Abstract
A significant number of multiple pregnancies and births worldwide continue to occur following treatment with Assisted Reproductive Technologies (ARTs). Whilst efforts have been made to increase the proportion of elective single embryo transfer (eSET) cycles, the multiple pregnancy rate or MPR remains at a level that most consider unacceptable given the associated clinical risks to mothers and babies, and the additional costs associated with neonatal care of premature and low birth weight babies. Northern Europe, Australia and Japan have continued to lead the way in the adoption of eSET. Randomised controlled trials or RCTs, meta-analyses and economic analyses support the implementation of an eSET policy, particularly in light of recent advances in ARTs. This paper provides a review of current evidence and an update to the eSET guidelines first published by CitationCutting et al. (2008) intended to assist ART clinics in the implementation of an effective eSET policy.
Introduction
In 2009, the Human Fertilisation and Embryology Authority (HFEA) introduced a policy to encourage fertility centres to adopt elective single embryo transfer (eSET) into routine use (CitationHFEA, 2008). This policy required a phased reduction in the multiple birth rate (MBR) from 24 % in 2009, 20 % in 2010, 15 % in 2011, to 10 % in 2012 and thereafter. Initially these targets formed a license condition, but in early 2014 a legal challenge to the HFEA by a UK-licensed fertility centre resulted in the removal of the license condition for all centres (CitationHFEA, 2013). Despite this, the HFEA, the Association of Clinical Embryologists (ACE), the British Fertility Society (BFS) and other stakeholder professional organisations remain fully supportive of the ‘One at a Time’ project supporting eSET and committed to the current 10 % MBR target.
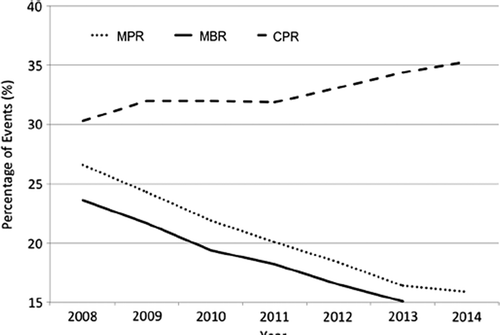
The most recent HFEA trends and figures report (CitationHFEA, 2014) revealed that clinics continue to replace two embryos in the majority of fresh or frozen IVF or ICSI procedures (51.0 % down from 56 % in 2012) with 27.0 % (up from 22.2 % in 2011) receiving eSET. An examination of the annual HFEA data published from 2008 to 2013 reveals an encouraging and sustained trend in reduction of multiple pregnancy rate (MPR) from 26.6 % in 2008 to 16.3 % in 2013. During this time period, the gradual improvement in live birth rate (LBR) from IVF and ICSI treatment, which has been observed since 1994, remained unaffected. What is clearly evident is that although an on-going downward trend in MBR has been observed, further adjustments to clinical practice must still be made if the 10 % recommended target is to be achieved whilst maintaining the LBR.
The message from Europe is clear: countries such as Sweden, Finland and Belgium have clearly demonstrated that a MBR of less than 10 % is achievable. Sweden continues to report the highest percentage of SET at 69.9 %. In 2010, CitationKallen et al. (2010b) examined trends in delivery and neonatal outcome from the Swedish National Heath Register dataset spanning over 25 years. The study included in excess of 25,000 women receiving IVF treatment over the 5-year period following the introduction of eSET and showed that the risk of neonatal death and morbidity was significantly reduced.
When the first edition of the ACE/BFS guidelines was published (CitationCutting et al., 2008), many of the Northern European countries had implemented eSET, but only Belgium and Sweden had legislation underpinning it. In 2010, Turkey introduced new legislation mandating SET in women less than 35 years of age in the first or second cycle of treatment and limiting clinics to the transfer of two embryos in the third or subsequent treatment cycles and in women over the age of 35 years (CitationKultursay et al., 2015). Since the implementation of this policy, preliminary results reported from a small multi-centre study have shown a significant reduction in MPR, with a modest downward trend in clinical pregnancy rate (CPR) from 39.9 % to 34.5 %, although this was not statistically significant (CitationKutlu et al., 2011).
In 2010 the provincial government of Quebec introduced eSET legislation with the aim of performing SET in every treatment cycle (CitationBissonnette et al., 2011). The legislation states that multiple embryos can only be transferred in cases where it is justified by the treating physician and on a case-by-case basis. In the first 3 months of the programme, 5 centres performed 1353 IVF cycles with an overall CPR of 32 % per embryo transfer (compared with 43 % prior to the policy being introduced), with 50 % of transfers using eSET. During the same time period, the MPR was reduced from 25.6 % to 3.7 %. Prior to this legislative change, eSET was performed in only 1.6 % of treatment cycles. The fact that central funding was allocated to this project by the provincial health programme must be recognised as a major contributing factor to the successful implementation of the policy in such a short period of time. CitationVelez et al. (2013) showed that the initiative in Quebec reaffirmed the policy of ‘1 plus 1 should equal 2’, with cumulative pregnancy rates from the sequential transfer of one fresh followed by one frozen embryo close to equalling that obtained with the transfer of two fresh embryos together.
In the USA only 1 % of fresh transfers prior to 2002 were eSET, with a modest increasing trend to 5.6 % overall in 2010, with a 9.6 % eSET rate and a 32.4 % twin rate in the < 35 age group. However, in 2012 the Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine published eSET guidance for patient selection (CitationASRM, 2012). These guidelines concluded that eSET should be promoted and offered to patients with a good prognosis, and to recipients of embryos from donated eggs and that eSET must be applied in association with an effective embryo cryopreservation programme. The most recent data from 2013 now show a more than doubling of eSET in the good prognosis < 35 age group to 22.5 %, and a modest reduction in the twin rate to 28.3 % (CitationSART, 2013).
In 2011, CitationMaheshwari et al. (2011) assessed global variation in the uptake of a SET policy (). They reported that it was not possible to distinguish eSET from non-elective SET from national ART reports but that in over 31 countries there had been a gradual increase in the rate of SET. They suggested that public funding, availability of effective cryopreservation, and national legislation remained the main contributory factors in the establishment of effective SET policy. Whilst legislation in the UK has defined targets the worldwide picture is very variable. Few countries have specific legislation, some have guidance, whist other countries still endorse double embryo transfer (DET). There does however seem to be consensus that embryo quality and age should be taken into account during the decision process ().
Table I. Summary of Global legislation in relation to eSET (adapted from CitationMaheshwari et al., 2011).
Aim
The aim of this guideline review is to provide updated evidence-based guidance, to assist UK fertility centres in the implementation of effective eSET policy with the intention of reducing MPR, whilst not discounting the patients’ right to choice and autonomy. The revised guideline remains highly supportive of the ‘One at a Time’ initiative in the UK, which continues to encourage the practice of eSET.
Scope
The guideline revision will address the need for eSET, patient and embryo selection, superovulation practices, cryopreservation strategies, the role of the HFEA and cost implications.
Levels of Evidence
The hierarchy of evidence and system for grading the strength of the evidence used in this paper are outlined in , as developed by the National Institute for Health and Care Excellence (NICE).
Table II. NICE hierarchy of evidence, and grading the strength of the evidence.
Safety
The safety of IVF procedures will be a major consideration throughout. IVF as an intervention occurs at a point in early human development when the embryo is uniquely vulnerable, due to the complete reorganisation of the genome and epigenome immediately after fertilisation. There is general appreciation now that the peri-conceptional environment can influence long-term programming of health in offspring, via largely epigenetic mechanisms (CitationWatkins et al., 2010; CitationLucas, 2013). This concern is consistent with the long-standing observation that IVF singleton babies are at increased risk of low birth weight (CitationHansen et al., 2002). With respect to technologies used in selection of embryos for eSET consideration should be given to the potential risk of the technology, set against the accepted benefit of reducing the incidence of multiple pregnancies (see above). The impact of ART and environmental stresses on embryo development and long-term disease is a priority area for research.
Recommendations – Safety
Further research is required (a) to validate the efficacy of eSET policies and (b) confirm the long-term safety of the culture and cryopreservation processes that constitute such policies. (C)
Is there still a role for DET?
Results from randomised controlled trials (RCTs) comparing eSET with DET clearly show that the chance of live birth is reduced, unless eSET is combined with an effective programme for the transfer of a subsequent frozen embryo (CitationLukassen et al., 2005; Citationvan Montfoort et al., 2006; CitationMoustafa et al., 2008). This supports the earlier study by CitationThurin et al., (2004) which reported that the LBR following eSET was significantly reduced compared with DET. However, the cumulative LBR following subsequent replacement of a single frozen embryo in the eSET group was equivalent to the DET group (39 % vs. 43 %). A Cochrane Database System Review has supported these findings (CitationPandian et al., 2009). More recently, CitationMcLernon et al., (2010) published a meta-analysis of 8 RCTs of cleavage-stage eSET versus DET, 5 of which had been published in peer-reviewed journals. This showed a cumulative LBR from 2 eSET (fresh + frozen) cycles with day 2/3 transfer slightly but not statistically significantly lower than that from a single DET cycle (38 % vs 42 %). In addition, statistical modelling of large treatment cycle datasets including the HFEA register has shown that SET would yield about a one-third lower LBR relative to DET, for any embryo transfer (CitationRoberts et al., 2011). However, this difference in LBR can be mitigated by patient and treatment cycle selection, resulting in an increased LBR with sequential eSET, compared with DET, with both fresh and frozen embryo transfers (FETs). Thus, it is undeniable that when combined with an effective cryopreservation programme eSET can yield cumulative success rates that at least match, and may exceed, those from DET (CitationRoberts et al., 2009; Citation2010b). Given the strength of the accumulating evidence in favour of a well-constructed eSET programme, why should DET still be considered, given the attendant risk of twin pregnancies?
Whilst cumulative cycles with cryopreservation maintain success rates, it is important to consider that this strategy requires multiple cycles and adds an emotional, physical and financial cost to purchasers, either through state funding or (depending on the costing models) patients. Interviews and focus groups conducted with patients undertaking fertility treatment continue to indicate a clear patient preference for twins despite the potential to maintain overall LBR with an eSET strategy (CitationLeese & Denton, 2010; CitationRoberts et al., 2010b). It is therefore vital when considering eSET that clinics ensure the needs of patients are taken into account and that patient preference is not seen as irrational. However, this must be considered in context with the short- and longer-term consequences of avoidable multiple pregnancy and birth. In particular, the desire for patients to have more than one child should be taken into consideration and eSET policies need to allow funding for the transfer of all embryos of transferrable quality, regardless of earlier outcome, if eSET is not to be disadvantageous in this respect.
Secondly, the extended treatment duration in a complete eSET programme may be particularly disadvantageous to older women whose fertility is declining rapidly. Although the evidence suggests that oocyte aneuploidy is the biggest limiting factor in older women, there is also some decline in uterine receptivity (CitationLegro et al., 1995; CitationStolwijk et al., 1997; CitationToner et al., 2002; CitationRoberts et al., 2010a). More significantly, repeat egg collections following failure will occur at later ages in an eSET programme, reducing the chances of a successful outcome. This could potentially be mitigated by offering multiple oocyte retrievals or ORs followed by cryopreservation and eSET.
Finally, although the economic costs from a total health service perspective are reduced with eSET (CitationScotland et al., 2011), this is not true of the direct costs to fertility service providers, or to patients in privately funded treatments.
Selection of patients for DET
Despite the evidence in favour of eSET it is likely that DET will remain part of clinical practice in the UK for the foreseeable future and the question arising is for which patients should DET still be considered. The available evidence suggests that the relative chance of twins is similar for all patients regardless of the usual prognostic factors (CitationRoberts et al., 2010b) with no specific risk factors for twinning, other than embryo quality, being identified. This of course means that the absolute chance of twins is greatest in patients with the best prognosis. In an extensive analysis of the UK HFEA register data, CitationLawlor and Nelson (2012) concluded that the transfer of three or more embryos at any age should be avoided and that the decision to transfer one or two embryos should be based on prognostic indicators, such as age.
Thus there is, at the time of writing, no clear high-quality evidence for selection of patients for inclusion in an eSET or DET strategy and there are no clear predictors of the likelihood of twins other than stage of embryo development at transfer, and embryo quality. Selection is therefore based on local or anecdotal evidence or a pragmatic financial decision to include those patients most likely to have twins because they are most likely to become pregnant following treatment.
A number of very similar policies for selecting patients for DET have been suggested, which essentially target eSET at those with the best prognosis and therefore reduce the overall twin rate, whilst allowing DET for the poor prognosis patients to maintain their single transfer success rate. In the CitationNICE (2013) guidelines, several factors were recommended which should be considered when determining an individual patient's suitability for eSET. These include maternal age, obstetric and gynaecological history, previous treatment history, ovarian response or reserve, number of embryos created and quality of embryos or blastocysts available for transfer, and cryopreservation. For women under the age of 37 years undertaking their first treatment cycle, the CitationNICE (2013) guidelines advocate eSET. These guidelines largely support the eSET algorithm proposed by CitationCutting et al. (2008), although they are not specific in terms of the day on which embryo transfer should be performed other than to recommend eSET in circumstances where at least one top-quality blastocyst has developed. The NICE guidelines propose additional recommendations for women in the age ranges of 37–39 and 40–42 years ().
Table III. CitationNICE (2013) Guidelines – Embryo transfer strategy summary table (including fresh & frozen embryos).
The relationship between LBR and maternal age is well established (CitationDessolle et al., 2011; CitationSifer et al., 2011). In the eSET algorithm published by CitationCutting et al., (2008), it was recommended that eSET should be routinely applied to patients < 37 years of age. However, a recent report has challenged whether eSET should be restricted solely to younger women. CitationNiinimaki et al., (2013) examined whether eSET for older women between 40 and 44 years could be feasible. This retrospective cohort study appears to be the first to show such a large number of older women consenting for eSET: 42 % of the study population. In fresh cycles, the CPRs were 23.5 % and 19.5 % in the eSET and DET group, respectively, with LBRs of 22.7 % and 13.3 %, respectively (P = 0.002). This study was limited by an inability to compare the two groups directly as the suitability for eSET had been individually assessed by a clinician and not randomised. These findings were challenged by CitationGleicher (2013), who in addition to questioning the rationale for eSET in older women with reduced prognosis questioned the ‘irrational attraction of eSET’. He commented on the ethics of urging older women to reduce their immediate pregnancy chances and suggested that under most circumstances women of advanced age cannot be considered to have a favourable prognosis. Gleicher highlighted the point that overall, the risk associated with a twin pregnancy (one live birth event) compared with two singleton pregnancies (two separate live birth events) becomes reduced.
A retrospective analysis of the outcome and feasibility of eSET in the first and second transfers was reported by CitationGremeau et al. (2012). The study showed that from a patient group of 783 couples embarking on a first cycle of IVF or ICSI treatment, the on-going pregnancy and cumulative delivery rates from eSET and DET cycles did not differ significantly (30.9 % and 34.6 %, respectively) with 67.6 % of the transfers being eSET. For the second treatment attempt, the eSET rate dropped to 16.9 % suggesting that eSET is less well accepted by patients for their second attempt, although it should be noted that patient acceptability and clinicians’ advice were not measured independently.
eSET has also been shown to yield a good LBR in oocyte donation programmes. A retrospective study which examined delivery rates before and after the implementation of an eSET policy showed a significant reduction in the twin rate, with no impact on the delivery rate (although the study was not powered to examine this) (CitationSoderstrom-Anttila et al., 2003). This observation was supported by another retrospective study, which suggested that when three embryos are available and eSET is performed, the cumulative LBR is not compromised (CitationClua et al., 2012). Following these studies, CitationNICE (2013) recommended using an embryo transfer strategy based on the age of the oocyte donor.
Recommendations – Selection of patients for DET
There should be a continued focus on minimising MBRs from IVF treatment by utilising an effective, dynamic eSET strategy. (GPP)
In situations where embryo quality is poor, clinics should consider the transfer of two embryos. An effective eSET policy should accommodate this. (B)
Centres should audit eSET rates, selection criteria, and MBRs and where their dataset is robust, consider adjusting their eSET algorithm in line with their results on at least an annual basis, focussing on good practice rather than small numbers of multiple births above or below a target rate. (GPP)
Superovulation strategies
Traditionally, stimulation protocols have induced pituitary downregulation with a gonadotrophin-releasing hormone (GnRH) agonist followed by gonadotrophin stimulation, or the use of a GnRH antagonist to block the luteinising hormone (LH) surge during the stimulatory phase. A Cochrane review of GnRH antagonists in assisted reproduction (CitationAl-Inany & Aboulghar, 2002) and a more recent update (CitationAl-Inany et al., 2011) suggest that overall the antagonist protocol gave a 1.5 % lower LBR and 2 % lower CPR compared with the agonist protocol. However GnRH antagonist use is associated with a lower risk of ovarian hyperstimulation syndrome (OHSS) compared with GnRH agonist (CitationMathur & Tan, 2014). The need for safety is paramount and the rationale for ‘aggressive’ stimulation protocols should be questioned in an effort to reduce OHSS and in association with an effective eSET strategy to reduce the incidence of multiple pregnancies. CitationHeijnen et al. (2007) showed that three mild stimulation protocols are as affective as two conventional stimulations in terms of outcome. CitationBaart et al. (2009) described how different ovarian stimulation regimens can affect oocyte and embryo quality.
Recommendations – Superovulation strategies
Maximising oocyte and embryo quality is an essential part of an effective eSET strategy; a focus should remain on optimising superovulation regimens. (A)
Selection of embryos or blastocysts for eSET
It is generally accepted that robust embryo selection is a key element in the implementation of an effective eSET programme. This has resulted in increased pressure and expectation on Clinical Embryologists to ensure that the embryo with the best potential to deliver a healthy live birth is selected for transfer. Significant progress has been made in recent years with the various techniques employed for embryo selection.
Morphological oocyte, embryo and blastocyst assessment
a) Oocyte assessment
In predicting embryo quality and implantation potential, it is wise to consider the assessment and quality of oocytes as predictors of embryo competence. However, historically there has been conflict in the literature as to the reliability of these observations, particularly during ICSI. CitationSerhal et al. (1997) suggested that anomalies including granularity and the presence of cytoplasmic inclusions such as vacuoles, smooth endoplasmic reticulum clustering, and refractile bodies had a negative impact on embryo implantation rates. In contrast, CitationBalaban et al. (1998) suggested the exact opposite: that oocyte morphology had no direct effect on fertilisation rate, embryo quality and implantation rate following ICSI. More recently, the Istanbul consensus on embryo assessment (CitationALPHA, 2011) published evidence suggesting oocyte assessment anomalies should be sub-divided into (i) intra-cytoplasmic (incorporations, refractile bodies, dense central granulation, vacuoles and aggregation of smooth endoplasmic reticulum); and (ii) extra-cytoplasmic dymorphisms (first polar body morphology, perivitelline space size and granularity, discolouration, zona pellucida defects, and shape anomalies). CitationALPHA (2011) also concluded that ‘giant’ oocytes and oocytes with smooth endoplasmic clusters should be selected against because of their likely abnormal genetic constitution and association with early foetal loss and imprinting disorders, respectively (CitationOtsuki et al., 2004; CitationEbner, 2008).
b) Pronuclear (PN) assessment
Assessment of pronuclear (PN) morphology is based on observations of features seen in zygotes 16–18 hours post-fertilisation. Grading systems were developed to assess the number, position, size and alignment of the nucleolar precursor bodies (NPB) in each embryo. Zygotes which showed pronuclei of similar size centrally located, containing NPB of equal size and number aligned at the pronuclear interface, were considered to have a good PN score and thus a good prognosis (CitationScott et al., 2000; CitationMachtinger & Racowsky, 2013). However, the relationship between PN score and embryo quality has not yet been fully established. Some early studies reported good correlation between the PN score and embryo development (CitationScott et al., 2000; CitationRienzi et al., 2002; CitationNagy et al., 2003) but more recent studies have questioned the validity of this form of assessment to predict embryo competency (CitationJames et al., 2006; CitationNicoli et al., 2007; CitationAhmad et al., 2008). A possible explanation for this conflicting data might be the highly dynamic nature of PN formation. With such rapid changes occurring within short periods of time, early assessments can change rapidly and may therefore be misleading. This has been demonstrated using time-lapse imaging (CitationMontag et al., 2011). A retrospective review by CitationNicoli et al. (2013) concluded that PN evaluation was only effective if embryo transfer is performed on day one of embryo culture.
c) Cleavage stage embryo assessment
Indicators of embryo development including cell number, evenness of size, and incidence of fragmentation have formed the basis of embryo assessment since the inception of our scientific discipline. More recently, parameters such as multi-nucleation of blastomeres and uneven cell division have enhanced such assessments, which can be performed at the cleavage stage of embryo development.
Cleavage rate
There is a considerable body of evidence suggesting that delayed or rapid embryo cleavage rate has a negative impact on implantation (CitationEdwards et al., 1980; CitationVan Royen et al., 1999; CitationDesai et al., 2000; CitationRoberts et al., 2010a). Two studies have suggested embryos that complete their first cleavage to the 2-cell stage between 25 and 27 hours post-insemination show increased implantation potential (CitationWharf et al., 2003; CitationGiorgetti et al., 2007). However, further recent data from a multi-centre prospective study has re-assessed the effect of early cleavage on embryo quality, implantation and LBR (Citationde los Santos et al., 2014). This study examined 700 embryo transfers and over 1000 early-stage embryos and showed that for conventional embryo culture and morphological evaluations, the assessment of early cleavage was not considered a valuable selection tool to improve embryo implantation. The Istanbul consensus on embryo assessment (CitationALPHA, 2011) states that the single most important indicator of embryo viability is the timely occurrence of cell divisions (CitationBalaban & Magli, 2011) as summarised in . This suggests that embryos that cleave more slowly than the expected rate have reduced implantation potential and those that cleave too rapidly are more likely to be abnormal and have a reduced implantation potential. This has been confirmed by large-scale analysis of UK data (CitationRoberts et al., 2010a).
Table IV. Timing of observation of fertilised oocytes and embryos and expected stage of development at each time point (CitationALPHA, 2011).
Fragmentation
A fragment is defined as an anuclear, membrane-bound extracellular cytoplasmic structure (CitationALPHA, 2011). CitationJohansson et al. (2003) defined fragments as cells that were < 45 μm in diameter for Day 2 embryos and < 40 μm for Day 3 embryos. The negative effect of increased fragmentation on embryo implantation potential is well established (CitationAlikani et al., 2008). Furthermore, the type and distribution of fragmentation may be related to implantation potential. Large fragments were found to be detrimental whilst small or scattered fragments did not have as much impact (CitationRienzi, 2005). However, fragments can be transient or migratory and can disappear or be reabsorbed during normal embryo development. For this reason, identification and evaluation of specific fragment patterns may not reflect the embryo's viability as part of a time-specific embryo assessment (CitationAntezak & Van Blerkom, 1999). Fragmentation levels of less than 10 % appear to have negligible impact on implantation (CitationVan Royen et al., 2001).
Cell size
For embryos at the 2-, 4- and 8-cell stages, it is expected that the blastomeres should be of equal size and shape. When blastomere size varies by more than 20 %, reduced implantation potential is observed due to an increased incidence of aneuploidy and multi-nucleation (CitationPlachot et al., 1998; CitationHardarson et al. 2001; CitationVan Royen et al., 2003).
Multi-nucleation
An embryo can be considered multinucleate when it contains at least one cell that has more than one interphase nucleus (CitationBalaban & Magli, 2011). There is considerable evidence to show that the presence of multi-nucleation correlates with chromosomal abnormalities, uneven cell size and division and lower implantation, pregnancy and LBRs (CitationHardarson et al., 2001; CitationVan Royen et al., 2003). This is supported by a recent retrospective cohort analysis, which showed that when multi-nucleation is present on Day 2 of development in at least one blastomere, there is an associated increase in the rate of aneuploidy even when a sibling blastomere is biopsied which showed no signs of multi-nucleation (CitationAmbroggio et al., 2011). There are certain factors which are known to affect the rate of multi-nucleation such as the type of culture medium (CitationWinston et al., 1991) and temperature fluctuations (CitationPickering et al., 1990), the impact of which should be kept to a minimum by good laboratory process control. The optimal stage to assess for this parameter is on day 2, but evaluation does not always confirm multi-nucleation, since the nuclei are only visible during the interphase stage and could potentially be missed (CitationRienzi, 2005). Time-lapse technology will serve to evaluate further the impact of multi-nucleation as evidence is published on the incidence and outcome of previously undetected multi-nucleated embryos.
Blastocyst assessment and transfer
Advances in embryo culture media and incubation systems have enabled the reliable extended culture of embryos to the blastocyst stage of development, 5 or 6 days following insemination. This allows embryologists flexibility in deciding when to perform the embryo transfer and theoretically should provide certain advantages over the transfer of cleavage-stage embryos. According to the CitationASRM, (2013b) committee opinion on blastocyst culture, these advantages include:
A higher implantation rate;
The opportunity to select the most viable embryo;
The potential decrease in the number of embryos transferred; and
Better temporal synchronisation between embryo and endometrium.
However, the potential downsides to blastocyst transfer can include the increased risk of couples not having a transfer when the procedure is applied in unselected patients having fewer embryos available for cryopreservation (CitationGlujovsky et al., 2012). In addition, there are concerns that blastocyst culture results in an increased risk in monozygotic twinning (MZT), including monochorionic and monoamniotic twinning, which are extremely high-risk pregnancies (CitationVitthala et al., 2009; CitationChang et al., 2009). This reported increase in MZT does not occur in all blastocyst transfer programmes (CitationMoayeri et al., 2007; CitationPapanikolaou et al., 2010); however, a notable recent analysis of a large number of cycles on the Society for Assisted Reproductive Technologies (SART) database confirmed the association between blastocyst transfer and increased incidence of MZT (CitationLuke et al., 2014). The causal factors are as of yet unknown and couples should be counselled about the possible increased risk of MZT in each particular clinic of blastocyst, compared with cleavage-stage embryo transfer. Counselling should include the advice that MZT carries greatly increased risks compared with dizygotic twinning and not just a higher incidence of twins. In addition, recent reports from national data registries in the US, Canada and Sweden show that babies born from blastocyst transfer are at significantly increased risk of pre-term and very pre-term birth (< 32 weeks) (CitationKallen et al., 2010b). The underlying cause of this phenomenon is unknown, but may include altered allocation of cells to the placenta in blastocysts developing in vitro. A meta-analysis of these studies (CitationMaheshwari et al., 2013) was not able to control for confounders, but patients should be counselled about the possible risk of pre- and very pre-term birth from blastocyst transfer. There is good evidence in animal models that embryos in prolonged culture are at risk of growth disorders such as Large Offspring Syndrome in sheep and cattle (CitationYoung et al., 1998) that arise from epigenetic disorders, with some data also available from human ART (CitationKallen et al., 2010a; CitationPark et al., 2011). But again, the evidence on this is incomplete and requires further high-quality prospective studies.
There does however appear to be consistent evidence to suggest that in good prognosis populations (defined by such factors as age, number of previous cycles, ovarian response and number and size of follicles at hCG trigger), there is an increased likelihood of live birth per embryo transfer after the transfer of fresh blastocysts compared with cleavage-stage embryos (CitationGardner et al., 1998; CitationPapanikolaou et al., 2005; CitationPapanikolaou et al., 2006; CitationGlujovsky et al., 2012), particularly when there are supernumerary blastocysts available for cryopreservation (CitationMullin et al., 2012). However the most recent Cochrane meta-analysis concluded that when both early cleavage and blastocyst stage embryo freezing are taken into account, early cleavage stage transfer results in a higher cumulative pregnancy rate compared with blastocyst transfer (CitationGlujovsky et al., 2012). It should be noted that this evidence was based on studies in which blastocysts were cryopreserved using controlled rate freezing rather than vitrification and this is an area which should be the subject of future review.
Recommendations – Selection of blastocysts for eSET
Where blastocyst transfer is performed, clinics should review their eSET data and criteria for extended embryo culture regularly. (GPP)
Prospective studies are required to determine the impact of embryo culture to the blastocyst stage on MZT, pre-term birth and associated epigenetic changes and on why MZT appears to be more prevalent in some clinics. (B)
Patients should be counselled that current best evidence indicates that blastocyst stage transfer may result in a successful outcome sooner, but with some specific increased risks, and may ultimately lead to a reduction in the overall chance of success. (A)
There are many studies that outline the importance of morphological grading of blastocysts as a predictor of outcome (CitationGardner et al., 2000; CitationBalaban et al., 2000; CitationGoto et al., 2011; CitationHeitmann et al., 2013). Blastocysts are usually assessed using a 3-part grading scheme which was first suggested by CitationGardner and Schoolcraft (1999) based on the degree of blastocyst expansion and characteristics of the inner cell mass (ICM) and trophectoderm (TE). More recently, there has been a focus on the particular importance of the TE with its grading correlating more closely with implantation and live birth than that of the ICM (CitationAhlstrom et al., 2011; CitationHill et al., 2013).
Following the Istanbul consensus on embryo grading (CitationALPHA, 2011), it was agreed that an optimal embryo at this developmental stage would be a fully expanded blastocyst with an ICM which is prominent and easily discernible, and consisting of many cells with the cells compacted tightly adhered together, and with a TE that comprises many cells forming a cohesive, even epithelium.
Standardising grading of embryos and blastocysts
The previous ACE/BFS guidelines for practice on eSET (CitationCutting et al., 2008) suggested a basic grading scheme which has been adopted both by the National External Quality Assurance Scheme or NEQAS embryo grading scheme and by NICE in the 2013 Fertility Guideline Review. The three components described in the grading scheme have been evaluated as predictors of pregnancy and it has been shown that embryo growth rate and fragmentation are by far the strongest predictors of pregnancy potential and that after including these, blastomere evenness did not improve the efficacy of the model to predict pregnancy further (CitationStylianou et al., 2012). However, it is important to take account of the subjective nature of morphological evaluation and that a laboratory should therefore have a robust approach to external and internal quality assurance (CitationBjorndahl et al., 2002).
The use of time-lapse imaging for embryo and blastocyst selection
Since the inception of IVF, the universally accepted method to select an embryo or embryos for transfer was via a manual morphological assessment. However, in recent years technological advances have allowed the integration of cameras into incubators to allow time-lapse embryo imaging of the process of morphometric evaluation in real time. This technology enables embryos to be cultured undisturbed, without the need for removal from the incubator for observation, allowing remote monitoring of development by serial image capture (CitationLemmen et al., 2008). There are many publications (the vast majority of which report on retrospective analyses), which suggest a benefit of morphometric evaluation for selection and de-selection of embryos at key stages in their development (see CitationAparicio et al., 2013; CitationBolton et al., 2015) as well as the benefit of having an undisturbed embryo incubation environment. However, CitationArmstrong et al., (2015) has recently published a critical appraisal of the design of time-lapse studies concluding they do not provide robust evidence in favour of time-lapse incubation as an undisturbed culture environment or as an embryo selection algorithm. They also point out the risks of introducing technology without critical appraisal.
Until recently, there had been no evidence based on randomised trials to support the use of time-lapse systems. CitationRubio et al. (2014) then described the findings of the first randomised controlled prospective study into the efficacy of time-lapse technology. The study, based on 843 cycles, indicated that while the use of time lapse microcopy combined with time-lapse embryo selection algorithm did not result in a statistically significant increase in the pregnancy rate (61.6 % vs. 56.3 %), the implantation rate was increased and early pregnancy loss was reduced (16.6 % vs. 25.8 %). This is without doubt an area where further high-quality large-scale prospective clinical trials are urgently required.
Current evidence does not discriminate between the effects of undisturbed culture and improved embryo selection afforded by time-lapse incubation systems. More recently there has been a focus on evaluating characteristics that are negative predictors for developmental competence and therefore allow the de-selection of embryos (CitationMontag et al., 2013). Negative predictors include direct cleavage from 1 cell to 3 cells, uneven blastomeres at the 2-cell stage and multi-nucleation at the 4-cell stage. CitationRubio et al. (2012) reported significantly lower implantation rates when embryos exhibited abnormal cleavage patterns. Combined with the work-developing eSET algorithms the information gained from the early cleavage observations could be used to select embryos on day 3 of culture, negating the need for blastocyst culture as a tool for selection (CitationHashimoto et al., 2012).
Whilst the studies to date show promise that time-lapse parameters have the potential to improve embryo selection beyond standard morphology, incorporation into routine practice will require the development of robust selection algorithms. It is not yet established whether a single algorithm would be appropriate for all centres or for all treatment types. Thus, the algorithms proposed have been based on surrogate endpoints (development to blastocyst stage) or on subsets of pregnancy outcome data where the embryo outcomes are known (all or none of the transferred embryos develop, which represents a biased approach) or restricted to ICSI cycles. With the exception of the work of CitationHerrero et al. (2013), all such studies have been far too small to enable robust algorithm development and validation: when the datasets produced include 20 or more parameters, datasets with more than 400 successful and unsuccessful outcomes are needed, and larger numbers still will be required to establish reliable thresholds.
From data currently available it appears likely that the use of time-lapse technology may facilitate the de-selection of embryos (CitationBolton et al., 2015). It is also clear that before firm conclusions can be drawn on the benefits of time-lapse technology there is an urgent need for further well-designed large-scale, independent, multi-centre studies with unselected patients to develop and validate selection criteria, followed by RCTs to demonstrate their clinical utility (CitationBolton et al., 2015). In addition, cost–benefit analyses should be performed to ascertain if benefits attributable to time-lapse incubation systems justify the significant costs involved in the procurement and implementation of the appropriate technology.
Recommendations – Use of time-lapse technology
Centres with the resources to support time-lapse morphometric analysis may use this information to improve their eSET algorithm. (GPP)
Technological advances including time-lapse imaging and morphometric assessment show promise as embryo selection and de-selection tools, but further high-quality prospective studies are required to develop and validate robust algorithms, followed by RCTs to demonstrate their role in an eSET strategy. (C)
Non-invasive testing of embryo viability
The correlation between metabolic activity and embryo viability was first reported by CitationRenard et al. (1980) in a study that looked at glucose uptake in advanced bovine blastocysts. Since then several studies have reported the use of metabolic, metabolomic, proteomic, genomic and transcriptomic markers as predictors of embryo viability (CitationLeese & Barton, 1984; CitationVan den Bergh et al., 2001; CitationGardner et al., 2001; CitationLeese, 2002; CitationBrison et al., 2004; Citation2007; CitationLeese, 2012; CitationBolton et al., 2015).
Recent papers by CitationGardner and Wale (2013) and CitationBolton et al., (2015) have reviewed the analysis of metabolism as a means of selecting viable embryos for transfer particularly with a view to replacing a single embryo. These papers review the data on amino acid turnover along with carbohydrate utilisation and suggest that there are distinct patterns of amino acid utilisation between viable and non-viable blastocysts as initially reported by CitationHoughton et al., (2002) and CitationBrison et al., (2004). Amino acid profiling has an established scientific basis (CitationBrison et al., 2004) but is yet to find a place in routine clinical practice. Refinements of the high-performance liquid chromatography (HPLC) technology to reduce the time it takes to carry out the amino acid profiling may make the process more efficient and attractive for clinical use. CitationGardner and Wale (2013) have shown that glucose consumption provides a further potential biomarker of the capacity of an embryo to give rise to a pregnancy. Raman and near-infrared spectroscopy have been used to analyse culture medium to examine metabolic profiles (the overall technique is known as ‘metabolomics’) of viable and non-viable embryos (CitationBrison et al., 2007; CitationSeli et al., 2007). However a prospective RCT of a method based on infrared spectroscopy failed to show an improvement in CPR (CitationHardarson et al., 2012). Other studies have utilised different technologies to assess the metabolomic profile of culture media including proton nuclear magnetic resonance (1H NMR) spectroscopy (CitationSeli et al., 2008). Respiration rates, in terms of oxygen consumption of oocytes and embryos, have been measured (CitationScott et al., 2008) and related to embryo viability (CitationLopes et al., 2007). However, as with the other non-invasive markers discussed, such measurements have yet to find a place in the IVF clinic.
Despite this, selection of the healthiest embryo for eSET is paramount and although not yet available for routine clinical use, these approaches show considerable promise. Further research and prospective trials are required to develop and validate these methods and translate them into clinical practice (CitationBolton et al., 2015)
Recommendations – Non-invasive testing of embryo viability
Further development and evaluation is required of the effectiveness of selecting embryos based on metabolic or related traits. (C)
Pre-implantation genetic diagnosis and screening
In cases where an individual or couple have a known risk of any resultant child being born with or developing a serious debilitating genetic disorder, the process of pre-implantation genetic diagnosis (PGD) can be applied to reduce this risk and ensure that embryos with the best implantation potential are selected for treatment. The procedure has obvious benefits and is not contested by most ART practitioners. However, the use of pre-implantation genetic screening (PGS) has to be one of the most controversial of all the developments in assisted reproduction over the last few years. For more than 10 years, PGS was performed at the cleavage stage of embryo development using Fluorescent in situ hybridization (FISH) yet was shown not to increase the live birth rate (CitationMastenbroek et al., 2011, CitationMastenbroek, 2013). This conclusion was supported by the European Society of Human Reproduction and Embryology or ESHRE PGD Consortium who issued a position paper suggesting that there is no improvement in LBRs in patients with advanced maternal age (CitationHarper et al., 2010). In 2008, an ASRM Practice Committee paper suggested that not only did PGS not improve LBRs in older women but also did not improve LBRs in couples experiencing implantation failure or recurrent pregnancy loss (CitationASRM 2008a).
PGS technology has developed very rapidly since these early papers were published with testing now ranging from polar body biopsy (CitationMagli et al., 2011) to TE biopsy (CitationMcArthur et al., 2008). Improved screening techniques have seen successful validation of array comparative genomic hybridisation or aCGH for PGS (CitationMagli et al., 2011; CitationGeraedts et al., 2011; CitationGutiérrez-Mateo et al., 2011; CitationMamas et al., 2012) for comprehensive chromosome analysis of embryos. In a rapidly changing science, perhaps the most recent advance in PGS technology has been the development of next-generation sequencing or NGS (CitationYin et al., 2013; CitationWells et al., 2014).
Several RCTs have been published, mainly applying PGS to good prognosis patients (CitationYang et al., 2012; CitationScott et al., 2013; CitationRubio et al., 2013; CitationForeman et al., 2013). Before widespread use of this procedure for embryo selection, it needs to be determined which patients would benefit (CitationBolton et al., 2015).
Assisted hatching
A committee opinion for ASRM in 2008 did not support the universal application of assisted hatching (AH) in all IVF cycles but did support, at that time, the use of AH in those with two or more failed cycles of treatment, when the woman was aged over 38 years (CitationASRM, 2008b). A Cochrane review in 2012 including 31 RCTs showed that the CPR in women who underwent AH was marginally improved but the ‘take home baby rate’ was not significantly different (CitationCarney et al., 2012). In addition AH increases the rate of MZT (CitationHershlag et al., 1999; CitationSchieve et al., 2000; CitationLuke et al., 2014), a finding that needs to be taken into consideration when applying an eSET strategy, particularly when considering the increased incidence of MZT associated with blastocyst transfer (CitationKnopman et al., 2014).
Cryopreservation strategies
Whilst the original ACE/BFS eSET guideline (CitationCutting et al., 2008) focussed on fresh embryo transfer the importance of implementing an effective strategy incorporating serial SET of cryopreserved embryos should not be underestimated. Cryopreservation is a well-established, routine component of a typical IVF cycle. An effective cryo-programme is essential to maximise the cumulative pregnancy rates, which can be achieved with eSET, irrespective of the day of fresh transfer (CitationGlujovsky et al., 2012).
Several authors have compared outcomes of eSET and DET frozen embryo replacement cycles. For example, CitationYanaihara et al. (2008) published data from 470 patients receiving vitrified blastocysts transferred in either SET or DET procedures. The results showed similar CPRs for both groups, with a significant reduction in the incidence of ectopic pregnancy in the SET group and a significant increase in twinning in the DET group. CitationIshihara et al. (2011) supported the use of frozen–thawed blastocyst transfer in reducing the risk of ectopic pregnancy. There is however conflicting evidence on the use of eSET in frozen cycles based on pregnancy outcome in the initial fresh cycle. For example, CitationEl-Toukhy et al. (2003) demonstrated a two-fold increase in implantation rate and an increased CPR from the transfer of cryo-thawed cleavage-stage embryos obtained from successful fresh treatment cycles compared with those obtained from cycles which failed. By contrast, a study of 243 frozen blastocyst transfer or FBT cycles by CitationBerin et al. (2011) indicated that fresh cycle outcome had no influence on the outcome of a subsequent frozen cycle.
A growing body of evidence from studies on FET cycles indicates that endometrial receptivity is impaired following ovarian stimulation, a phenomenon which is not observed when undergoing a natural frozen embryo replacement cycle (CitationShapiro et al., 2011). A matched-cohort comparison of SET patients in fresh and FET cycles showed that overall the on-going CPR was significantly greater in the frozen–thawed group than the fresh group, for both Day 5 and Day 6 blastocysts (CitationShapiro et al., 2013). Further such evidence is provided by CitationRoque et al. (2013) and CitationCohen and Alikani (2013). Overall, the data suggest that clinics should consider both the timing of cryopreservation and the possible negative effect of ovarian stimulation on endometrial receptivity associated with fresh cleavage-stage embryo or blastocyst transfer during a stimulated treatment cycle.
Provision of a safe, effective IVF programme is paramount. A recent meta-analysis by CitationMaheshwari et al. (2012) reported that the risks of perinatal mortality, low birth weight, pre-term birth and antepartum haemorrhage are lower in pregnancies resulting from cryopreserved embryos when compared with pregnancies from fresh embryo transfers. Significantly improved neonatal outcomes were observed in a study comparing SET of vitrified-warmed blastocysts to fresh transfers (CitationRoy et al., 2014). Although LBRs were similar, improvements were also seen for gestational age and birth weight. This is important, since ovarian stimulation in fresh cycles may be one contributor to underlying low birth weight in IVF babies (CitationSchieve et al., 2002; CitationImudia et al., 2012), and correspondingly, FETs might show improved perinatal outcomes (CitationImudia et al., 2013). In particular, research should be directed at the routine use of cryopreservation in clinical IVF treatment, including assessments of efficacy, safety and cost-effectiveness. Although outcomes appear improved for FETs, greater understanding and research into modifiable risk factors during the process is also required to reduce disparity between the outcome of ART and non-ART infants (CitationHansen & Bower, 2014). Long-term follow-up studies of children would also be beneficial to evaluate the outcome of such strategies.
As evidence gathers in support of FET as a front-line treatment, significant developments have been observed in cryopreservation protocols. There is increasing usage of vitrification worldwide (CitationBrison et al., 2012) particularly for blastocyst cryopreservation, with several studies reporting high survival rates and implantation rates for embryos and blastocysts (CitationEdgar & Gook, 2012). Whilst evidence suggests that cleavage-stage embryos can be cryopreserved equally effectively using slow cooling and vitrification, blastocyst survival rates seem consistently higher with vitrification (CitationEdgar & Gook, 2012). The ACE consensus meeting (CitationBrison et al., 2012) and the ALPHA consensus meeting (CitationALPHA, 2012) highlighted the lack of well-designed prospective studies to determine whether one method should be preferred clinically to the other and stressed the need for further research to optimise process and methodology with respect to outcome and safety.
Whichever method is used for cryopreservation and thawing, it is recommended that centres should continually audit their performance and develop their MBR strategy to include FET cycles.
Recommendations – Cryopreservation strategies
An effective eSET strategy should include both fresh and subsequent frozen cycles, i.e. a ‘full cycle’ of treatment. (A)
Clinics should optimise cryopreservation methodology to ensure good survival rates and LBRs are maintained throughout a full cycle of treatment. (B)
Prospective RCTs are required to evaluate the efficacy of different cryopreservation strategies when adopted in an eSET programme. (B)
Long-term follow-up studies of children following treatment involving the use of frozen embryos and blastocysts are required. (B)
Further research is required to investigate the highly variable results obtained from frozen treatment cycles in different IVF centres. (C)
Further research is required into the efficacy of the ‘freeze all, replace later’ strategy. (C)
Patient education
A review of patients’ views on eSET was carried out by CitationLeese and Denton (2010), and CitationRoberts et al. (2010b) presented further research into UK patient perspectives. Many patients perceive patient selection (be it mandatory or voluntary) included in an SET programme as unfair and view the risk of twins from DET as acceptable within the boundaries of their own personal circumstances. The requirement for additional treatment cycles to achieve the same probability of success is regarded by some patients as physically and emotionally burdensome, and to some extent unnecessary. Inadequate patient education has often been held responsible for couples rejecting a clinical recommendation in favour of eSET, and even when fully informed of the available options surrounding eSET, many patients would still opt for DET. That said, it is encouraging that although some patients still expressed a desire for twins rather than singletons, the evidence suggested that eSET could become increasingly accepted especially if success rates approached or improved upon those of DET. CitationRai et al. (2011) concluded that the wish to have DET was driven by a desire to achieve a pregnancy and the perceived increase in success when replacing two embryos. Providing couples with more information about the potential risks of DET is an effective way to change their attitude towards eSET. It was reported that advice from the patient's clinician strongly influenced their decision regarding the number of embryos to replace. The paper also highlighted that women failed to recognise the social impact of a twin birth, suggesting that promoting the effectiveness of SET through emphasising tangible benefits such as improved long-term offspring health would be beneficial. Clearly patients need robust, detailed information to help them make an informed decision about eSET. CitationHope et al. (2008) showed that by exposing patients to information via an educational DVD on the outcomes and risk of twin pregnancies they were much more likely to accept eSET compared with those provided with standard reading material alone.
Recommendations – Patient education
The desire for patients to have more than one child needs to be taken into consideration and eSET policies and funding should allow for the transfer of all embryos of suitable quality regardless of earlier outcome if eSET is not to be perceived as disadvantageous. (B)
Multiple birth reduction strategies
UK clinics are required to have a MBR strategy and to monitor and audit this strategy. As evidence emerges on the issues of embryo selection and cryopreservation technologies, it is clearly necessary to audit and revise this strategy regularly. However as multiple births are a relatively rare event in any single clinic the absolute numbers of twins in a given audit period of 12 months or less will be too low to reveal anything but the most gross departures from a target value. Thus a more rounded, multifactorial approach to audit and review is required which examines the policy as implemented compared with the best practice in the sector. Such a review will include the selection of patients for DET, particularly the use of policy exceptions and discretionary areas, the use of cryopreservation, and patients’ views on acceptability.
Recommendations – Multiple birth reduction strategies
The individualisation of an eSET algorithm based solely on MBRs in an individual clinic is not recommended. Clinics should review their policies in the light of best practice determined across the sector. (GPP)
Centres should avoid basing decisions to change practice on small numbers of events within the normal range of statistical variation. (GPP)
Centres committed to eSET who are working towards achieving, or have attained and continue to maintain, a low MBR should take steps to evaluate the effectiveness of their eSET algorithm with respect to the timing of transfer, the selection of embryos for transfer and their cryopreservation strategy. (GPP)
Although there is evidence of a sustained reduction in the UK MBR it is imperative that centres audit their own policies and practice at least annually with the long-term aim of continually and sustainably reducing their MBR over time. Audit should focus on ensuring good practice rather than responding to small numbers of multiple births above or below a target rate. (GPP)
Cost implications and commissioning considerations
Multiple pregnancies are associated with a broad range of complications for both mother and child which in turn lead not only to higher antenatal and postnatal care costs but potential lifelong costs due to requirements for both medical and social support. The cost-effectiveness of implementing an eSET strategy has been comprehensively analysed in many different countries. Early work by CitationGerris et al. (2004) showed, in a non-randomised study, that the transfer of a top-quality embryo was associated with significantly lower costs in women under 38 years during their first cycle. This observation was confirmed by CitationKjellberg et al. (2006) with respect to the costs associated with complications of multiple pregnancy. Many studies support the cost-effectiveness of eSET especially when cumulative rates with frozen embryo replacement cycles are taken into consideration with CitationScotland et al., (2011) providing a UK perspective.
An attitude of responsibility must be adopted to ensure avoidable costs are limited through an eSET policy so burden is not placed on an already stretched NHS. This in turn may allow more effective allocation of already stretched and limited NHS funding resources to the commissioning of assisted conception treatment services.
Recommendations – Cost implications
Ensure commissioners are aware of the cost savings for both neonatal and long-term offspring care from adopting an eSET policy. (B)
Clinics should continue to support the campaign for NICE guidelines for funding provision and fair and equal provision of fertility treatment to all. (GPP)
The role of the HFEA
Achieving HFEA multiple birth targets
Since the HFEA established MBR limits in 2008, a significant shift in practice has been observed in favour of eSET, with the effect of reducing MBR in the vast majority of UK-licensed fertility centres. The key shifts in practice are summarised by the HFEA in their Multiple Births Year 4 Target Authority Paper (CitationDarby, 2012):
Since January 2008, the proportion of eSETs performed has increased across the sector. In 2008, 39,000 embryo transfers were performed, of which 1,919 (4.9 %) were eSET. In 2012, 50,219 embryo transfers were performed, of which 11,152 (22.2 %) were eSET.
Since 2008 there has been a steady increase in the proportion of embryos transferred at the blastocyst stage. This has increased from 8.7 % of all embryo transfers in January 2008, to 48.7 %, in December 2012.
MPR has decreased from 26.6 % in 2008 to 18.4 % in 2012. The decrease is most pronounced in women aged 18–34 years, who saw the greatest increase in eSET during this time.
MBR closely follows the MPR, showing a continuing decline between 2009 and 2013, most notably in women aged 18–34 years, summarised in .
Pregnancy and LBRs have remained broadly steady since the introduction of the eSET initiative in 2008 through to 2013.
Compliance with MBR recommendations
Since the introduction of the MBR limit in 2008–2009 and an annual reduction in the target MBR thereafter, it is clear that clinics continue to find difficulty in meeting the target rate given that the national MBR rate remains at 15 % (CitationHFEA, 2015).
Although the majority of clinics cannot be shown statistically to exceed the 15 % and 10 % target rates, the sector has still found compliance a challenge. The small numbers of twins seen in any one clinic mean that only a very few clinics are showing MBR rates which are statistically significantly above the target. The issue of how to deal with clinics that fail to meet the HFEA recommended MBR limit, some of which may well have adopted an eSET policy which is compliant with the best practice recommendations, remains a challenge for the sector. Conversely the low power to detect non-compliance based on individual clinic MBR implies that poor MBR reduction policies are unlikely to be detected by simple monitoring of outcomes. Only by working together can the sector and the HFEA ensure that best standards of patient safety and treatment efficacy are achieved in fertility centres across the UK and that the data currently published via the Choose a fertility Centre (CaFC) tool provide a fair and meaningful reflection of an individual clinic's performance irrespective of its size and treatment cycle throughput.
Reporting of treatment outcome results
The original ACE/BFS eSET guidance (CitationCutting et al., 2008) concluded that the HFEA reporting of results was not conducive to an eSET strategy and in the intervening period the situation has not much improved. That said, at the time of writing, the HFEA are undertaking a stakeholder review and consultation process on the future presentation of clinic results via CaFC, as part of the Information for Quality (IfQ) Programme. The intention is to highlight those clinics demonstrating an effective, safe eSET policy and to provide information to patients in a more meaningful, transparent way. A shift away from reporting LBR per cycle started towards LBR per embryo transferred and cumulative LBR from full cycles of treatment (including the transfer of fresh and subsequent frozen embryos) would improve the value of the data for patients trying to select a safe and effective treatment provider. There is also a need for more rounded provision of information on the outcomes of the component processes that make up an IVF treatment cycle. This is a clear priority area for research as this is the first time the UK will have changed the method of results presentation for more than 20 years.
Recommendations - HFEA
The HFEA should make changes to the way clinic data are reported. Clinic outcome data should be represented as LBR per embryo transferred and as cumulative LBR from fresh and FETs, which constitute a ‘full cycle’ of treatment. (C)
Future amendments to the way the HFEA present their ‘Choose a Fertility Clinic’ data to the public should highlight those clinics whose MBR is and remains low as being good examples of safe and effective practice. (C)
Further research is required into appropriate outcome measures which capture the complexity of the IVF process and their presentation. (C)
Multiple pregnancy is one of the most significant risks of fertility treatment and is to some extent avoidable. Fertility centres must continue to show a sustained commitment to reduce their MBR year-on-year to its lowest achievable value. (A)
Summary of Recommendations
Recommendations for fertility clinics
There should be a continued focus on minimising MBRs from IVF treatment by utilising an effective, dynamic eSET strategy. (GPP)
In situations where embryo quality is poor, clinics should consider the transfer of two embryos. An effective eSET policy should accommodate this. (B)
Centres should audit eSET rates, selection criteria and MBRs and where their dataset is robust, consider adjusting their eSET algorithm in line with their results on at least an annual basis, focussing on good practice rather than small numbers of multiple births above or below a target rate. (GPP)
Maximising oocyte and embryo quality is an essential part of an effective eSET strategy; a focus should remain on optimising superovulation regimens. (A)
Patients should be counselled that current best evidence indicates that blastocyst stage transfer may result in a successful outcome sooner, but with some specific increased risks, and may ultimately lead to a reduction in the overall chance of success. (A)
An effective eSET strategy should include both fresh and subsequent frozen cycles, i.e. a ‘full cycle’ of treatment. (A)
Clinics should optimise cryopreservation methodology to ensure good survival rates and LBRs are maintained throughout a full cycle of treatment. (B)
The desire for patients to have more than one child needs to be taken into consideration and eSET policies and funding should allow for the transfer of all embryos of suitable quality regardless of earlier outcome if eSET is not to be perceived as disadvantageous. (B)
The individualisation of an eSET algorithm based solely on MBRs in an individual clinic is not recommended. Clinics should review their policies in the light of best practice determined across the sector. (GPP)
Ensure commissioners are aware of the cost savings for both neonatal and long-term offspring care from adopting an eSET policy. (B)
Clinics should continue to support the campaign for NICE guidelines for funding provision and fair and equal provision of fertility treatment to all. (GPP)
The HFEA should make changes to the way clinic data are reported. Clinic outcome data should be represented as LBR per embryo transferred and as cumulative LBR from fresh and FETs, which constitute a ‘full cycle’ of treatment. (C)
Future amendments to the way the HFEA present their ‘Choose a Fertility Clinic’ data to the public should highlight those clinics whose MBR is and remains low as being good examples of safe and effective practice. (C)
Multiple pregnancy is one of the most significant risks of fertility treatment and is to some extent avoidable. Fertility centres must continue to show a sustained commitment to reduce their MBR year-on-year to its lowest achievable value. (A)
Recommendations for the management and review of clinic eSET algorithm
Where blastocyst transfer is performed, clinics should review their eSET data and criteria for extended embryo culture regularly. (GPP)
Centres with the resources to support time-lapse morphometric analysis may use this information to improve their eSET algorithm. (GPP)
Centres should avoid basing decisions to change practice on small numbers of events within the normal range of statistical variation. (GPP)
Centres committed to eSET who are working towards achieving, or have attained and continue to maintain, a low MBR should take steps to evaluate the effectiveness of their eSET algorithm with respect to the timing of transfer, the selection of embryos for transfer and their cryopreservation strategy. (GPP)
Although there is evidence of a sustained reduction in the UK MBR it is imperative that centres audit their own policies and practice at least annually with the long-term aim of continually and sustainably reducing their MBR over time. Audit should focus on ensuring good practice rather than responding to small numbers of multiple births above or below a target rate. (GPP)
Research recommendations
Further research is required (a) to validate the efficacy of eSET policies and (b) confirm the long-term safety of the culture and cryopreservation processes that constitute such policies. (C)
Prospective studies are required to determine the impact of embryo culture to the blastocyst stage on MZT, pre-term birth and associated epigenetic changes and on why MZT appears to be more prevalent in some clinics. (B)
Technological advances including time-lapse imaging and morphometric assessment show promise, as embryo selection and de-selection tools, but further high-quality prospective studies are required to develop and validate robust algorithms followed by RCTs to demonstrate their role in an eSET strategy. (C)
Further development and evaluation is required of the effectiveness of selecting embryos based on metabolic or related traits. (C)
Prospective RCTs are required to evaluate the efficacy of different cryopreservation strategies when adopted in an eSET programme. (B)
Long-term follow-up studies of children following treatment involving the use of frozen embryos and blastocysts are required. (B)
Further research is required to investigate the highly variable results obtained from frozen treatment cycles in different IVF centres. (C)
Further research is required into the efficacy of the ‘freeze all, replace later’ strategy. (C)
Further research is required into appropriate outcome measures, which capture the complexity of the IVF process and their presentation. (C)
Acknowledgements
The authors would like to thank the members of ACE and the BFS involved in reviewing this paper pre-submission, and Henry Leese and Allan Pacey for their review of this paper prior to final submission. We would also like to thank Suzanne Hodgson from the HFEA for providing us with the most up-to-date relevant national information available at the time of submission.
Declaration of interest: None
References
- Ahlstrom, A., Westin, C., Reismer, E., Wikland, M., & Hardarson, T. (2011). Trophectoderm morphology: An important parameter for predicting live birth after single blastocyst transfer. Human Reproduction, 26, 3289–3296.
- Ahmad, K., Goorbarry, J., Cho, M., Loughlin, J., & McCulloh, D.H. (2008). Z scores of embryos at the pronuclear stage are not predictive of IVF pregnancy outcome. Fertility & Sterility, 90, S430.
- Alikani, M., Tomkin, G., Ferry, K., & Garrisi, M. (2008). High pregnancy loss rate following implantation of IVF human embryos with extensive fragmentation. Fertility & Sterility, 92, S83.
- Al-Inany, H. & Aboulghar, M. (2002). GnRH Antagonist in assisted reproduction: a Cochrane review. Human Reproduction, 17, 874–885.
- Al-Inany, H., Youssef, M.A.F.M., Aboulghar, M., Broekmans, F., Sterrenburg, M., Smit, J., & Abou-Setta, A.M. (2011). GnRH antagonists are safer than agonists: an update of a Cochrane review. Human Reproduction Update, 17, 435–436.
- Alpha Scientists in Reproductive Medicine, ESHRE Special Interest Group in Embryology. (2011). Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reproductive BioMedicine Online, 22, 632–646.
- Alpha Scientists in Reproductive Medicine. (2012). The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reproductive BioMedicine Online, 25, 146–167.
- Ambroggio, J., Gindoff, P.R., Molina, B., Dayal, M., Khaldi, R., Peak, D., et al. (2011). Multinucleation of a sibling blastomere on day 2 suggests unsuitability for embryo transfer in IVF – pre-implantation genetic screening cycles. Fertility & Sterility, 96, 856–859.
- Antezak, M. & Van Blerkom, J. (1999). Temporal and spatialects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Human Reproduction, 14, 429–447.
- Aparicio, B., Cruz, M., & Mesegeur, M. (2013). Is Morphokinetic Analysis the Answer? Reproductive BioMedicine Online, 27, 654–63.
- Armstrong, S., Vail, A., Mastenbroek, S., Jordan, V., & Farquhar, C. (2015). Time-lapse in the IVF-lab: how should we assess potential benefit? Human Reproduction, 30, 3–8.
- ASRM. (2008a). Practice Committee of the Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Pre-implantation genetic testing: a Practice Committee opinion. Fertility & Sterility, 90, S136–143.
- ASRM. (2008b). Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. The role of assisted hatching in in vitro Fertilisation: A review of the literature. A Committee opinion. Fertility & Sterility, 90, S196–S198.
- ASRM. (2012). Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Elective Single Embryo Transfer. Fertility & Sterility, 97, 835–842.
- ASRM. (2013a). Practice Committee of American Society for Reproductive Medicine; Practice Committee of Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertility & Sterility, 99, 44–46.
- ASRM. (2013b). Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Blastocyst culture and transfer in clinical-assisted Reproduction: a committee opinion. Fertility & Sterility, 99, 667–672.
- Baart, E., Macklon, N., & Fauser, B.J.C.M. (2009). Ovarian Stimulation and Embryo Quality. Reproductive BioMedicine Online, 18, S45–50.
- Balaban, B., Urman, B., Sertac, A., Alatas, C., Aksoy, S., & Mercan, R. (1998). Oocye morphology does not affect fertilitisation rate, embryo quality and implantation rate after ICSI. Human Reproduction, 13, 3431–3433.
- Balaban, B., Urman, B., Sertac, A., Alatas, C., Aksoy, S., & Mercan, R. (2000). Blastocyst quality affects the success of blastocyst stage transfer. Fertility & Sterility, 74, 282–287.
- Balaban, B. & Magli, C.M. (2011). The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human Reproduction, 26, 1270–1283.
- Berin, I., McLellan, S.T., Macklin, E.A., Toth, T.L., & Wright, D.L. (2011). Frozen- thawed embryo transfer cycles: clinical outcomes of single and double blastocyst transfer. Journal of Assisted Reproduction & Genetics, 28, 575–581.
- Bissonnette, F., Phillips, S.J., Gunby, J., Holzer, H., Mahutte, N., St-Michel, P., & Kadoch, I.J. (2011). Working to eliminate multiple pregnancies: a success story in Quebec. Reproductive Biomedicine Online, 23, 500–504.
- Bjorndahl, L., Barratt, C., Fraser, L., Kvist, U., & Mortimer, D. (2002). ESHRE basic semen analysis course 1995–1999: Immediate beneficial effects of standardized training. Human Reproduction, 17, 1299–1305.
- Bolton, V.N., Leary, C., Harbottle, S., Cutting, R., & Harper, J.C. (2015). How should we choose the ‘best’ embryo? A commentary on behalf of the British Fertility Society and the Association of Clinical Embryologists. Human Fertility18, 156–164.
- Brison, D.R., Houghton, F.D., Falconer, D., Roberts, S.A., Hawkhead, J.A., Humpherson, P.G., et al. (2004). Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Human Reproduction, 19, 2319–2324.
- Brison, D., Hollywood, K., Arneson, R., & Goodacre, R. (2007). Predicting Human embryo viability: the road to non-invasive analysis of the secretome using metabolic footprinting. Reproductive Biomedicine Online, 15, 296–302.
- Brison, D., Cutting, R., Clarke, H., & Wood, M. (2012). ACE consensus meeting Reproduction: oocyte and embryo cryopreservation Sheffield. Human Fertility, 15, 69–74.
- Carney, S.K., Das, S., Blake, D., Farquhar, C., Seif, M.M., & Nelson, L. (2012). Assisted hatching on assisted conception (in-vitro Fertilitization IVF) and intra-cytoplasmic sperm injection (ICSI). Cochrane Database of Systematic Reviews, Issue 12 Art. No: CD001894 DOI: 10.1002/14651858.CD001894.pub5.
- Chang, H.J., Lee, J.R., Jee, B.C., Suh, C.S., & Kim, S.H. (2009). Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rat: a systematic review and meta-analysis. Fertility & Sterility, 91, 2381–90.
- Clua, E., Tur, R., Coroleu, B., Boda, M., Rodriguez, I., Barri, P.N., & Viega, A. (2012)Elective single-embryo transfer in oocyte donation programmes: Should it be the rule? Reproductive BioMedicine Online, 25, 642–648.
- Cohen, J. & Alikani, M. (2013). The time has come to radically rethink assisted reproduction. Reproductive BioMedicine Online, 27, 323–324.
- Cutting, R., Morroll, D., Roberts, S., Pickering, S., & Rutherford, A. (on behalf of BFS and ACE). (2008). Elective Single Embryo Transfer: Guidelines for Practice British Fertility Society and Association of Clinical Embryologists. Human Fertility, 11, 131–146.
- Darby, H. (2012). Authority Committee Paper on the Year 4 multiple births target. http://www.hfea.gov.uk/Multiple-births-after-IVF.html. Last accessed 13th May 2015.
- de los Santos, M.J., Arroyo, G., Busquet, A., Calderon, G., Cuadros, J., Hurtado de Mendoza, M.V., et al. (2014). A multi-centre prospective study to assess the effect of early cleavage on embryo quality, implantation, and live-birth rate. Fertility & Sterility, 101, 981–987.
- Desai, N.N., Goldstein, J., Rowland, D.Y., & Goldfarb, J.M. (2000). Morphological evaluation of Human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Human Reproduction, 15, 2190–2196.
- Dessolle, L., Freour, T., Ravel, C., Jean, M., Colombel, A., Darai, E., & Barriere, P. (2011). Predictive Factors of healthy term birth after single blastocyst transfer. Human Reproduction, 26, 1220–1226.
- Ebner, T. (2008). Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reproductive BioMedicine Online, 16, 1591–1597.
- Edgar, D.H. & Gook, D.A. (2012). A critical appraisal of cryopreservation (slow cooling versus vitrification) of Human oocytes and embryos. Human Reproduction Update, 18, 536–554.
- Edwards, R.G., Steptoe, P.C., & Purdy, J.M. (1980). Establishing full-term Human pregnancies using cleaving embryos grown in vitro. British Journal of Obstetretics & Gynaecology, 87, 737–756.
- El-Toukhy, T., Khalaf, Y., Al-Darazi, K., Andritsos, V., Taylor, A., & Braude, P. (2003).
- Effect of blastomere loss on the outcome of frozen embryo Replacement cycles. Fertility & Sterility, 79, 1106–1111.
- Foreman, E.J., Hong, K.H., Ferry, K.M., Tao, X., Taylor, D., Levy, B., et al. (2013). In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertility & Sterility, 100, 100–107.
- Gardner, D.K., Schoolcraft, W.B., Wagley, L., Schlenker, T., Steens, J., & Hesla, J. (1998). A prospective randomized trial of blastocyst culture and transfer in in vitro Fertilisation. Human Reproduction, 13, 3434–3440.
- Gardner, D.K. & Schoolcraft, W.B. (1999). Culture and transfer of human blastocyst. Current opinion in Obstetrics and Gynaecology, 11, 307–311.
- Gardner, D., Lane, M., Stevens, J., Schenkler, T., & Schoolcraft, M. (2000). Blastocyst scores affects implantation and pregnancy outcome: towards single blastocyst transfer. Fertility & Sterility, 73, 1555–1558.
- Gardner, D., Lane, M., Stevens, J., & Schoolcraft, M. (2001). Non invasive assessment of human embryo nutrient consumption is a measure of developmental potential. Fertility & Sterility, 73, 1175–1180.
- Gardner, D.K. & Wale, P.L. (2013). Analysis of metabolism to select viable human embryos for transfer. Fertility & Sterility, 99, 1062–1072.
- Geraedts, J., Montag, M., Magli, M.C., Repping, S., Handyside, A., Staessen, C., et al. (2011). Polar body array CGH for prediction of the status of the corresponding oocyte. I. Clinical results. Human Reproduction, 26, 3173–3180.
- Gerris, J., De Sutter, P., De Neubourg, D., Van Royen, E., Vander Elst, J., Mangelschots, K., et al. (2004). A real-life prospective health economic study of elective single embryo transfer versus two-embryo transfer in first IVF/ICSI cycles. Human Reproduction, 19, 917–923.
- Giorgetti, C., Hans, E., Terriou, P., Salzmann, J., Barry, B., Chabert-Orsini, V., et al. (2007). Early cleavage: an additional predictor of high implantation rate following elective single embryo transfer. Reproductive BioMedicine Online, 14, 85–91.
- Gleicher, N. (2013). The Irrational Attraction of Elective single-embryo Transfer (eSET). Human Reproduction, 28, 294–297.
- Glujovsky, D., Blake, D., Farquhar, C., & Bardach, A. (2012). Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews, CD002118.
- Goto, S., Kadowaki, T., Tanaka, S., Hashimoto, H., Kokeguchi, S., & Shiotani, M. (2011). Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertility & Sterility, 95, 948–952.
- Gremeau, A.S., Brugnon, F., Bouraoiu, Z., Pekrishvili, R., Janny, L., & Pouly, J.L. (2012). Outcome and feasibility of elective single embryo transfer eSET policy for the first and second IVD/ICSI attempts. European Journal of Obstetric and Gynaecological Reproductive Biology, 160, 45–50.
- Gutiérrez-Mateo, C., Colls, P., Sanchez-Garcia, J., Escudero, T., Prates, R., Ketterson, K., et al. (2011). Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertility & Sterility, 95, 953–958.
- Hansen, M., Kurinczuk, J., Bower, C., & Webb, S. (2002). The risk of major birth defects after intracytoplasmic sperm injection and in vitro Fertilisation. New England Journal of Medicine, 346, 725–730.
- Hansen, M. & Bower, C. (2014). The impact of assisted Reproductive technologies on intra-uterine growth and birth defects in singletons. Seminars in Fetal and Neonatal Medicine, 19, 228–233.
- Hardarson, T., Hanson, C., Sjogren, A., & Lundin, K. (2001). Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indication for aneuploidy and multi-nucleation. Human Reproduction, 16, 313–318.
- Hardarson, T., Ahlstom, A., Rogberg, L., Botros, L., Hillensjo, T., Westlander, G., et al. (2012). Non-invasive metabolomics profiling of Day 2 and Day 5 embryo culture medium: a prospective randomized trial. Human Reproduction, 27, 89–96.
- Harper, J., Coonen, E., De Rycke, M., Fiorentino, F., Geraedts, J., Goossens, V., et al. (2010). What next for pre-implantation genetic screening (PGS)? A position statement from the ESHRE PGD Consortium Steering Committee. Human Reproduction, 25, 821–823.
- Hashimoto, S., Kato, N., Saeki, K., & Morimoto, Y. (2012). Selection of high-potential embryos by culture in poly (dimethylsiloxane) microwells and time-lapse imaging. Fertility & Sterility, 97, 332–337.
- Heijnen, E.M., Eijkemans, M.J., DE Klerk, C., Polinder, S., Beckers, N.G., Klinkert, E.R., et al. (2007). A mild treatment strategy for in-vitro Fertilisation: a randomized non-inferiority trial. Lancet, 369, 743–749.
- Heitmann, R.J., Hill, M.J., Richter, K.S., Decherney, A.H., & Widra, E.A. (2013). The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. Journal of Assisted Reproduction and Genetics, 30, 563–567.
- Herrero, J., Tejera, A., Albert, C., Vidal, C., de los Santos, M, Meseguer M. (2013). A time to look back: analysis of morphokinetic characteristics of Human embryo development. Fertility & Sterility, 100, 1602–09.
- Hershlag, A., Paine, T., Cooper, G.W., Scholl, G.M., Rawlinson, K., & Kvapil, G. (1999). Monozygotic twinning associated with mechanical assisted hatching. Fertility & Sterility, 71, 144–6.
- HFEA. (2008). Chairs Letter, Multiple Births, Single Embryo Transfer policy. Available online at http://www.hfea.gov.uk/489.html. Last accessed 14th June 2015.
- HFEA. (2013). Chairs Letter, Removal of the licence condition relating to multiple births. Available online at http://www.hfea.gov.uk/8380.html. Last accessed 14th June 2015.
- HFEA. (2014). Fertility treatment in 2013: trends and figures. Available online at http://www.hfea.gov.uk/9463.html. Last accessed 14th June 2015.
- HFEA. (2015). Improving outcomes for fertility patients: Multiple Births 2015. Available online at http://www.hfea.gov.uk/docs/Multiple_Births_Report_2015.pdf. Last accessed 22nd July 2015.
- Hill, M.J., Richter, K.S., Heitman, R.J., Graham, J.R., Tucer, M.J., DeVherney, A.H., et al. (2013). Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertility & Sterility, 99, 1283–1289.
- Hope, N.J., Philips, S.J., & Rombauts, L. (2008). Can an educational DVD improve the acceptability of elective single embryo transfer?: a randomized controlled study. Fertility & Sterility, 90, Suppl, S67.
- Houghton, F.D., Hawkhead, J., Humpherson, P.G., Hogg, J., Balen, A.H., Rutherford, A.J., & Leese, H.J. (2002). Non invasive amino acid turnover predicts Human embryo developmental capacity. Human Reproduction, 17, 999–1005.
- Imudia, A., Awonuga, A., Doyle, J.O., Kaimal, A., Wright, D.L., Toth, T.L., & Styer, A.K. (2012). Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilisation. Fertility & Sterility, 97, 1374–1379.
- Imudia, A., Awonuga, A., Doyle, J.O., Kaimal, A., Wright, D.L., Styer, A.K., & Toth, T.L. (2013). Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertility & Sterility, 99, 168–173.
- Ishihara, O., Kuwahara, M.D., & Saitoh, H. (2011). Frozen thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertility & Sterility, 95, 1966–1969.
- James, A.N., Hennessy, S., Reggio, B., Weimer, K., Larsen, F., & Cohen, J. (2006). The limited importance of pronuclear scoring of Human zygotes. Human Reproduction 21, 1599–1604.
- Johansson, M., Hardarson, T., & Lundin, K. (2003). There is a cut off limit in diameter between a blastomere and a small anucleate fragment. Journal of Assisted Reproduction and Genetics, 20, 309–313.
- Kallen, B., Finnstrom, O., Lindam, A., Nilsson, E., Nygren, K.G., & Olausson, P.O. (2010a). Blastocyst versus cleavage stage transfer in vitro Fertilisation; differences in neonatal outcome? Fertility & Sterility, 94, 1860–1863.
- Kallen, B., Finnstrom, O., Lindam, A., Nilsson, E., Nygren, K.G., & Olausson, P.O. (2010b). Trends in delivery and neonatal outcome after in vitro Fertilisation in Sweden: data for 25 years. Human Reproduction, 25, 1026–1034.
- Kjellberg, A.T., Carlsson, P., & Bergh, C. (2006). Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Human Reproduction, 21, 210–216.
- Knopman, J.M., Krey, L.C., Oh, C., Lee, J., McCaffrey, C., & Noyes, N. (2014). What makes them split? Identifying risk factors that lead to monozygotic twins after in vitro fertilisation. Fertility & Sterility, 102, 82–89.
- Kultursay, N., Yalaz, M., & Koroglu, O.A; MAR Neonatal Study Group. (2015). Neonatal outcome following new assisted reproductive technology regulations in Turkey - a nationwide multicenter point prevalence study. Journal of Maternal and Fetal Neonatal Medicine, 28, 204–209. doi: 10.3109/14767058.2014.908844.
- Kutlu, P., Atvar, O., Vanlioglu, O.F., Kutlu, U., Arici, A., Yilmaz, E., et al. (2011). Effect of the new legislation and single-embryo transfer policy in turkey on assisted reproduction outcomes: preliminary results. Reproductive Biomedicine Online, 22, 208–14.
- Lawlor, D.A. & Nelson, S.M. (2012). Effect of age on decisions about the numbers of embryos to transfer in assisted conception: a prospective study. Lancet, 379, 521–527.
- Leese, B. & Denton, J. (2010). Attitudes towards single embryo transfer, twin and higher order pregnancies in patients undergoing infertility treatment: a review. Human Fertility, 13, 28–34.
- Leese, H.J. & Barton, A.M. (1984). Pyruvate and glucose uptake by mouse ova and preimplantation embryos. Journal of Reproduction & Fertility, 72, 9–13.
- Leese, H.J. (2002). Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays, 24, 845–849.
- Leese, H.J. (2012). Metabolism of the preimplantation embryo: 40 years on. Reproduction, 143, 417–427.
- Legro, R.S., Wong, I.L., Paulson, R.J., Lobo, R.A., Sauer, M.V. (1995). Recipients age does not adversely affect pregnancy outcome after oocyte donation. American Journal of Obstetretics & Gynaecology, 172, 96–100.
- Lemmen, J.G., Agerholm, I., & Ziebe, S. (2008). Kinetic markers of Human embryo quality using time-lapse recordings of IVF/ICSI-Fertilityized oocytes. Reproductive BioMedicine Online, 17, 385–391.
- Lopes, A.S., Madsen, S.E., Ramsing, N.B., Løvendahl, P., Greve, T., & Callesen, H. (2007). Investigation of respiration of individual bovine embryos produced in vivo and in vitro and correlation with viability following transfer. Human Reproduction, 22, 558–566.
- Lucas, E. (2013). Epigenetic effects on the embryo as a result of periconceptional environment and assisted reproductive technology. Reproductive BioMedicine Online, 27, 477–485.
- Lukassen, H.G., Braat, D.D., Wetzels, A.M., Zielhuis, G.A., Adang, E.M., Scheenjes, E., & Kremer, J.A. (2005). Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial. Human Reproduction, 20, 702–708.
- Luke, B., Brown, M.B., Wantman, E., & Stern, J.E. (2014). Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles. Fertility & Sterility, 101, 683–689.
- Machtinger, R. & Racowsky, C. (2013). Morphological systems of human embryo assessment and clinical evidence. Reproductive BioMedicine Online, 26, 210–221.
- Magli, M.C., Montag, M., Köster, M., Muzi, L., Geraedts, J., Collins, J., et al. (2011). Polar body array CGH for prediction of the status of the corresponding oocyte. Part II: Technical aspects. Human Reproduction, 26, 3181–3185.
- Maheshwari, A., Griffiths, S., & Bhattacharya, S. (2011). Global variation in the uptake of single embryo transfer. Human Reproduction Update, 17, 107–120.
- Maheshwari, A., Pandey, S., Shetty, A., Hamilton, M., & Bhattacharya, S. (2012). Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilisation treatment: a systematic review and meta-analysis. Fertility & Sterility, 98, 368–377.
- Maheshwari, A., Kalampokas, T., Davidson, J., & Bhattacharya, S. (2013). Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst stage versus cleavage stage embryos generated through in vitro fertilisation: a systematic review and meta-analysis. Fertility & Sterility, 100, 1615–1621.
- Mamas, T., Gordon, A., Brown, A., Harper, J., & Sengupta, S. (2012). Detection of aneuploidy by array comparative genomic hybridization using cell lines to mimic a mosaic trophectoderm biopsy. Fertility & Sterility, 97, 943–947.
- Mastenbroek, S., Twisk, M., van der Veen, F., & Repping, S. (2011). Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Human Reproduction Update, 17, 454–466.
- Mastenbroek, S. (2013). One swallow does not make a summer. Fertility & Sterility, 99, 1205–1206.
- Mathur, R.S., & Tan, B.K. (2014)British Fertility Society Policy and Practice Committee: prevention of ovarian hyperstimulation syndrome. Human Fertility, 17, 257–268.
- McArthur, S.J., Leigh, D., Marshall, J.T., Gee, A.J., De Boer, K.A., & Jansen, R.P. (2008). Blastocyst trophectoderm biopsy and preimplantation genetic diagnosis for familial monogenic disorders and chromosomal translocations. Prenatal Diagnosis, 28, 434–442.
- McLernon, D.J., Harrild, K., Bergh, C., Davies, M.J., de Neubourg, D., Dumoulin, J.C.M., et al. (2010). Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomized trials. British Medical Journal, 342, 34–35.
- Moayeri, S, Beht, B., Lathi, R., Westphal LM, Milki AA. (2007). Risk of monozygotic twinning with blastocyst transfer decreases over time: an 8 year experience. Fertility & Sterility, 87, 1028–32.
- Montag, M., Liebenthron, J., & Koster, M. (2011). Which morphological scoring system is relevant in Human development? Placenta, 32, S252–S256.
- Montag, M., Toth, B., & Strowitzki, T. (2013). New approaches to embryo selection. Reproductive BioMedicine Online, 27, 539–546.
- Moustafa, M.K., Sheded, S.A., El Aziz Mousta, M.A. (2008). Elective single embryo transfer versus double embryo transfer in assisted Reproduction. Reproductive BioMedicine Online, 17, 82–87.
- Mullin, C., Berkeley, A.S., & Grifo, J.A. (2012). Supernumerary blastocyst cryopreservation: a key prognostic indicator for patients opting for an elective single blastocyst transfer (eSBT). Journal of Assisted Reproduction and Genetics, 29, 783–788.
- Nagy, Z.P., Dozortsev, D., Diamond, M., Rienzi, L., Ulbaldi, F., Abdelmassih, R., & Greco, E. (2003). Pronuclear morphology evaluation with subsequent evaluation of embryo morphology significantly increases implantation rates. Fertility & Sterility, 80, 67–74.
- National Institute for Health and Care Excellence. (2013). Fertility: assessment and treatment for people with Fertility problems. Clinical Guidelines CG156, http://www.nice.org.uk/guidance/cg156. Last accessed 13th May 2015.
- Nicoli, A., Valli, B., Di Girolamo, R., Tommaso, B., Gallinelli, A., & La Sala, G.B. (2007). Limited importance of pre embryo pronuclear morphology (zygote score) in assisted reproduction outcome in the absence of embryo cryopreservation. Fertility and Sterility, 88 (4 suppl.), 1167–1173.
- Nicoli, A., Capodanno, F., Rondini, I., Valli, B., Villani, M., Morini, D., et al. (2013). Pronuclear morphology evaluation in in vitro Fertilisation (IVF)/intra-cytoplasmic sperm injection (ICSI) cycles: a retrospective clinical review. Journal of Ovarian Reserve, 6, 1–6.
- Niinimaki, M., Suikari, A.M., Makinen, S., Soderstrom-Anttila, V., & Martikainen, H. (2013). Elective single embryo transfer in women aged 40–44 years. Human Reproduction, 28, 331–335.
- Otsuki, J., Okada, A., Morimoto, K., Nagi, Y., & Kubo, H. (2004). The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in Metaphase 2 Human oocytes. Human Reproduction, 19, 1591–1597.
- Pandian, Z., Bhattacharaya, S., Ozturk, O., Serour, G., & Templeton, A. (2009). Number of embryos for transfer following in-vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Systematic Review, 15, CD0003416.
- Papanikolaou, E.G., D'haeseleer, E., Verheyen, G., Vaan de Velde, H., Camus, M., Steirteghem, A., et al. (2005). Live birth rate is significantly better than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Human Reproduction, 20, 3198–3203.
- Papanikolaou, E.G., Camus, M., Kolibinakis, E.M., Van Landuyt, L., Van Steirteghem, A., & Devroey, P. (2006). In vitro Fertilisation with single blastocyst stage versus single cleavage stage embryos. New England Journal of Medicine, 354, 1139–46.
- Papanikolaou, E.G., Fatemi, H., Venetis, C., Donos, P., Kolibianakis, E., Tournaye, H., et al. (2010). Monozygotic twinning is not increased after single blastocyst transfer compared with single cleavage-stage embryo transfer. Fertility & Sterility, 93, 592–597.
- Park, C., Ahn, J., Yoon, Y., & Park, S. (2011). A multi-sample based method for identifying common CNVs in normal Human genomic structure using high-resolution aCGH data. PLoS One, 6, e26975.
- Pickering, S.J., Braude, P.R., Johnson, M.H., Cant, A., & Currie, J. (1990). Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the Human oocyte. Fertility & Sterility, 54, 102–108.
- Plachot, M., Grouchy, J., Junca, A.M., Mandlebaum, J., Salat-Baroux, J., & Cohen, J. (1998). Chromosomal analysis of human oocytes and embryos in an in vitro fertilisation program. Annals of the New York Academy of Sciences, 541, 384–397.
- Rai, V., Betswort, A., Beer, C., Ndukwe, G., & Glazebrook, C. (2011). Comparing patients’ and clinicians’ perceptions of elective single embryo transfer using the attitudes to a twin IVF pregnancy scale (ATIPS). Journal of Assisted Reproduction and Genetics, 28, 65–72.
- Renard, J.P., Phillipon, A., & Menezo, Y. (1980). In-vitro uptake of glucose by bovine blastocyst. Journal of Reproduction and Fertility, 58, 161–164.
- Rienzi, L., Ubaldi, F., Iacobelli, M., Ferrero, S., Minasi, M.G., Martinez, F., et al. (2002). Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Human Reproduction, 17, 1852–1855.
- Rienzi, L. (2005). Significance of morphological attributes of the early embryo. Reproductive BioMedicine Online, 10, 669–681.
- Roberts, S.A., Fitzgerald, C.T., & Brison, D.R. (2009). Modelling the impact of single embryo transfer in a national health service IVF programme. Human Reproduction, 24, 122–131.
- Roberts, S.A., Hirst, W.M., Brison, D.R., & Vail, A. (2010a). Embryo and uterine influences on IVF outcomes: an analysis of a UK multi-centre cohort. Human Reproduction, 25, 2792–2802.
- Roberts, S.A., McGowan, L., Hirst, W.M., Brison, D.R., Vail, A., & Lieberman, B.A. (2010b). Towards single embryo transfer? Modelling clinical outcomes of potential treatment choices using multiple data sources: predictive models and patient perspectives. Health Technology Assessment, (14), No. 38.
- Roberts, S.A., McGowan, L., Hirst, W.M., Vail, A., Rutherford, A., Lieberman, B.A., & Brison, D.A. (2011). Reducing the incidence of twins from IVF treatments: predictive modelling from a retrospective cohort. Human Reproduction, 26, 569–575.
- Roque, M., Lattes, K., Serra, S., Solà, I., Geber, S., Carreras, R., & Checa, M.A. (2013). Fresh embryo transfer versus frozen embryo transfer in in vitro fertilisation cycles: a systematic review and meta-analysis. Fertility & Sterility, 99, 156–162.
- Roy, T.K., Bradley, C.K., Bowman, M., & McArthur, S. (2014). Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertility & Sterility, 101, 1294–1301.
- Rubio, I., Kuhlmann, R., Agerholm, I., Kirk, J., Herrero, J., Escribá, M.J., et al. (2012). Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertility & Sterility, 98, 1458–1463.
- Rubio, C., Bellver, J., Rodrigo, L., Bosch, E., Mercader, A., Vidal, C., et al. (2013). Preimplantation genetic screening using fluorescence in situ hybridisation in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertility & Sterility, 99, 1400–1407.
- Rubio, I., Galan, A., Larreategui, Z., Ayerdi, F., Bellver, J., Herrero, J., & Meseguer, M. (2014). Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertility & Sterility, 102, 1287–1294.
- Schieve, L.A., Meikle, S.F., Peterson, H.B., Jeng, G., Burnett, N.M., & Wilcox, L.S. (2000). Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro Fertilisation? Fertility & Sterility, 74, 288–294.
- Schieve, L., Meikle, S.F., Ferre, C., Peterson, H.B., Jeng, G., & Wilcox, L.S. (2002). Low and very low birth weight in infants conceived with use of assisted Reproductive technology. New England Journal of Medicine, 346, 731–737.
- Scotland, G.S., McLernon, D., Kurinczuk, J.J., McNamee, P., Harrild, K., Lyall, H., et al. (2011). Minimising twins in in-vitro fertilitisation: a modelling study assessing the costs, consequences and cost-utility of elective single versus double embryo transfer over a 20-year time horizon. International Journal of Obstretrics & Gynaecology, 118, 1073–1083.
- Scott, L.A., Alvero, R., Leondires, M., & Miller, B. (2000). The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Human Reproduction, 15, 2394–2403.
- Scott, L., Berntsen, J., Davies, D., Gundersen, J., Hill, J., & Ramsing, N. (2008). Symposium: innovative techniques in human embryo viability assessment. Human oocyte respiration rate measurement potential to improve oocyte and embryo selection? Reproductive BioMedicine Online, 17, 461–469.
- Scott, R.T., Upham, K.M., Forman, E.J., Zhao, T., & Treff, N.R. (2013). Cleavage stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertility & Sterility, 100, 624–630.
- Seli, E., Sakkas, D., Scott, R., Kwok, S.C., Rosendahl, S.M., & Burns, D.H. (2007). Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilisation. Fertility & Sterility, 88, 1350–1357.
- Seli, E., Botros, L., Sakkas, D., & Burns, D.H. (2008). Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in-vitro fertilization. Fertility & Sterility, 90, 2183–2189.
- Serhal, P.F., Ranieri, D.M., Kinis, A., Marchant, S., Davies, M., & Khadum, I.M. (1997). Oocyte morphology predicts outcome of intra-cytoplasmic sperm injection. Human Reproduction, 12, 1267–1270.
- Shapiro, B.S., Daneshmand, M.D., Garner, F.C., Aguirre, M., Hudson, C., & Thomas, S. (2011). Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilisation: a prospective randomized trial comparing fresh and frozen- thawed embryo transfer in normal responders. Fertility & Sterility, 96, 344–348.
- Shapiro, B.S., Daneshmand, M.D., Restrepro, H., Garner, F.C., Aguirre, M., & Hudson, C. (2013). Matched cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertility & Sterility, 99, 389–392.
- Sifer, C., Sermondade, N., Poncelet, C., Hafhouf, E., Porcher, R., Cedrin-Durnerin, I., et al. (2011). Biological predictive criteria for clinical pregnancy after elective single embryo transfer. Fertility & Sterility, 95, 427–430.
- Society for Assisted Reproductive Technology Clinic Summary Report (2013). https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID = 0. Last accessed 13th May 2015.
- Soderstrom-Anttila, V., Vilska, S., Makinen, S., Foudila, T., & Suikkari, A.M. (2003). Elective single embryo transfer yields good delivery rates on oocyte donation. Human Reproduction, 18, 1858–1863.
- Stolwijk, A.M., Zielhuis, G.A., Sauer, M.V., Hamilton, C.J.C.M., & Paulson, R.J. (1997). The impact of the woman's age on the success of standard and donor in-vitro Fertilisation. Fertility & Sterility, 67, 702–710.
- Stylianou, C., Critchlow, D., Brison, D.R., & Roberts, S.A. (2012). Embryo morphology as a predictor of IVF success: An evaluation of the proposed UK ACE grading scheme for cleavage stage embryos. Human Fertility, 15, 11–17.
- Thurin, A., Hausken, J., Hillensjö, T., Jablonowska, B., Pinborg, A., Strandell, A., & Bergh, C. (2004). Elective Single-Embryo Transfer versus Double-Embryo Transfer in in vitro Fertilisation. New England Journal of Medicine, 351, 2392–402.
- Toner, J.P., Grainger, D.A., & Frazier, L.M. (2002). Clinical outcomes among recipients of donated eggs: an analysis of the US national experience, 1996–1998. Fertility & Sterility, 78, 1038–1045.
- Van den Bergh, M., Devreker, F., Emiliani, S., & Englert, Y. (2001). Glycolytic activity: a possible tool for Human blastocyst selection. Reproductive BioMedicine Online, 3(suppl 1), 8.
- Van Montfoort, A.P., Fiddelers, A.A., Janssen, J.M., Derhaag, J.G., Dirksen, C.D., Dunselman, G.A., et al. (2006). In unselected patients, elective single embryo transfer prevents all multiples, but results in significantly lower pregnancy rates compared with double embryo transfer: a randomized controlled trial. Human Reproduction, 21, 338–343.
- Van Royen, E., Mangelschots, K., De Neubourg, D., Valkenburg, M., Van de Meerssche, M., Ryckaert, G., et al. (1999). Characterization of a top quality embryo, a step towards single-embryo transfer. Human Reproduction, 14, 2345–2349.
- Van Royen, E., Mangelschots, K., De Neubourg, D., Laureys, I., Ryckaert, G., & Gerris, J. (2001). Calculating the implantation potential of day 3 embryos in women younger than 38 years of age: a new model. Human Reproduction, 16, 326–332.
- Van Royen, E., Mangleschots, K., Vercruyssen, M., De Neubourg, D., Valkenburg, M., Ryckaert, G., & Gerris, J. (2003). Multi-nucleation in cleavage stage embryos. Human Reproduction, 18, 1062–1069.
- Velez, M.P., Kadoch, I.J., Phillips, S.J., & Bissonette, F. (2013). Rapid policy change to single-embryo transfer while maintaining pregnancy rates per initiated cycle. Reproductive Biomedicine Online, 26, 506–511.
- Vitthala, S., Gelbaya, T.A., Brison, D.R., Fitzgerald, C.T., & Nardo, L.G. (2009). The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Human Reproduction Update, 15, 45–55.
- Watkins, A.J., Lucas, E.S., & Fleming, T.P. (2010). Impact of the peri-conceptional environment on the programming of adult disease. Journal of Developmental Origins of Health & Disease, 1, 87–95.
- Wells, D., Kaur, K., Jamie Grifo, J., Glassner, M., Taylor, J.C., Fragouli, E., & Munne, S. (2014). Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. Journal of Medical Genetics, 51, 553–562.
- Wharf, E., Dimitrakopoulos, A., Khalaf, Y., & Pickering, S. (2003). Early embryo development is an indicator of implantation potential. Reproductive BioMedicine Online, 8, 212–218.
- Winston, N.J., Braude, P.R., Pickering, S.J., George, M.A., Cant, A., Currie, J., & Johnson, M.H. (1991). The incidence of abnormal morphology and nucleocytoplasmic ratios in 2,3 and 5 day human pre-embryos. Human Reproduction, 6, 17–24.
- Yanaihara, A., Yorimitsu, T., Motoyama, H., Ohara, M., & Kawamura, T. (2008). Clinical outcome of frozen blastocyst transfer; single vs. double transfer. Journal of Assisted Reproduction and Genetics, 25, 531–534.
- Yang, Z., Liu, J., Collins, G.S., Salem, S.A., Liu, X., Lyle, S.S., et al. (2012). Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Molecular Cytogenetics, 5, 24. doi: 10.1186/1755–8166-5-24. Open Access.
- Yin, X., Tan, K., Vajta, G., Jiang, H., Tan, Y., Zhang, C., et al. (2013). Massively Parallel Sequencing for Chromosomal Abnormality Testing in Trophectoderm Cells of Human Blastocysts. Biology of Reproduction, 88, 69.
- Young, L.E., Sinclair, K.D., & Wilmut, I. (1998). Large Offspring syndrome in cattle and sheep. Reviews of Reproduction, 3, 155–163.

