Abstract
Compounds 4-(4-morpholinophenyl)-6-phenylpyrimidin-2-amine 20, 4-(4-methoxyphenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine 23, 4-(4-bromophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine 25, 4-(3-chlorophenyl)-6-(4-morpholinophenyl) pyrimidin-2-amine 27, and 4-(3-fluorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine 28 exerted excellent antibacterial activity against V. cholerae. Compounds 4-(4-chlorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine 22, 25, 4-(4-morpholinophenyl)-6-(3-nitrophenyl)pyrimidin-2-amine 26, and 28, which contained electron-withdrawing chloro, bromo, nitro, and fluoro functional groups, respectively, at the para/meta position of the phenyl ring attached to the pyrimidine ring promoted much activity against S. aureus. Compounds 4-(4-fluorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine 24 and 25 (against β-hemolytic Streptococcus) and compound 28 (against S. felxneri) showed pronounced activity. Compounds 26 and 28 (against K. pneumoniae) and compounds 24, 25, and 28 (against P. aeruginosa) exerted strong antibacterial activity. Compounds 22 and 25 promoted much antifungal activity against A. flavus, while compounds 24 and 25 registered maximum activity against Mucor. Compounds 23 (against C. albicans) and 27 and 28 (against Rhizopus) promoted good activity.
Introduction
Pyrimidines consititute the basic nucleus in nucleic acids and have been associated with a number of biological activities. Some notable biological activities of pyrimidine derivatives include as adenosine receptor antagonistsCitation1, as kinase inhibitorsCitation2, analgesicCitation3, anti-inflammatoryCitation3, as inhibitors of cyclin-dependent kinases 1 and 2Citation4, as calcium channel antagonistsCitation5, antihistaminicCitation6, and antitubercularCitation7 activities. Substituted aminopyrimidine nuclei are common in marketed drugs such as anti-atherosclerotic aronixil, anti-histaminic thonzylamine, anti-anxiolytic buspirone, anti-psoriatic enazadrem, and other medicinally relevant compounds.
Morpholine is a simple heterocyclic compound with great industrial importance. It is used as an anticorrosive agent and as a chemical intermediate: catalyst, solvent, and antioxidant, in the production of various pharmaceuticals and pesticides. 4-Phenyl morpholine derivativesCitation8 are reported to possess antimicrobial, anti-inflammatory, and central nervous system activities. Linezolide (commercially available antimicrobial) also possesses a 4-phenyl-morpholine substituent. These derivatives are reported to exert a number of important physiological activities such as antidiabeticCitation9, antiemeticCitation10, platelet aggregation inhibition, antihyperlipoproteinemicCitation9, bronchodilatory, growth stimulatoryCitation11, and antidepressantCitation12. They have also been used in the treatment of inflammatory diseases, pain, migraine, and asthmaCitation13.
Recently, we exploited the synthesis of 6-aryl-1,2,4,5-tetrazinane-3-thionesCitation14, fused indazolesCitation15, pyrimidinyl thiazolidin-4-onesCitation16, and 2,6-diarylpiperidin-4-one derivativesCitation17–19 with a view to incorporating various other bioactive heterocyclic nuclei such as 1,2,3-selenadiazoles, 1,2,3-thiadiazoles, and diazepans, intact, for the evaluation of associated antibacterial and antifungal activities. In view of the above and as part of the ongoing research on antimicrobials, we planned to synthesize a system that comprises both N-functionalized morpholine and 2-amino-4,6-diarylpyrimidine components together to give a compact structure as the title 4-(4-morpholinophenyl)-6-arylpyrimidin-2-amines.
Experimental
Chemistry
Thin layer chromatography (TLC) was performed to assess the reactions and the purity of the products. All the reported melting points were taken in open capillaries and are uncorrected. Infrared (IR) spectra were recorded in KBr (pellet form) on a Nicolet Avatar-330 Fourier transform (FT)-IR spectrophotometer, and noteworthy absorption values (cm−1) alone are listed. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 400 MHz and 100 MHz, respectively, on a Bruker AMX 400 NMR spectrometer using dimethylsulfoxide (DMSO)-d as solvent. Electrospray ionization (ESI)-+ve mass spectra (MS) were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on a Carlo Erba 1106 CHN analyzer.
General procedureCitation20 for the synthesis of (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19)
To an ethanolic solution of 1-(4-morpholinophenyl) ethanone (0.001 mol) and substituted benzaldehyde (0.001 mol), aqueous sodium hydroxide (0.005 mol) was added dropwise with stirring on a mechanical stirrer for 10 min, and stirring was continued for 4–6 h. After completion of reaction, the crude product isolated by suction was washed with water, dried, and recrystallized from ethanol.
(E)-1-(4-Morpholinophenyl)-3-phenyl-prop-2-en-1-one (11) Reaction time: 4 h; m.p.: 148–150°C; Yield: 95%; IR (KBr) ν (cm−1): 3007, 2962, 2924, 2852, 1646, 1606, 1190, 769; MS: m/z 294 (M + 1)+; Molecular formula: C19H19NO2; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2), 3.87–3.89 (t, 4H, O(CH2)2), 6.93–6.95 (d, 1H, H2, J = 8.9 Hz); 7.38–7.82 (m, 10H, Harom); 8.01–8.03 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.7 N(CH2), 66.5 O(CH2), 122.3 C-2, 143.2 C-3, 113.6, 128.8–130.3 -Carom, 128.2, 135.4, 154.1 ipso-C, 188.1 C-1.
(E)-1-(4-Morpholinophenyl)-3-p-tolyl-prop-2-en-1-one (12) Reaction time: 5 h; m.p.: 178–180°C; Yield: 92%; IR (KBr) ν (cm−1): 3012, 2923, 2924, 2851, 1645, 1600, 1194, 810; MS: m/z 308 (M + 1)+; Molecular formula: C20H21NO2; 1H NMR (δ ppm): 1.57 (s, 3H, CH3 at phenyl ring), 3.32–3.35 (t, 4H, N(CH2)2), 3.86–3.89 (t, 4H, O(CH2)2), 6.92–6.94 (d, 1H, H2, J = 8.8 Hz); 7.21–7.80 (m, 9H, Harom); 8.00–8.02 (d, 1H, H3, J = 8.8 Hz); 13C NMR (δ ppm): 21.0 CH3 at phenyl ring, 47.7 N(CH2), 66.5 O(CH2), 121.2 C-2, 143.3 C-3, 113.5, 129.3–130.5 -Carom, 128.2, 132.7, 140.5, 154.1 ipso-C, 188.2 C-1.
(E)-3-(4-Chlorophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one (13) Reaction time: 5 h; m.p.: 142–144°C; Yield: 90%; IR (KBr) ν (cm−1): 3087, 2967, 2920, 2859, 1597, 1654, 1202, 817; MS: m/z 328 (M + 1)+; Molecular formula: C19H18NO2Cl; 1H NMR (δ ppm): 3.34–3.37 (t, 4H, N(CH2)2), 3.89–3.91 (t, 4H, O(CH2)2), 6.97–6.99 (d, 1H, H2, J = 8.8 Hz); 7.35–7.76 (m, 9H, Harom); 8.00–8.02 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.6 N(CH2), 66.5 O(CH2), 121.2 C-2, 141.8 C-3, 113.5, 129.4–130.6 -Carom, 129.1, 133.8, 135.0, 154.0 ipso-C, 192.2 C-1.
(E)-3-(4-Methoxyphenyl)-1-(4-morpholinophenyl)prop-2-en-1-one (14) Reaction time: 4 h; m.p.: 110–112°C; Yield: 90%; IR (KBr) ν (cm−1): 3010, 2961, 2918, 2841, 1645, 1601, 1225; MS: m/z 324 (M + 1)+; Molecular formula: C20H21NO3; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2), 3.87–3.90 (t, 4H, O(CH2)2), 3.86 (s, 3H, OCH3 at phenyl ring), 7.59–7.61 (d, 1H, H2, J = 8.6 Hz); 6.92–7.46 and 7.75–7.79 (m, 9H, Harom); 8.00–8.02 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.6 N(CH2), 55.3 OCH3 at phenyl ring, 66.5 O(CH2), 119.6 C-2, 143.1 C-3, 113.5, 129.2–130.4 -Carom, 127.9, 129.9, 153.9, 161.3 ipso-C, 187.8 C-1.
(E)-3-(4-Fluorophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one (15) Reaction time: 4 h; m.p.: 159–161°C; Yield: 95%; IR (KBr) ν (cm−1): 3009, 2969, 2919, 2849, 1650, 1602, 1227; MS: m/z 312 (M + 1)+; Molecular formula: C19H18NO2F; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2), 3.87–3.89 (t, 4H, O(CH2)2), 6.93–6.95 (d, 1H, H2, J = 8.9 Hz); 7.08–7.78 (m, 9H, Harom); 8.00–8.02 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.5 N(CH2), 66.5 O(CH2), 121.6 C-2, 141.9 C-3, 113.4, 115.8, 130.1, 131.5 -Carom, 128.8, 130.6, 154.1, 162.5 ipso-C, 187.8 C-1.
(E)-3-(4-Bromophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one (16) Reaction time: 2 h; m.p.: 138–139°C; Yield: 94%; IR (KBr) ν (cm−1): 3001, 2960, 2923, 2845, 1657, 1612, 1227; MS: m/z 372 (M + 1)+; Molecular formula: C19H18NO2Br; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2), 3.86–3.87 (t, 4H, O(CH2)2), 6.94–6.96 (d, 1H, H2, J = 8.8 Hz); 7.18–7.82 (m, 9H, Harom); 8.01–8.03 (d, 1H, H3, J = 8.6 Hz); 13C NMR (δ ppm): 47.9 N(CH2), 65.6 O(CH2), 121.8 C-2, 142.3 C-3, 113.8, 115.1, 130.7, 131.7 -Carom, 128.5, 131.2, 154.7, 162.7 ipso-C, 188.8 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-nitrophenyl)prop-2-en-1-one (17) Reaction time: 6 h; m.p.: 134–316°C; Yield: 87%; IR (KBr) ν (cm−1): 3087, 2966, 2923, 2862, 1651, 1608, 1224; MS: m/z 339 (M + 1)+; Molecular formula: C19H18N2O4; 1H NMR (δ ppm): 3.36–3.38 (t, 4H, N(CH2)2), 3.88–3.90 (t, 4H, O(CH2)2), 6.95–6.97 (d, 1H, H2, J = 8.9 Hz); 7.27–7.91 and 8.23–8.25 (m, 9H, Harom); 8.03–8.05 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.3 N(CH2), 66.9 O(CH2), 122.0 C-2, 140.1 C-3, 113.3, 124.2–134.2 -Carom, 128.9, 137.1, 148.7, 154.3 ipso-C, 187.8 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-chlorophenyl)prop-2-en-1-one (18) Reaction time: 6 h; m.p.: 138–140°C; Yield: 90%; IR (KBr) ν (cm−1): 3093, 2969, 2928, 2857, 1593, 1652, 1212, 830; MS: m/z 328 (M + 1)+; Molecular formula: C19H18NO2Cl; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2), 3.89–3.91 (t, 4H, O(CH2)2), 6.96–6.98 (d, 1H, H2, J = 8.9 Hz); 7.33–7.81 (m, 9H, Harom); 7.98–8.00 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.8 N(CH2), 66.4 O(CH2), 121.2 C-2, 141.6 C-3, 113.3, 128.8–130.1 -Carom, 129.3, 133.7, 145.2, 154.1 ipso-C, 192.3 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-fluorophenyl)prop-2-en-1-one (19) Reaction time: 5 h; m.p.: 152–157°C; Yield: 91%; IR (KBr) ν (cm−1): 3018, 2974, 2924, 2843, 1649, 1605, 1226; MS: m/z 312 (M + 1)+; Molecular formula: C19H18NO2F; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2), 3.86–3.88 (t, 4H, O(CH2)2), 6.92–6.94 (d, 1H, H2, J = 8.8 Hz); 7.18–7.68 (m, 9H, Harom); 7.92–7.94 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.5 N(CH2), 66.6 O(CH2), 121.3 C-2, 141.8 C-3, 113.4, 125.3–130.1 -Carom, 128.6, 130.5, 154.3, 162.7 ipso-C, 187.5 C-1.
General method for the synthesis of 4-(4-morpholinophenyl)-6-arylpyrimidin-2-amines (20–28)
A mixture of (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) (0.001 mol) and guanidine nitrate (0.001 mol) in ethanol (50 mL) was refluxed, while a solution of sodium hydroxide (0.005 mol) in water (10 mL) was added portion-wise for 2 h. Refluxing was continued for a further 10 h and the mixture was poured into ice-cold water. The formed solid was separated by filtration, and purified by column chromatography using silica gel (100–200 mesh), with ethyl acetate–petroleum ether (b.p. 40–60°C) in the ratio 2:8 as eluent.
4-(4-Morpholinophenyl)-6-phenylpyrimidin-2-amine (20) IR (KBr) (cm−1): 3355, 3459, 3060, 2961, 2920, 1661, 1599, 1229, 928, 824, 776, 697, 634; 1H NMR (δ ppm): 3.33–3.38 (t, 4H, N(CH2)2), 3.88–3.89 (t, 4H, O(CH2)2), 5.23 (s, 2H, NH2), 7.38–7.85 (m, 10H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 46.3 N(CH2), 67.3 O(CH2), 103.4 C-5, 163.8 C-2, 164.7 C-6, 165.0 C-4, 127.0–131.5 -Carom, 142.1, 153.9 ipso-C.
4-(4-Morpholinophenyl)-6-p-tolylpyrimidin-2-amine (21) IR (KBr) (cm−1): 3432, 3200, 2967, 2923, 1625, 1599, 1229, 928, 815, 645; 1H NMR (δ ppm): 2.31 (s, 3H, CH3), 3.34–3.38 (t, 4H, N(CH2)2), 3.86–3.89 (t, 4H, O(CH2)2), 5.25 (s, 2H, NH2), 7.20–8.12 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 25.4 CH3, 46.7 N(CH2), 67.5 O(CH2), 104.1 C-5, 163.8 C-2, 164.1 C-6, 164.3 C-4, 126.0–131.4 -Carom, 143.8, 152.4 ipso-C.
4-(4-Chlorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (22) IR (KBr) (cm−1): 3396, 3217, 3027, 2962, 2920, 1656, 1229, 930, 819, 684; 1H NMR (δ ppm): 3.35–3.39 (t, 4H, N(CH2)2), 3.89–3.91 (t, 4H, O(CH2)2), 5.28 (s, 2H, NH2), 7.26–7.85 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 47.6 N(CH2), 66.5 O(CH2), 104.5 C-5, 163.8 C-2, 164.1 C-6, 164.7 C-4, 127.0–139.1 -Carom, 141.5, 152.5 ipso-C.
4-(4-Methoxyphenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (23) IR (KBr) (cm−1): 3447, 3200, 2972, 2922, 1659, 1600, 1243, 929, 821, 607; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2), 3.86 (s, 3H, OCH3), 3.87–3.90 (t, 4H, O(CH2)2), 5.26 (s, 2H, NH2), 7.18–7.82 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 46.7 N(CH2), 55.0 OCH3, 67.3 O(CH2), 103.9 C-5, 163.7 C-2, 164.7 C-6, 165.0 C-4, 127.5–142.1 -Carom, 153.8, 154.2 ipso-C.
4-(4-Fluorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (24) IR (KBr) (cm−1): 3434, 3200, 2967, 2923, 1624, 1599, 1226, 928, 815, 645; 1H NMR (δ ppm): 3.34–3.36 (t, 4H, N(CH2)2), 3.87–3.88 (t, 4H, O(CH2)2), 5.29 (s, 2H, NH2), 7.30–8.03 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 46.6 N(CH2), 67.3 O(CH2), 103.5 C-5, 163.8 C-2, 164.2 C-6, 164.4 C-4, 126.5–140.0 Carom, 140.6, 154.0 ipso-C.
4-(4-Bromophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (25) IR (KBr) (cm−1): 3398, 3219, 3029, 2965, 2922, 1659, 1231, 934, 821, 687; 1H NMR (δ ppm): 3.36–3.39 (t, 4H, N(CH2)2), 3.87–3.91 (t, 4H, O(CH2)2), 5.29 (s, 2H, NH2), 7.29–7.87 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 47.8 N(CH2), 66.6 O(CH2), 104.8 C-5, 163.9 C-2, 164.3 C-6, 164.8 C-4, 127.5–139.7 -Carom, 152.6, 141.6 ipso-C.
4-(4-Morpholinophenyl)-6-(3-nitrophenyl)pyrimidin-2-amine (26) IR (KBr) (cm−1): 3400, 3200, 3060, 2961, 2920, 1661, 1566, 1229, 928, 824, 776, 697; 1H NMR (δ ppm): 3.36–3.39 (t, 4H, N(CH2)2), 3.88–3.92 (t, 4H, O(CH2)2), 5.28 (s, 2H, NH2), 7.27–8.28 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 47.3 N(CH2), 67.6 O(CH2), 104.4 C-5, 163.8 C-2, 164.2 C-6, 164.4 C-4, 125.2–131.5 -Carom, 146.7, 153.9 ipso-C.
4-(3-Chlorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (27) IR (KBr) (cm−1): 3398, 3219, 3028, 2963, 2923, 1655, 1228, 929, 817, 686; 1H NMR (δ ppm): 3.36–3.38 (t, 4H, N(CH2)2), 3.88–3.90 (t, 4H, O(CH2)2), 5.29 (s, 2H, NH2), 7.16–7.74 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 47.7 N(CH2), 66.6 O(CH2), 104.4 C-5, 163.6 C-2, 164.3 C-6, 164.8 C-4, 126.2–138.8 -Carom, 146.5, 152.3 ipso-C.
4-(3-Fluorophenyl)-6-(4-morpholinophenyl)pyrimidin-2-amine (28) IR (KBr) (cm−1): 3437, 3204, 2965, 2928, 1623, 1597, 1225, 921, 811, 649; 1H NMR (δ ppm): 3.33–3.35 (t, 4H, N(CH2)2), 3.87–3.88 (t, 4H, O(CH2)2), 5.27 (s, 2H, NH2), 7.28–8.05 (m, 9H, Harom), the signal for H-5 proton may be merged with the aromatic protons; 13C NMR (δ ppm): 46.8 N(CH2), 67.4 O(CH2), 103.7 C-5, 163.9 C-2, 164.4 C-6, 164.5 C-4, 125.9–140.3 -Carom, 146.6, 154.2 ipso-C.
Microbiology
Materials
All the clinically isolated bacterial strains, namely Staphylococcus aureus, β-hemolytic Streptococcus, Vibreo cholerae, Shigella felxneri, Klebsiella pneumoniae, and Pseudomonas aeruginosa and fungal strains, namely Aspergillus flavus, Candida albicans, Mucor, and Rhizopus were obtained from the Faculty of Medicine, Annamalai University, Annamalainagar, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
Minimum inhibitory concentration (MIC) values in μg/mL were determined by the two-fold serial dilution methodCitation21. The respective test compounds (20–28) were dissolved in DMSO to obtain 1 mg mL−1 stock solution. Seeded broth (broth containing microbial spores) was prepared in nutrient broth (NB) from 24-h-old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C, while fungal spores from 1- to 7-day-old Sabouraud agar (Hi-media) slant cultures were suspended in Sabouraud dextrose broth (SDB). The colony forming units (cfu) of the seeded broth were determined by the plating technique and adjusted in the range of 104–105 cfu/mL. The final inoculum size was 105 cfu/mL for the antibacterial assay and 1.1–1.5 × 102 cfu/mL for the antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at pH 5.6 for fungi (SDB). Exactly 0.4 mL of test compound solution was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution, and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth were kept as control. The tubes were incubated in biochemical oxygen demand (BOD) incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observation after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin was used as the standard for bacterial studies and fluconazole was used as the standard for fungal studies.
Results and discussion
Chemistry
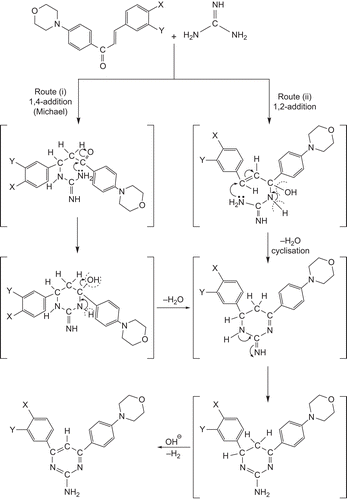
(E)-1-(4-Morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) were synthesized by stirring commercially available 1-(4-morpholinophenyl) ethanone and substituted benzaldehyde in the presence of sodium hydroxide in ethanol at 20°C for 1 h. Treatment of compounds 11–19 with guanidine nitrate22 in the presence of sodium hydroxide in refluxing ethanol for 8 h yielded the respective 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28). A schematic representation and analytical data of compounds 20–28 are shown in and , respectively. The structure of the newly synthesized compounds 20–28 was confirmed by the melting points, elemental analysis, and MS, FT-IR, and one-dimensional NMR (1H and 13C) spectroscopic data.
Scheme 1. Synthesis reaction pathway for formation of 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines.
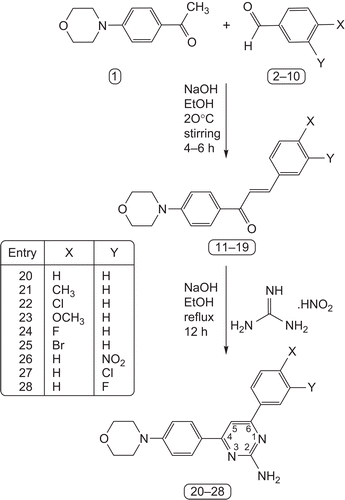
Table 1. Physical and analytical data of compounds 11–28.
Antibacterial activity
Novel 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28) were tested for their antibacterial activity in vitro against S. aureus, β-hemolytic Streptococcus, V. cholerae, S. felxneri, K. pneumoniae, and P. aeruginosa. Ciprofloxacin was used as the standard drug. Minimum inhibitory concentration (MIC) values are reproduced in . Compound 20, which had no substitution at the para position of the phenyl ring, only exerted moderate activities against all the used bacterial strains except against V. cholerae, with pronounced activity at 12.5 μg/mL, besides compounds 23, 25, 27, and 28, which were also potently active. Compounds 21 (against S. felxneri) and 23 (against β-hemolytic Streptococcus) did not exhibit antibacterial activity even at higher concentrations, i.e. 200 μg/mL. Compounds 22, 25, 26, and 28, which contained electron-withdrawing chloro, bromo, nitro, and fluoro functional groups, respectively, at the para/meta position of the phenyl ring attached to the pyrimidine ring promoted much activity against S. aureus. Compounds 24 and 25 (against β-hemolytic Streptococcus) and compound 28 (against S. felxneri) showed pronounced activity. Compounds 26 and 28 (against K. pneumoniae) and compounds 24, 25, and 28 (against P. aeruginosa) exerted strong antibacterial activity.
Table 2. In vitro antibacterial activity (MIC) values for compounds 20–28.
Antifungal activity
The in vitro antifungal activity of 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28) was studied against the fungal strains A. flavus, C. albicans, Mucor, and Rhizopus. Fluconazole was used as the standard drug. The MIC values are reproduced in . Compound 20, which had no substitution at the para position of the phenyl ring, compound 21, which had an electron-donating CH3 group, and compound 26, which had an electron-withdrawing NO2 group attached at the meta position of the phenyl ring attached to the pyrimidine ring, were less active against all tested fungal strains, whereas compound 27 did not exhibit antifungal activity against A. flavus and C. albicans. Further introduction of an electron-withdrawing chloro/bromo functional group at the para position of the phenyl ring attached to the pyrimidine ring in compounds 22 and 25 promoted much activity against A. flavus, while against Mucor compounds 24 and 25 registered maximum activity. Compound 23, which had an electron-donating methoxy group at the para position of the phenyl ring attached to the pyrimidine ring, was more potent against C. albicans. Instead, where the chloro/fluoro group was attached at the meta position of the phenyl ring attached to the pyrimidine ring, 27 and 28 promoted good activity against Rhizopus.
Table 3. In vitro antifungal activity (MIC) values for compounds 20–28.
Conclusion
Microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the newly synthesized 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28) are clearly illustrated by and . Close inspection of the in vitro antibacterial and antifungal activity profiles in different electron-donating (CH3 and OCH3) and electron-withdrawing (-F, -Cl, -Br, and -NO2) functional group-substituted phenyl rings of the novel 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28) showed that they exerted strong antibacterial activity against all tested bacterial strains. Compounds 20, 23, 25, 27, and 28 exerted excellent antibacterial activity against V. cholerae, and compounds 21 (against S. felxneri) and 23 (against β-hemolytic Streptococcus) did not exhibit antibacterial activity even at higher concentrations, i.e. 200 μg/mL. Compounds 22, 25, 26, and 28, which contained electron-withdrawing chloro, bromo, nitro, and fluoro functional groups, respectively, at the para/meta position of the phenyl ring attached to the pyrimidine ring promoted much activity against S. aureus. Compounds 24 and 25 (against β-hemolytic Streptococcus) and compound 28 (against S. felxneri) showed pronounced activity. Compounds 26 and 28 (against K. pneumoniae) and compounds 24, 25, and 28 (against P. aeruginosa) exerted strong antibacterial activity. Results of the antifungal activity study showed that the nature of the substituent on the phenyl ring, namely chloro, fluoro, and bromo, and methoxy functions at the para/meta positions of the aryl moieties, were determinants of the nature and extent of the antifungal activity of all synthesized compounds 20–28 on the fungal strains A. flavus, C. albicans, Mucor, and Rhizopus. The introduction of an electron-withdrawing chloro/bromo functional group at the para position of the phenyl ring attached to the pyrimidine ring in compounds 22 and 25 promoted much activity against A. flavus, while compounds 24 and 25 registered maximum activity against Mucor. Compound 23, which had an electron-donating methoxy group at the para position of the phenyl ring attached to the pyrimidine ring, was more potent against C. albicans. Instead, when the chloro/fluoro group was attached at the meta position of the phenyl ring attached to the pyrimidine ring, compounds 27 and 28 promoted pronounced activity against Rhizopus. The method of action of these compounds is unknown. These observations may promote progress of our research in this field. Further development of this group of 4-(4-morpholinophenyl)-6-aryl-pyrimidin-2-amines (20–28) may lead to compounds with better pharmacological profiles than those of standard antibacterial and antifungal drugs.
Acknowledgments
The authors are thankful to the NMR Research Center, Indian Institute of Science, Bangalore for recording spectra.
Declaration of interest: One of the authors (V.K.) is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of a CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. Another author (J.T.) wishes to thank the Annamalai University authorities for providing financial support in the form of a Research Fellowship.
References
- Van Veldhoven JP, Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Struensee-Link R, Beukers MW, et al. A new generation of adenosine receptor antagonists: from di- to trisubstituted aminopyrimidines. Bioorg Med Chem 2008;16:2741–52.
- Hughes TV, Emanuel SL, Beck AK, Wetter SK, Connolly PJ, Karnachi P, et al. 4-Aryl-5-cyano-2-aminopyrimidines as VEGF-R2 inhibitors: synthesis and biological evaluation. Bioorg Med Chem Lett 2007;17:3266–70.
- Chhabria MT, Bhatt HG, Raval HG, Oza PM. Synthesis and biological evaluation of some 5-ethoxycarbonyl-6-isopropylamino-4-(substitutedphenyl)amino pyrimidines as potent analgesic and anti-inflammatory agents. Bioorg Med Chem Lett 2007;17:1022–4.
- Sayle KL, Bentley JF, Boyle T, Calvert AH, Cheng Y, Curtin NJ, et al. Structure-based design of 2-arylamino-4-cyclohexylmethyl-5-nitroso-6-aminopyrimidine inhibitors of cyclin-dependent kinases 1 and 2. Bioorg Med Chem Lett 2003;13:3079–82.
- Pastor A, Alajarin R, Vaquero JJ, Alvarez-Builla J, Fau de Casa-Juana M, Sunkel C, et al. Synthesis and structure of new pyrido[2,3-d]pyrimidine derivatives with calcium channel antagonist activity. Tetrahedron 1994;50:8085–98.
- Youssouf MS, Kaiser P, Singh GD, Singh S, Bani S, Gupta VK, et al. Anti-histaminic, anti-inflammatory and bronchorelaxant activities of 2, 7-dimethyl-3-nitro-4H pyrido [1,2-a] pyrimidine-4-one. Int Immunopharmacol 2008;8:1049–55.
- Gasse C, Douguet D, Huteau V, Marchal G, Munier-Lehmann H, Pochet S. Substituted benzyl-pyrimidines targeting thymidine monophosphate kinase of Mycobacterium tuberculosis: synthesis and in vitro anti-mycobacterial activity. Bioorg Med Chem 2008;16:6075–85.
- Panneerselvam P, Nair RR, Vijayalakshmi G, Subramanian EH, Sridhar SK. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur J Med Chem 2005;40:225–9.
- Manfred R, Michael M, Robert S, Wolfgang G. 1H and 13C NMR spectra of N-substituted morpholines. Eur Pat Appl EP 334146:28. Chem Abstr 1989;112:178999.
- Hale JJ, Mills SG, MacCross M, Dorn CP, Finke PE, Budhu RJ, et al. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J Med Chem 2000;43:1234–7.
- Fisher MH, Wyvratt MJ. Morpholine derivatives: compositions and uses. US Patent 5077290:10. Chem Abstr 1991;116:214513.
- Avramova P, Danchev N, Buyukliev R, Bogoslovova T. Synthesis, toxicological, and pharmacological assessment of derivatives of 2-aryl-4-(3-arylpropyl)morpholines. Arch Pharm 1998;331:342–6.
- Dorn CP, Hale JJ, MacCross M, Mills SG. Morpholine compounds are prodrugs useful as tachykinin receptor antagonists. US Patent 5691336:82. Chem Abstr 1997;128:48231.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Design, “one-pot” synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J Sulf Chem 2007;28:383–92.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Synthesis, spectral analysis, antibacterial and antifungal activities of some 4,6-diaryl-4,5-dihydro-3-hydroxy-2[H]-indazole—a novel fused indazole derivative. J Enzyme Inhib Med Chem 2008;23:974–9.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Design, synthesis, spectral analysis and in vitro microbiological evaluation of biolabile 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones. J Enzyme Inhib Med Chem 2009; in press.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J Enzyme Inhib Med Chem 2008;23:347–51.
- Gopalakrishnan M, Thanusu J, Kanagarajan V. Synthesis and biological evaluation of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-thiadiazoles. Med Chem Res 2007;16:392–401.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V, Govindaraju R, Jayasri G. A convenient “one-pot” synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enzyme Inhib Med Chem 2007;22:709–15.
- Guthrie W, Wang XP. The aldol condensation of acetophenone with acetone. Can J Chem 1991;69:339–40.
- Dhar MH, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants biological activity. Part I. Indian J Exp Biol 1968;6:232–47.
- Kothari S, Singhal M, Vijayvergia D, Vyas R, Verma BL. Synthesis and biocidal activity of some new 2-amino-4,6-diaryl-substituted-pyrimidines. J Indian Chem Soc 2000;77:329–31.