Abstract
Objectives. Cardiotrophin-1 (CT-1) is closely linked to many cardiovascular diseases, such as myocardial infarction and heart failure, and exhibits cardioprotective effect in ischemia-reperfusion injury. The present study was designed to investigate the course of CT-1 in patients undergoing on-pump coronary artery bypass grafting (CABG), and to evaluate the relationship between plasma CT-1 levels and postoperative cardiac function. Methods. Twenty-four patients undergoing elective CABG were studied. Radial artery blood samples were collected before cardiopulmonary bypass (CPB), 5 min and 20 min after reperfusion, and 1 h, 6 h, 12 h and 24 h after CPB. Coronary sinus blood samples were collected before CPB, 5 min and 20 min after reperfusion. Plasma CT-1 levels were measured using the ELISA method. Hemodynamic data were collected. Results. Peripheral CT-1 levels did not change significantly postoperatively. Trans-myocardial CT-1 levels increased significantly 5 min and 20 minutes after reperfusion as compared to baseline. A weak positive correlation (r = 0.408, p = 0.048) was found between trans-myocardial CT-1 levels at 20 min after reperfusion and CI at 12 h after CPB. Conclusions. The heart secretes CT-1 after ischemic injury. The precise effect of CT-1 in CABG needs further investigation.
Cardiotrophin-1, a novel member of the interleukin-6 superfamily, is identified based on its ability to induce hypertrophic responses in cultured neonatal rat cardiac myocytes (Citation1). The myocyte hypertrophy stimulated by CT-1 is mediated via gp130 signaling pathway, which stimulates the assembly of sarcomeric units in series in cardiac myocytes resulting in eccentric hypertrophy (Citation2,Citation3). Another important biological activity of CT-1 is the inhibition of cardiomyocyte apoptosis, which maybe mediated via PI3K and MAPK pathway. Pre- or postconditioning with CT-1 was able to limit the injury induced by ischemia and reperfusion both in isolated rat cardiac myocytes and in vitro rat heart (Citation4–6).
Expression of CT-1 has been observed in normal and disease states. In humans, a 1.7 kb mRNA encoding CT-1 was found in heart, skeletal muscle, prostate and ovary (Citation7). Elevated serum levels of CT-1 have been observed in patients with unstable angina, acute myocardial infarction and heart failure, and the level of CT-1 expression is correlated with the degree of left ventricular systolic dysfunction (Citation8–11).
Although CT-1 is greatly related to cardiovascular disease, such as myocardial infarction and heart failure and exhibits cardioprotective effect in ischemia-reperfusion injury, little attention has been paid to it in cardiac surgery. The present study was designed to document the perioperative course of CT-1 in patients undergoing CABG, and evaluate the relationship between plasma CT-1 levels and postoperative cardiac function.
Material and methods
Patients
The study was approved by the Ethics Committee of Tampere University Hospital, and written informed consent was obtained from each patient. All blood samples of this study were collected from patients in our previous studies. Twenty-four patients with stable angina were scheduled for isolated elective CABG operations. Exclusion criteria were patients receiving corticosteroid or sulfonylurea medication, recent myocardial infarction (<1 month), prior CABG surgery, severe non-cardiac disease, or unstable angina ().
Table I. Clinical data.
Anesthesia and surgical methodology
A radial artery line and a pulmonary artery catheter were inserted for hemodynamic monitoring. Anesthesia was inducted with sufentanil, midazolam, and pancuronium. CABG operation was undertaken using one internal thoracic artery and one to four peripheral vein grafts obtained from the lower extremities in each case. The patients were perfused at a temperature of 32°C with non-pulsative flow from a membrane oxygenator (Dideco, Mirandola, Italy). The circuit was primed with 2000 ml of Ringer's lactate solution. A cold-blood antegrade-retrograde cardioplegic solution (6–8°C) was delivered through a BCD-Plus device (Dideco), which mixed blood with a sanguineous solution at a ratio of 4:1. The potassium concentration of the induction cardioplegic solution was 21 mmol/l. After each distal anastomosis, additional cardioplegic solution was delivered for 1 min through the vein graft and coronary sinus catheter. A warm-blood retrograde cardioplegic solution was given at the end of cross-clamping. Aortic cross clamping time and total time of CPB were documented. After weaning from CPB, pharmacological therapy with inotropic agents was used to maintain a cardiac index greater than 2.0 l/min/m. Cumulative dosages of inotropic agents were recorded immediately after operation, 1 h, 6 h, 12 h and 24 h after operation, respectively.
Hemodynamic data was serially collected at the following five time points: before induction of anesthesia as baseline, 1 h, 6 h, 12 h and 24 h after CPB. Heart rate (HR), mean arterial pressure (MAP), mean pulmonary artery pressure (MPAP), pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), and cardiac output (CO) were measured. Derived cardiovascular variables, including cardiac index (CI), systemic vascular resistance index (SVRI), and pulmonary vascular resistance index (PVRI) were calculated by using standard formulas.
Blood samples for creatine kinase-MB (CK-MB) measurements were taken before the operation, 6 h, 12 h and 24 h after CPB. Samples for CK-MB measurement were collected and cooled in 4°C immediately, then centrifuged. Serum samples were measured with a Chiron ACS180 analyzer (ACS: 180; Chiron/Diagnostics, Emeryville, CA), using a direct chemiluminescence method. Blood samples for CT-1 measurements were collected from radial artery before CPB (baseline), 5 min and 20 min after reperfusion, 1 h, 6 h, 12 h and 24 h after CPB. Three coronary sinus blood samples were collected before CPB, and 5 min and 20 min after reperfusion. All CT-1 samples were anticoagulated with EDTA acid, immediately cooled in ice, and centrifuged within 30 min (4000 g for 10 min); plasma was transferred to polypropylene test tubes and stored at −70°C until assay. CT-1 concentration was determined using ELISA kit (Bio Vender Laboratory Inc, Czech) according to the manufacturer's protocol. CT-1 concentrations were corrected for hemodilution. Trans-myocardial change of CT-1 was calculated as sinus CT-1 levels minus artery levels.
Statistics
Statistical analysis was performed by using SPSS (version 13.0; SPSS for Windows) software package. The Mann-Whitney U-test was used to distinguish differences in demographic parameters between the groups. Continuous variables were analyzed by analysis of variance (ANOVA) for repeated measures. Correlation between different variables was assessed by Spearman's coefficient. Statistical significance was attributed to p-values lower than 0.05. All results are expressed as mean ± standard deviation.
Results
There were no deaths or any major complications (myocardial infarction, cerebral complications, renal and hepatic dysfunction) in all patients (). Hemodynamic parameters are presented in .
Table II. Hemodynamic parameters.
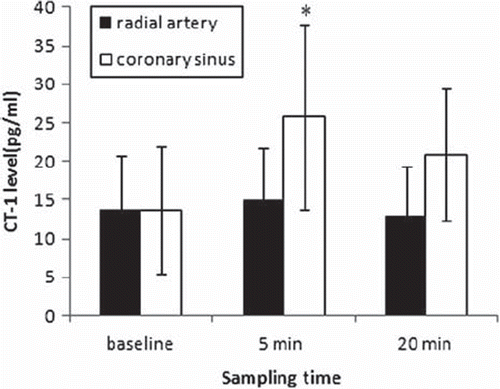
Peripheral CT-1 levels did not change significantly after operation (, p = 0.264). Levels of CT-1 in coronary sinus blood samples were not different from those in radial artery before CPB (13.767 ± 6.95 vs. 13.708 ± 8.18, p = 0.982). Trans-myocardial CT-1 levels increased significantly 5 (14.893 ± 6.88 vs. 25.824 ± 12.04, p = 0.000) and 20 minutes (13.067 ± 6.27 vs. 20.883 ± 8.55, p = 0.001) after reperfusion as compared to baseline (). CK-MB increased after operation ().
Figure 1. Perioperative arterial CT-1 levels. CT-1 levels were measured before CPB, 5 min and 20 minutes after reperfusion, 1 h, 6 h, 12 h, 24 h after CPB.
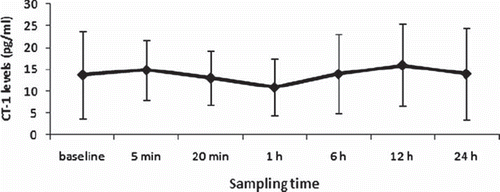
Figure 2. CT-1 levels from radial artery and coronary sinus. *p < 0.05, as compared to CT-1 level from radial artery.
There was no correlation between CT-1 levels and duration of aortic cross clamping or CPB at any time point. A weak positive correlation was found between transcardiac CT-1 levels at 20 min after reperfusion and CI at 12 h after CPB (r = 0.408, p = 0.048). Transcardiac and peripheral CT-1 levels did not correlate with CKMB levels and postoperative cumulative dosages of inotropic medication at any time point.
Discussion
Earlier evidence showed that CT-1 mRNA is expressed in various tissues, including the heart, lung, kidney and muscle. All of these organs are candidates for the source of circulating CT-1 (Citation7). Asai and colleagues demonstrated that there was a significant transcardiac difference of CT-1 in patients with angina pectoris and concluded that the heart is a source of CT-1 in human beings (Citation13). However, the type of angina pectoris was not described in detail in their study. Although transcardiac CT-1 concentration increased significantly after aortic declamping in the present study, there was no transcardiac CT-1 difference in patients with stable angina and normal heart function. Thus, our present results indicated that an ischemic but not a stable heart secretes CT-1 via the coronary sinus into the peripheral circulation.
It has been reported that hypoxic stress induces cardiotrophin-1 expression in isolated cardiac myocytes (Citation8) and plasma levels of CT-1 were significantly elevated in patients with unstable angina or myocardial infarction (Citation9,Citation10). Asai (Citation13) and associates found that plasma CT-1 levels from the femoral vein in patients with angina pectoris were similar to those from cubital vein in healthy volunteers, though a significant transcardiac CT-1 elevation was found in angina patients. The present study showed no peripheral CT-1 change in patients with stable angina, although significant transcardiac CT-1 elevation was found after operation. This may contribute to less and shorter myocardial ischemia in CABG patients, as the heart was arrested with cardioplegia. Our previous study also showed no changes of postoperative peripheral CT-1 after off-pump coronary artery bypass, but high preoperative CT-1 levels were associated with recent myocardial infarction (Citation12).
Talwar and associates found that CT-1 concentration was 142.5 fmol/ml (42.2–527.4 fmol/ml) in unstable angina, 73.2 fmol/ml (41.5–102.1 fmol/ml) in stable angina (p < 0.05 v unstable angina), and 27 fmol/ml (6.9–54.1 fmol/ml) in controls (p < 0.0005 v stable angina; p < 0.0001 v unstable angina) (Citation9). The heart may secret more CT-1 in longer or more severe myocardial ischemic, such as unstable angina pectoris and myocardial infarction, and local myocardial production of CT-1 may be reflected by peripheral CT-1 levels in these setting. The present study detected transcardiac (20 min) and peripheral CT-1 (24 h) levels in a short period. Further CT-1 investigation will be of interest in low ejection fraction CABG patients with longer study period.
CT-1 is identified based on its ability to induce hypertrophy response of cardiomyocyte. Recent studies reported that CT-1 is cardioprotective to ischemia-reperfusion injury, and exhibit an anti-apoptotic effect in different tissues (Citation14–19). CT-1 was able to protect isolated rat cardiac myocytes from apoptosis when added before ischemia or at the onset of the reoxygenation (Citation6). Yin and his colleagues demonstrated that CT-1 pretreatment is capable of significantly improving hemodynamic parameters and cardiac function, reducing myocardial apoptosis in a rat model of acute myocardial infarction (Citation16). In accordance with these results, the present results found that cardiac release of CT-1 in reperfusion period did not relate to degree of cardiac damage, but weakly related to postoperative cardiac index. CT-1 release in reperfusion period may be cardioprotective in patients undergoing CABG. Ghosh and his colleagues (Citation20) demonstrated that CT-1 has a protective effect against ischemia in human adult myocardium, which can significantly decrease CK leakage and increase MTT reduction. They found that the protection is only afforded when tissue is exposed to CT-1 for 24 h but not for a shorter period (2 h). Further study with longer study period of transcardiac CT-1 levels may define the relationship between CT-1 and cardiac damage in CABG.
In conclusion, the present study showed that the heart secretes CT-1 after CABG. However, peripheral CT-1 levels do not change postoperatively. The precise effect of CT-1 in CABG needs further investigation.
Acknowledgements
The present study was supported by grants from Fund of the Tianjin Municipal Science and Technology Commission (05YFSZSF02700, 06YFJMJC08700), Tianjin, P. R. China and Fund from Tampere University Hospital, Tampere, Finland. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, . Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 1995;92:1142–6.
- Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, . Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/ leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271: 9535–45.
- Pennica D, Wood WI, Chien KR. Cardiotrophin-1: A multifunctional cytokine that signals via LIF receptor-gp 130 dependent pathways. Cytokine Growth Factor Rev. 1996;7:81–91.
- Kuwahara K, Saito Y, Kishimoto I, Miyamoto Y, Harada M, Ogawa E, . Cardiotrophin-1 phosphorylates akt and BAD, and prolongs cell survival via a PI3K-dependent pathway in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1385–94.
- Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272:5783–91.
- Brar BK, Stephanou A, Pennica D, Latchman DS. CT-1 mediated cardioprotection against ischaemic re-oxygenation injury is mediated by PI3 kinase, Akt and MEK1/2 pathways. Cytokine. 2001;16:93–6.
- Pennica D, Swanson TA, Shaw KJ, Kuang WJ, Gray CL, Beatty BG, . Human cardiotrophin-1: Protein and gene structure, biological and binding activities, and chromosomal localization. Cytokine. 1996;8:183–9.
- Hishinuma S, Funamoto M, Fujio Y, Kunisada K, Yamauchi-Takihara K. Hypoxic stress induces cardiotrophin-1 expression in cardiac myocytes. Biochem Biophys Res Commun. 1999;264:436–40.
- Talwar S, Squire IB, Downie PF, Davies JE, Ng LL. Plasma N terminal pro-brain natriuretic peptide and cardiotrophin 1 are raised in unstable angina. Heart. 2000;84:421–4.
- Talwar S, Squire IB, Downie PF, O'Brien RJ, Davies JE, Ng LL. Elevated circulating cardiotrophin-1 in heart failure: Relationship with parameters of left ventricular systolic dysfunction. Clin Sci (Lond). 2000;99:83–8.
- Talwar S, Squire IB, O'Brien RJ, Downie PF, Davies JE, Ng LL. Plasma cardiotrophin-1 following acute myocardial infarction: Relationship with left ventricular systolic dysfunction. Clin Sci (Lond). 2002;102:9–14.
- Wei M, Ren S, Liu J, Li P, Qian H, Tarkka M. Perioperative plasma brain natriuretic peptide and cardiotrophin-1 in off-pump coronary artery bypass. Scand Cardiovasc J. 2008;42:399–404.
- Asai S, Saito Y, Kuwahara K, Mizuno Y, Yoshimura M, Higashikubo C, . The heart is a source of circulating cardiotrophin-1 in humans. Biochem Biophys Res Comm. 2000;279:320–3.
- Stejskal D, Ruzicka V. Cardiotrophin-1 review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:9–19.
- Liao Z, Brar BK, Cai Q, Stephanou A, O'Leary RM, Pennica D, . Cardiotrophin-1 (CT-1) can protect the adult heart from injury when added both prior to ischaemia and at reperfusion. Cardiovasc Res. 2002;53:902–10.
- Ruixing Y, Dezhai Y, Jiaquan L. Effects of cardiotrophin-1 on hemodynamics and cardiomyocyte apoptosis in rats with acute myocardial infarction. J Med Invest. 2004;51: 29–37.
- Ruixing Y, Jinzhen W, Dezhai Y, Jiaquan L. Cardioprotective role of cardiotrophin-1 gene transfer in a murine model of myocardial infarction. Growth Factors. 2007;25:286–94.
- Song J, Zhang YW, Yao AH, Yu Y, Hua ZY, Pu LY, . Adenoviral cardiotrophin-1 transfer improves survival and early graft function after ischemia and reperfusion in rat small-for-size liver transplantation model. Transpl Int. 2008;21:372–83.
- Marqučs JM, Belza I, Holtmann B, Pennica D, Prieto J, Bustos M. Cardiotrophin-1 is an essential factor in the natural defense of the liver against apoptosis. Hepatology. 2007;45:639–48.
- Ghosh S, Ng LL, Talwar S, Squire IB, Galiñanes M . Cardiotrophin-1 protects the human myocardium from ischemic injury. Comparison with the first and second window of protection by ischemic preconditioning. Cardiovasc Res. 2000;48:440–7.
