Abstract
The plasma membrane H+-ATPase pump (Pma1p) has been proposed as a viable target for antifungal drugs since this high capacity proton pump plays a critical role in the intracellular regulation of pH and in nutrient uptake of yeast and other fungi. In recent years, this and other laboratories have verified that the antifungal activity of 2-phenylbenzisoselenazol-3(2H)-one, an organoselenium compound commonly referred to as ebselen (1), stems, at least in part, from its inhibitory action on the fungal Pma1p. In the present study, the antifungal efficacy of 2-(3-pyridinyl)-benzisoselenazol-3(2H)-one (2) and 2-phenylbenzisoselenazol-3(2H)-one 1-oxide (3), two ebselen analogs, was evaluated using a strain of S. cerevisiae and compared against that of 1. In addition, the study also examined the inhibitory potential of these three compounds toward the Pma1p of S. cerevisiae. Based on mean IC50 values, the antifungal potency was found to decrease in the order 3 > 1 > 2. However, in terms of inhibitory action on Pma1p, the potency decreased in the order 1 > 3 > 2. The magnitude of these activities appears to be correlated with the corresponding log P values, with compound 2 being the most hydrophilic and the least active of the three.
| Abbreviations: | ||
| Pma1p, | = | yeast plasma membrane H+-ATPase; |
| TLC, | = | thin layer chromatography |
Introduction
The majority of antifungal agents in current use for the treatment of systemic fungal infections achieve their therapeutic effect by disrupting critical yeast cell wall components, by interfering with the biosynthetic pathway leading to cell membrane ergosterol, or by interfering with DNA synthesisCitation1. In spite of the availability of such antifungal drug options, recent studies have indicated that the rates of morbidity and mortality associated with systemic fungal infections remain highCitation2–4. In recent years, this therapeutic problem has become compounded by the emergence of drug-resistant strains of fungi for which newer and more effective antifungal agents and treatment strategies are being sought. In this context, several stable organoselenium compounds with therapeutic potential toward a wide range of human diseases, including infections, have become available for biological testingCitation5–6. Among these compounds, ebselen, 2-phenylbenzisoselenazol-3(2H)-one (1), is the one that has attracted the most interest owing to its multiple biological effectsCitation5,7,8, such as antifungalCitation9–13, antibacterialCitation10,Citation11,Citation13,Citation14, and antiviralCitation11,Citation13 actions. Two additional reasons that make this selenium compound a subject of interest as an antimicrobial agent are its apparent lack of toxicity in mammalian systemsCitation15 and moderate antifungal activity toward selected strains of SaccharomycesCitation9,Citation10, CandidaCitation9,Citation13,Citation16, and CryptococcusCitation9. The antifungal activity of ebselen appears to be related, at least in part, to its ability to inhibit the fungal plasma membrane H+-ATPase, a major plasma membrane protein with low homology to human adenosine triphosphatases (ATPases) that is used by yeast and other fungi to establish an electrochemical proton gradient across the plasma membrane and to maintain a proper intracellular pHCitation17–19. Inhibition of this H+-ATPase by ebselen could result from its covalent interaction with the thiol groups of cysteine residues critical to the normal activity of this pumpCitation10. Two lines of evidence support this mechanism of action. One is the extreme facility with which ebselen reacts with endogenous thiol groupsCitation7,Citation20, such as those found in certain enzymes and other proteins, to form a selenium–sulfur bond with concomitant opening of the isoselenazol ringCitation21; and the other is the close correlation that exists between a decrease in medium acidifying ability and a decrease in H+-ATPase activityCitation9,Citation10.
The present study was undertaken with two specific aims: (a) to examine the growth-inhibiting activity of the ebselen analogs 2-(3-pyridinyl)-benzisoselenazol-3(2H)-one (2) and 2-phenylbenzisoselenazol-3(2H)-one 1-oxide (ebselen Se-oxide) (3) toward S. cerevisiae; and (b) to compare the antifungal activity of these ebselen analogs against that of the parent compound. To our knowledge, the effect of these two ebselen analogs on the yeast plasma membrane H+-ATPase has not been previously demonstrated.
Materials and methods
Yeast strains and culture medium
The S. cerevisiae strain AH109 is a PMA1-proficient haploid yeast strain obtained from Clontech (Palo Alto, CA). YPD medium (yeast extract, peptone, dextrose) was prepared from Bacto™ Tryptone (20 g), Bacto™ yeast extract (10 g), and distilled water (950 mL), followed by adjustment of the pH to 6.5 with HCl. After autoclaving at 121°C for 15 min, the medium was supplemented with 40% filter-sterilized dextrose (50 mL) and 0.2% filter-sterilized adenine hemisulfate (15 mL).
Chemicals
Samples of ebselen (compound 1) and ebselen Se-oxide (compound 3) were purchased from Cayman Chemical (Ann Arbor, MI). Alternatively, these compounds can be easily obtained from anthranilic acidCitation22,Citation23. The 3-pyridinyl analog of ebselen (compound 2) was synthesized as previously described in the literatureCitation13. A stock solution of ebselen or of one of its analogs was prepared by dissolving an appropriate amount of each compound in dimethylsulfoxide (DMSO) to give a final concentration of 100 mM (compounds 1 and 3) or 18 mM (compound 2). The concentration of the stock solution of compound 2 was lower than that of ebselen or compound 3 due to its lower solubility in DMSO.
Evaluation of effect of ebselen and its analogs on growth of S. cerevisiae
AH109 cells were cultured in 200 mL of YPD to obtain an initial absorbance reading of 0.005 at 600 nm (Abs600) on a spectrophotometer. The yeast suspension was then divided into 10.0 mL aliquots, to which a different concentration of compound 1 (0.001–30.0 µM), 2 (0.001–18.0 µM), or 3 (0.001–30.0 µM) was added. The samples were placed in a shaking incubator and agitated at 200 rpm and 30°C. After 18–24 h, fungal growth was assessed by measuring Abs600 on a spectrophotometer. Each concentration of test compound was tested in triplicate.
Determination of ATPase activity of Pma1p
The effect of an organoselenium compound on ATPase activity was evaluated using a partially purified sample of Pma1p from S. cerevisiae graciously provided by Dr David Perlin (The Public Health Research Institute Center, Newark, NJ). This aim was accomplished by measuring the ATPase activity of Pma1p in the absence and presence of compound 1 (1, 3, or 10 μM), compound 2 (5, 9, or 18 μM), or compound 3 (2, 5, or 10 μM) by the method of Wang et al.Citation24. In these experiments, Pma1p was pre-incubated with each of the test compounds at 30°C for 30 min before assessing its ATPase activity. The reaction medium used for this purpose, with or without an inhibitor, contained 10 mM of MES-Tris (pH 6.5), 5 mM of MgSO4, 25 mM of NH4Cl, 2 mM of ATP, 1–2 μg of partially purified Pma1p, and 2% of dextrose. Further additions included 50 mM of KNO3 (to inhibit vacuolar ATPase), 0.2 mM of ammonium molybdate (to inhibit acid phosphatase), and 5 mM of NaN3 (to inhibit mitochondrial ATPase). The reaction was stopped after 15 min by adding the malachite green reagent. In the presence of molybdate and free phosphate, a green color complex will form upon the addition of malachite green reagent; the intensity of the color complex is proportional to the amount of free phosphate. The green complex that formed between malachite green and free phosphate was measured with a microplate reader set at 630 nm.
Thin layer chromatography
Thin layer chromatography (TLC) was carried out in a glass chamber lined with Whatman No. 2 filter paper (Whatman Inc., Florham Park, NJ), and pre-saturated with the mobile phase (1-propanol/H2O, 7 + 3, by volume) for 2 h. The samples (30.0 mM ebselen, 18.0 mM compound 2, 30.0 mM compound 3, 30.0 mM l-cysteine, and a 1:1 mixture of each compound with l-cysteine) were spotted with capillary tubes along a line drawn 2 cm above one of the edges of a 20 × 20 cm silica gel Whatman polyester plate (Whatman Ltd., Maidstone, Kent, England). The plate was placed in the glass chromatographic chamber, and allowed to develop until the mobile phase reached a distance of 14 cm from the starting line. The TLC plate was dried in a 70°C oven and sprayed lightly with 0.2% ninhydrin in acetone, and then placed back into the oven until the appearance of colored spots. After the ninhydrin treatment, the TLC plate was exposed to iodine vapors in a closed chamber for 2 min and any brown spot, corresponding to unsaturated or aromatic compounds, was outlined in pencil. As a confirmatory step, the plate was also examined under short wavelength ultraviolet (UV) light for the presence of fluorescence-quenching spots.
Statistical analysis of data
Unless otherwise indicated, all experiments were carried out in triplicate and their results are reported as the mean ± SEM from at least three representative experiments. All statistical analyses were carried out with the help of GraphPad Prism® 4.0 (GraphPad Software, Inc., San Diego, CA) using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple group comparisons. Differences were considered to be significant at p < 0.05.
Results
Effect of ebselen and its analogs on growth of S. cerevisiae strain AH109
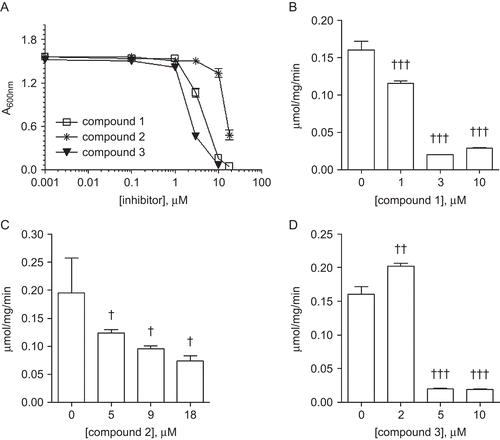
To determine the effect of ebselen and its analogs on the growth of nonpathogenic yeast, the S. cerevisiae strain AH109 was grown in YPD medium containing various concentrations of compound 1, 2, or 3. Growth was assessed by measuring the Abs600 of the medium after 18–24 h. As graphically shown in , all the compounds tested here were found to inhibit the growth of S. cerevisiae cells in a concentration-dependent manner. Whereas the half maximal (50%) inhibitory concentration (IC50) value of compound 1 was ∼4.6 µM, that of 3 was nearly one-half lower (∼2.1 μM), and that of compound 2 about four-fold higher (∼18 μM). These data are summarized in .
Table 1. Growth inhibitory effect of ebselen (compound 1) and its analogs on AH109 cells.
Assessment of effect of ebselen and its analogs on yeast plasma membrane H+-ATPase (Pma1p) activity
The Pma1p of fungi acts as an electrogenic proton pump that couples the hydrolysis of ATP to ion transport, specifically the transport of protons (H+). The effect of an organoselenium compound on this pump was tested using a partially purified sample of Pma1p from S. cerevisiae following its pre-incubation with a test compound. In each instance, the activity was assessed based on the amount of Pi released from ATP into the extracellular medium. From the results graphically presented in , all of the test compounds showed an ability to reduce the activity of the partially purified Pma1p in direct proportion to the amount of organoselenium compound present. These data are summarized in . It is evident that the yeast plasma membrane H+-ATPase is sensitive to ebselen and its analogs, with the IC50 value of Pma1p decreasing in the order compound 2 (∼7.0 μM) > compound 3 (∼4.0 μM) > compound 1 (∼2.5 μM).
Table 2. Inhibition of the yeast plasma membrane H+-ATPase pump by ebselen (compound 1) and two of its analogs.
Thin layer chromatography of compounds 1, 2, and 3 in absence or presence of l-cysteine
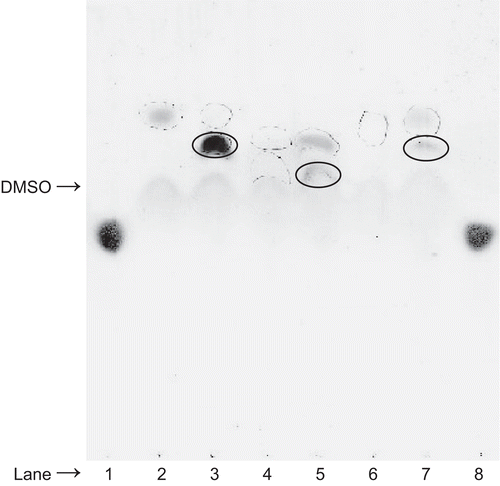
Ebselen can react with free sulfhydryl groups and can modify critical cysteine residues, such as those necessary for normal fungal cell growth and survival. Therefore, to determine whether or not compounds 2 and 3 exhibit a propensity to react with l-cysteine, thin layer chromatography (TLC) analysis was performed. Equal volumes of compounds 1, 2, and 3, in the absence or presence of l-cysteine, were repeatedly spotted on the same application point of the TLC plate to find out whether the two compounds would interact in situ in a spontaneous and rapid manner. Each of the compounds was also separately spotted alongside the reaction mixtures for comparative purposes. A representative TLC chromatograph is depicted in . The dark spot detected by exposure to iodine vapors, and located at the top of lane 2 (dotted circle), is ebselen. The dotted circles in lanes 4 and 6 show the spots detected under UV light for compounds 2 and 3, respectively. The lone ninhydrin spot toward the bottom of lanes 1 and 8 is l-cysteine. The ninhydrin-positive spot in lane 3, which migrates more slowly than the spot for ebselen but ahead of the spot for l-cysteine, is ascribed to an ebselen–l-cysteine condensation product (solid circle). Condensation of L-cysteine with compound 2 is detected in lane 5 (solid circle) and with compound 3 is shown in lane 7 (solid circle). Each of the condensation products exhibited a 77Se-nuclear magnetic resonance (NMR) chemical shift of ∼550 ppm, characteristic for selenosulfides (data not shown).
Figure 2. Thin layer chromatographic analysis of the products formed by reacting (30 mM) ebselen, (18 mM) compound 2, or (30 mM) compound 3 with (30 mM) l-cysteine. Lane 1, l-cysteine; lane 2, ebselen (dotted circle); lane 3, ebselen overspotted with l-cysteine; lane 4, compound 2 (dotted circle); lane 5, compound 2 overspotted with l-cysteine; lane 6, compound 3 (dotted circle); lane 7, compound 3 overspotted with l-cysteine; lane 8, l-cysteine. Note that lanes 3, 5, and 7 reveal that the l-cysteine spot has disappeared and that new spots (solid circles) with a slower migration rate than compounds 1, 2, and 3, respectively, are observed. l-cysteine was visualized with ninhydrin reagent followed by warming at 70°C in an oven.
Discussion
Based on the IC50 values for the growth inhibitory action of ebselen and its analogs on S. cerevisiae strain AH109, it is evident that compound 3 was more potent than ebselen, and ebselen more potent than compound 2. Although the antifungal and antibacterial properties of ebselen and some of its analogs have been previously notedCitation9–Citation14, there appears to be a lack of information on the antifungal activity of an ebselen analog such as compound 3, and in which the valence of the selenium atom is tetravalent rather than the usual divalent. At the same time, this finding suggests the potential value of further organoselenium compounds bearing tetravalent selenium as ebselen analogs with a greater antifungal activity than those containing divalent selenium.
The lower fungal growth inhibitory activity of compound 2 in comparison to both ebselen and compound 3 is in line with the findings of an earlier studyCitation13, and may be inherent to its particular structural features. Indeed, in compound 2, the 3-pyridinyl nitrogen can enter in hydrogen bond association with water by acting as a hydrogen bond acceptor (O—H⋯:N). This situation may not be operative in the case of ebselen, since this compound bears a non-hydrogen bond-forming phenyl substituent at the 2-position. As a result, compound 2 will be expected to be more soluble in water, to exhibit a lower diffusibility across the yeast plasma membrane, and to exert a lower antifungal potency than ebselen. Although the Se-oxide moiety of compound 3 may also form hydrogen bonds with water (O—H⋯:O), the bond energy of the water—H⋯:O=Se-(compound 3) bond is likely to be lower and, thus, less stable, than that of the water—H⋯:N-(compound 2) bondCitation25. Moreover, on the basis of their estimated log P values in 1-octanol-water (), compound 2 will be expected to be less hydrophobic than either ebselen or compound 3 and, hence, to be the benzisoselenazol-3(2H)-one with the lowest capability of the three to permeate through the yeast cell membrane. This correlation may further explain the need for a higher concentration of compound 2 to inhibit yeast growth than of ebselen or of compound 3.
The present results on ebselen and its analogs represented by compounds 2 and 3 corroborate the ability of organoselenium compounds to inhibit the fungal H+-ATPase. The same results also indicate that the concentration of ebselen or of an ebselen analog that is required to inhibit the growth of AH109 cells by 50% is roughly the same as that needed to strongly inhibit the ATPase activity of the H+-ATPase from yeast. Moreover, compound 3, an analog of ebselen bearing a tetravalent selenium atom, was about equipotent to ebselen in terms of its inhibitory power on H+-ATPase. The similarity in antifungal activity of the Se-oxide of ebselen (i.e., compound 3) to that of ebselen (compound 1) might be a consequence of its conversion to ebselen subsequent to its uptake into a fungal cell. If this were the case, then ebselen Se-oxide would be serving as an ebselen prodrug. However, this possibility will require future confirmation since, to our knowledge, the use of an ebselen prodrug to combat fungal infections has not been reported in the scientific literature. The present results point to the need for a future study specifically aimed at assessing the merits of novel benzisoselenazoles bearing a tetravalent selenium atom as useful inhibitors of the fungal H+-ATPase.
The lower inhibitory potency of compound 2 on the H+-ATPase relative to ebselen and compound 3 may be due to the proximity of the pyridinyl nitrogen to the reactive Se atom. By virtue of its electron-withdrawing power, the pyridinyl nitrogen may negatively impact on the antifungal activity of this benzisoselenazole compound by reducing the affinity of the selenium atom for cysteine residues. An additional noteworthy finding for compound 3 was that, while it was clearly inhibitory of H+-ATPase at concentrations ≥ 5 μM, at a lower concentration (2 μM) it induced a small but significant increase in ATPase activity relative to control values (). Although the exact reason underlying the biphasic activity of compound 3 is presently unknown, this behavior is not new, since at least two recent studies have also verified a dual effect for organoslenium compounds on biological processesCitation26,Citation27. Ebselen in known to be highly reactive toward thiol compounds such as glutathione, N-acetylcysteine, and dithiothreitolCitation7,Citation28,Citation29. The H+-ATPase of S. cerevisiae contains nine l-cysteine residues (Entrez Protein Database accession #P05030). The binding of ebselen to the thiol group of any one or all of the cysteine residues may account for its inhibitory activity on yeast H+-ATPase activityCitation10. Evidence on a similar interaction between the two analogs of ebselen tested and critical cysteine residues of H+-ATPase can be inferred from the results for TLC () and 77Se-NMR spectroscopic studies (data not shown) of the products formed upon interaction of the selenenamide moiety of compounds 1–3 and the thiol group of l-cysteine at ambient temperature. Future studies are warranted to demonstrate the occurrence of the same types of interaction with cysteinyl residues in H+-ATPase and the consequences of the interactions on the pump activity.
In short, the present work shows that analogs of ebselen can modulate the activity of the fungal plasma membrane H+-ATPase, possibly through a covalent interaction with thiol groups at critical cysteinyl residues, and that the valence of the organoselenium atom plays a determining role in the extent of the inhibitory activity.
Acknowledgments
Contributors: Author Billack designed all of the studies presented in this manuscript. Authors Billack, Santoro and Lau-Cam carried out the literature searches and summaries of previous work. Authors Billack, Santoro, and Nicholson carried out all experiments, collected the data, and performed statistical analyses of the collected data. Authors Piętka-Ottlik and Młochowski synthesized compound 2. Authors Billack and Santoro wrote the first draft of the manuscript, and authors Lau-Cam, Młochowski, and Piętka-Ottlik contributed to correcting, revising, and rewriting the manuscript. Authors Santoro and Billack prepared the graphs and figures. All authors contributed to and have approved the final manuscript.
Decleration of interest: This work was funded by the Department of Pharmaceutical Sciences (St. John’s University).
References
- Sheppard D, Lampiris HW. Antifungal agents. In: Katzung B, ed. Basic and Clinical Pharmacology, 10th ed. New York: McGraw-Hill, 2007:814–22.
- Mavor AL, Thewes S, Hube B. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets 2005;6:863–74.
- Dimopoulos G, Karabinis A, Samonis G, Falagas ME. Candidemia in immunocompromised and immunocompetent critically ill patients: a prospective comparative study. Eur J Clin Microbiol Infect Dis 2007;26: 377–84.
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 2007;45:321–46.
- Młochowski J, Kloc K, Lisiak R, Potaczek P, Wójtowicz H. Developments in the chemistry of selenaheterocyclic compounds of practical importance in synthesis and medicinal biology. ARKIVOC 2007;6:14–46.
- Narajji C, Karvekar MD, Das AK. Biological importance of organoselenium compounds. Indian J Pharm Sci 2007;69:344–51.
- Schewe T. Molecular actions of ebselen—an antiinflammtory antioxidant. Gen Pharmacol 1995;26:1153–69.
- Mugesh G, du Mont W-W, Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem Rev 2001;101:2125–79.
- Soteropoulos P, Vaz T, Santangelo R, Paderu P, Huang DY, Tamas MJ, et al. Molecular characterization of the plasma membrane H(+)-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob Agents Chemother 2000;44:2349–55.
- Chan G, Hardej D, Santoro M, Lau-Cam C, Billack B. Evaluation of the antimicrobial activity of ebselen: role of the yeast plasma membrane H+-ATPase. J Biochem Mol Toxicol 2007;21:252–64.
- Piętka-Ottlik M, Wójtowicz-Młochowska H, Kołodziejczyk K, Piasecki E, Młochowski J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem Pharm Bull 2008;56:1423–7.
- Bień M, Blaszczyk B, Kalinowska K, Młochowski J, Inglot AD. Antifungal activity of 2-(4-chlorophenyl)-1,2-benzisoselenazol-3(2H)-one, the analog of ebselen. Arch Immunol Ther Exp (Warsz) 1999;47:185–93.
- Wójtowicz H, Kloc K, Maliszewska I, Młochowski J, Piętka M, Piasecki E. Azaanalogues of ebselen as antimicrobial and antiviral agents: synthesis and properties. Farmaco 2004;59:863–8.
- Nozawa K, Yokota T, Fujimoto T. Susceptibility of methicillin-resistant Staphylococcus aureus to the selenium-containing compound 2-phenyl-1,2-benzoisoselenazol-3(2H)-one (PZ51). Antimicrob Agents Chemother 1989;33:1388–90.
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 1998;29:12–17.
- Bouhafs RK, Jarstrand C. Effects of antioxidants on surfactant peroxidation by stimulated human polymorphonuclear leukocytes. Free Radic Res 2002;36:727–34.
- Serrano R, Kielland-Brandt MC, Fink GR. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+-K+), K+-, and Ca2+- ATPases. Nature 1986;319:689–93.
- Monk BC, Perlin DS. Fungal plasma membrane proton pumps as promising new antifungal targets. Crit Rev Microbiol 1994;20:209–23.
- Perlin DS, Seto-Young D, Monk BC. The plasma membrane H(+)-ATPase of fungi. A candidate drug target? Ann NY Acad Sci 1997;834:609–17.
- Cotgreave IA, Morgenstern R, Engman L, Ahokas J. Characterisation and quantitation of a selenol intermediate in the reaction of ebselen with thiols. Chem Biol Interact 1992;84:69–76.
- Glass RS, Farooqui F, Sabahi M, Ehler KW. Formation of thiocarbonyl compounds in the reaction of ebselen oxide with thiols. J Org Chem 1989;54:1092–7.
- Młochowski J, Kloc K, Syper L, Inglot AD, Piasecki E. Aromatic and azoaromatic diselenides, benzisoselenazolones and related compounds as immunomodulators active in humans: synthesis and properties. Liebigs Ann Chem 1993;12:1239–44.
- Palus J, Młochowski J, Juchniewicz L. 2,2′-Diselenobisbenzoates and 2,2′-diselenobisbenzenesulfonates: new chiral aryl diselenides. Pol J Chem 1998;72:1931–6.
- Wang G, Tamas MJ, Hall MJ, Pascual-Ahuir A, Perlin DS. Probing conserved regions of the cytoplasmic LOOP1 segment linking transmembrane segments 2 and 3 of the Saccharomyces cerevisiae plasma membrane H+-ATPase. J Biol Chem 1996;271:25438–45.
- Emsley J. Very strong hydrogen bonding. Chem Soc Rev 1980;9:91–124.
- Morin D, Zini R, Ligeret H, Neckameyer W, Labidalle S, Tillement JP. Dual effect of ebselen on mitochondrial permeability transition. Biochem Pharmacol 2003;65:1643–51.
- Porciúncula LO, Rocha JB, Ghisleni G, Tavares RG, Souza DO. The effects of ebselen on [3H]glutamate uptake by synaptic vesicles from rat brain. Brain Res 2004;1027:192–5.
- Sarma BK, Mugesh G. Glutathione peroxidase-like antioxidant activity of the organoselenium drug ebselen: unexpected complications with thiol exchange reactions. J Am Chem Soc 2005;127:11477–85.
- Lisiak R, Mlochowski J, Palus J. Nucleophilic cleavage of selenaheterocyclic ring in benzisoselenaol-3(2H)-ones and 1,3,2-benzodiselenazoles. Pol J Chem 2007;81:1403–11.