this plant to treat illness respiratory is not related to essential oils. There are reports about relaxant effect of some constituent of L. thymoides essential oil, as β-caryophyllene on rat ileum (Leonhardt et al., Citation2010) and borneol on rat aorta (Silva-Filho et al., Citation2011), but their mechanism of action were not elicited.
Thus, this work demonstrated that essential oils obtained from leaves of L. thymoides, collected at the four seasons, had as the main compound the sesquiterpene β-caryophyllene with a low variation in other constituents. In vitro assays showed that these essential oils do not have antioxidant activity, but relax isolated aorta and uterus rat, and in minor effectiveness, isolated guinea-pig trachea. Antimicrobial selectivity to Gram-positive bacteria and yeast fungus was observed. All these data suggest that L. thymoides is a source of natural products of pharmaceutical interest, but additional studies are necessary to determine the substances involved in the biological activities.
Declaration of interest
The authors report that they have no conflicts of interest. This study was supported by grants from Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB).
References
- Abena AA, Diatewa M, Gakosso G, et al. (2003). Analgesic, antipyretic and anti-inflammatory effects of essential oil of Lippia multiflora. Fitoterapia 74:231–6
- Adorjan B, Buchbauer G. (2010). Biological properties of essential oils: An updated review. Flavour Fragr J 25:407–26
- Albuquerque CC, Camara TR, Mariano RLR, et al. (2006). Antimicrobial action of essential oil of Lippia gracilis Schauer. Braz Arch Biol Technol 49:527–35
- Almeida VS, Bandeira FPSF. (2010). O significado cultural do uso de plantas da caatinga pelos quilombolas do Raso da Catarina, município de Jeremoabo, Bahia, Brasil. Rodriguesia 61:195–209
- Alva M, Popich S, Borkosky S, et al. (2012). Bioactivity of the essential oil of an argentine collection of Acanthospermum hispidum (Asteraceae). Nat Prod Commun 7:245–8
- Anthony KP, Deolu-Sobogun SA, Saleh MA. (2012). Comprehensive assessment of antioxidant activity of essential oils. J Food Sci 77:C839–43
- Arana-Sánchez A, Estarrón-Espinosa M, Obledo-Vázquez EM, et al. (2010). Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Lett Appl Microbiol 50:585–90
- Aruoma OI. (2003). Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 523–524:9–20
- Avlessi F, Alitonou G, Sohounhloue DK, et al. (2005). Aromatic plants of tropical West Africa. Part XIV. Chemical and biological investigation of Lippia multiflora Mold. essential oil from Benin. J Essential Oil Res 17:405–7
- Bassolé IH, Lamien-Meda A, Bayala B, et al. (2010). Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 15:7825–39
- Botelho, MA, Nogueira, NAP, Bastos GM, et al. (2007). Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res 40:349–56
- Bueno J, Escobar P, Martínez JR, et al. (2011). Composition of three essential oils, and their mammalian cell toxicity and antimycobacterial activity against drug resistant-tuberculosis and nontuberculous mycobacteria strains. Nat Prod Commun 6:1743–8
- Camurça-Vasconcelos ALF, Bevilaqua CML, Morais SM, et al. (2007). Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet Parasitol 148:288–94
- Camurça-Vasconcelos ALF, Bevilaqua CML, Morais SM, et al. (2008). Anthelmintic activity of Lippia sidoides essential oils on sheep gastrointestinal nematodes. Vet Parasitol 154:167–70
- Cárdenas J, Rojas J, Rojas-Fermin L, et al. (2012). Essential oil composition and antibacterial activity of Monticalia greenmaniana (Asteraceae). Nat Prod Commun 7:243–4
- Carvalho AFU, Melo VMM, Craveiro AA, et al. (2003). Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti Linn. Mem Inst Oswaldo Cruz 98:569–71
- Cavalcanti ESB, Morais SI, Lima MAA, Santana EW. (2004). Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz 99:541–4
- CLSI, Clinical and Laboratory Standards Institute. (2006). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard – Seventh Edition. Clinical and Laboratory Standards Institute document M7-A7. Wayne (PA): Clinical and Laboratory Standards Institute
- CLSI, Clinical and Laboratory Standards Institute. (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard – Third Edition. Clinical and Laboratory Standards Institute document M27-A3. Wayne (PA): Clinical and Laboratory Standards Institute
- Cristani M, D’Arrigo M, Mandalari G, et al. (2007). Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J Agric Food Chem 55:6300–8
- Cruz EM, Pinto JA, Fontes SS, et al. (2014). Water deficit and seasonality study on essential oil constituents of Lippia gracilis Schauer germplasm. Sci World J 2014:314626
- Edris AE. (2007). Pharmaceutical and therapeutical potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21:308–23
- Elakovich SD, Stevens K. (1985). Volatile constituents of Lippia nodiflora. J Nat Prod 48:504–6
- Funch LS, Harley R, Funch R, et al. (2004). Plantas úteis da Chapada Diamantina. São Carlos: RiMa Editora, São Paulo, Brazil
- Görnemann T, Nayal R, Pertz HH, et al. (2008). Antispasmodic activity of essential oil from Lippia dulcis Trev. J Ethnopharm 117:166–9
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. (2003). Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem 83:547–50
- Köllner TG, Held M, Lenk C, et al. (2008). A maize (E)-β-caryophyllene synthase implicated in indirect defence responses against herbivores is not expressed in most American maize varieties. Plant Cell 20:482–94
- Kuroda M, Nagasaki S, Ohta T. (2007). Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to β-lactams in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 59:425–32
- Lahlou M. (2004). Methods to study the phytochemistry and bioactivity of essential oils. Phytother Res 18:435–48
- Lampasona MEP, Catalan CAN. (2002). The chemistry of the genus Lippia. In: Kintzios SE, ed. Oregano: The Genera Origanum and Lippia. New York (NY): Taylor and Francis, INC, 127–49
- Leonhardt V, Leal-Cardoso, JH, Lahlou S, et al. (2010). Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundam Clin Pharmacol 24:749–58
- Lima B, López S, Luna L, et al. (2011). Essential oils of medicinal plants from the Central Andes of Argentina: Chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem Biodivers 8:924–36
- Macêdo CL, Vasconcelos LHC, Correia, ACC, et al. (2011). Spasmolytic effect of galetin 3,6-dimethyl ether, a flavonoid obtained from Piptadenia stipulacea (Benth) Ducke. J Smooth Muscle Res 47:123–34
- Maynard LG, Santos KC, Cunha PS, et al. (2011). Chemical composition and vasorelaxant effect induced by the essential oil of Lippia alba (Mill.) N.E. Brown. (Verbenaceae) in rat mesenteric artery. Indian J Pharmacol 43:694–8
- Mendes SS, Bonfim RR, Jesus HCR, et al. (2010). Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J Ethnopharmacol 129:391–7
- Mensor LL, Menezes FS, Leitao GG, et al. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–30
- Miguel MG. (2010). Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr J 25:291–312
- Miltiadous G, Elisaf M. (2011). Native valve endocarditis due to Micrococcus luteus: A case report and review of the literature. J Med Case Rep 5:251
- Moreno-Murillo B, Quijano-Célis C, Romero AR, Pino JA. (2010). Essential oil from leaves of Lippia dulcis grown in Colombia. Nat Prod Commun 5:613–14
- O’Riordan K, Lee JC. (2004). Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 17:218–34
- Pascual ME, Slowing K, Carretero E, et al. (2001). Lippia: Traditional uses, chemistry and pharmacology: A review. J Ethnopharm 76:201–14
- Pessoa ODL, Carvalho CBM, Silvestre JOVL, et al. (2005). Antibacterial activity of the essential oil from Lippia aff. Gracilis. Fitoterapia 76:712–14
- Ribeiro LAA, Tavares JF, Andrade NC, et al. (2007). O ácido (8)17,12E,14-labdatrieno-18-óico (labdano302), diterpeno tipo labdano isolado de Xylopia langsdorffiana St. Hil. & Tul. (Annonaceae) relaxa a traquéia isolada de cobaia. Rev Bras Farmacogn 17:197–203
- Rivero-Cruz I, Duarte G, Navarrete A, Pino JA. (2011). Chemical composition and antimicrobial and spasmolytic properties of Poliomintha longiflora and Lippia graveolens essential oils. J Food Sci 76:C309–17
- Rocha-Guzmán NE, Gallegos-Infante JA, González-Laredo RF, et al. (2007). Antioxidant effect of oregano (Lippia berlandieri v. Shauer) essential oil and mother liquors. Food Chem 102:330–5
- Salimena FRG. (2002). Novos sinônimos e tipificações em Lippia Sect. Rhodolippia (Verbenaceae). Darwiniana 40:121–5
- Salimena FRG. (2010). Uma nova espécie de Lippia L. (Verbenaceae) do cerrado brasileiro. Acta Bot Bras 24:232–4
- Silva-Filho JC, Oliveira NNPM, Arcanjo DDR, et al. (2011). Investigation of mechanism involved in (-)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin Pharmacol Toxicol 110:171–7
- Terpinc P, Čeh B, Ulrih NP, Abramovič H. (2012). Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind Crops Prod 39:210–17
- Trombetta D, Castelli F, Sarpietro MG, et al. (2005). Mechanism of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 49:2474–8
- Villaño D, Fernández-Pachón MS, Moyá ML, et al. (2007). Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 71:230–5
- Wannes WA, Mhamdi B, Sriti J, et al. (2010). Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italic L.) leaf, stem and flower. Food Chem Toxicol 48:1362–70
- Wang R-L, Staehelin C, Peng S-L, et al. (2010). Responses of Mikania micrantha, an invasive weed to elevated CO2: Induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-caryophyllene. J Chem Ecol 36:1076–82
- Wang P, Kong CH, Zhang CX. (2006). Chemical composition and antimicrobial activity of the essential oil from Ambrosia trifida L. Molecules 11:549–55

