Abstract
Conferin (1), a new isoflavone, has been isolated from the ethyl acetate soluble fraction of Caragana conferta Benth. along with seven known compounds, namely biochanin A (2), p-hydroxybenzoic acid (3), 3,5- dimethoxybenzoic acid (4), ursolic acid (5), erythrodiol (6), pinoresinol (7), and syringresinol (8), reported for the first time from this species. The structure of the new isoflavone was deduced on the basis of spectroscopic studies. Compounds 1 and 2 were investigated for biological activities and showed significant anti-inflammatory activity in carrageenan induced paw edema of rats. Evaluation of antioxidant activity by the radical scavenging method indicated that compound 1 is a potent antioxidant while 2 is moderately active. It was also shown that the reducing capability of compound 2 was remarkably increased in a concentration dependent manner as compared to 1. Compound 1 showed moderate inhibitory activity against the enzyme lipoxygenase, while 2 showed weak activity.
Introduction
The family Papilionaceae comprises 55 genera and well over 7000 species. The genus Caragana has over 80 species, of which 10 have so far been identified in PakistanCitation1. Caragana conferta is a shrub which grows in Asia, Africa, and south east Europe. In Pakistan it is mainly found in the Gilgit and Kashmir valleys at an altitude of 7000–12,000 feet above sea levelCitation1. Plants of the genus Caragana are used as folk medicine in China and Korea for the treatment of neuralgia, rheumatism, arthritis, and hypertensionCitation2. A literature survey revealed that few compounds have so far been reported from this speciesCitation3,Citation4. A methanolic extract of this plant showed strong toxicity in the brine shrimp lethality test, and on subsequent fractionation the major toxicity was observed in the ethyl acetate soluble sub-fraction. Further pharmacological screening of this fraction revealed potent antioxidant activity. In this article we report the isolation of a new isoflavone (1), named conferin, along with several known compounds: biochanin A (2)Citation5, p-hydroxybenzoic acid (3)Citation6, 3,5-dimethoxybenzoic acid (4)Citation6, ursolic acid (5)Citation7, erythrodiol (6)Citation8, pinoresinol (7)Citation9, and syringresinol (8)Citation10. This study was undertaken to investigate the antioxidant potentials using different tests including 1,1-diphenyl-2- picryl-hydrazyl (DPPH) radical scavenging activity and reducing power. In vivo anti-inflammatory activity was determined by observing the carrageen induced paw edema of rats for compounds 1 and 2, while the lipoxygenase inhibitory assay was also performed.
Materials and methods
Plant material
The whole plant of Caragana conferta Benth. was collected from Gilgit (Pakistan) and identified by Dr. Rubina Ashraf of the National Agriculture Research Center, Islamabad, Pakistan. A voucher specimen has been deposited in the herbarium of the Department of Botany, University of Karachi (voucher no. 319).
General experimental procedures
1H- and 13C-nuclear magnetic resonance (NMR), heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond coherence (HMBC) spectra were recorded on Bruker spectrometers operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR, respectively. The chemical shift values are reported in units of ppm (δ) and the coupling constants (J) are in Hz. Electron impact mass spectra (EIMS) and high resolution EIMS (HREIMS) were recorded on JMS-HX-110 and JMS-DA-5000 mass spectrometers. Ultraviolet (UV) spectra were recorded on a Hitachi UV-3200 spectrophotometer. Infrared (IR) spectra were recorded on a 460 Shimadzu spectrometer. Aluminum sheets precoated with silica gel 60 F254 (20 × 20 cm, 0.2 mm thick; E. Merck) were used for thin layer chromatography (TLC) and silica gel (230–400 mesh) was used for column chromatography. Visualization of the TLC plates was carried out under UV light at 254 and 366 nm and by spraying with ceric sulfate reagent solution (with heating).
Extraction and isolation
Shade dried whole plant material (15 kg) was extracted with MeOH (3 × 60 L) at room temperature. The combined methanolic extract was evaporated under reduced pressure to obtain a thick gummy mass (700 g). It was suspended in water and successively extracted with n-hexane, ethyl acetate, and n-butanol. The ethyl acetate soluble fraction (150 g) was subjected to column chromatography, eluting with CHCl3, CHCl3–MeOH, and MeOH in increasing order of polarity to obtain five fractions (A–E). Fraction A obtained from CHCl3–MeOH (9.9:0.1) was further purified by column chromatography, eluting with CHCl3–MeOH (9.7:0.3) to afford compounds 3 (16 mg) and 4 (15 mg). Fraction B obtained from CHCl3–MeOH (9.8:0.2) was further purified by column chromatography, eluting with CHCl3–MeOH (9.5:0.5) to afford compounds 5 (22 mg) and 6 (25 mg). Fraction C obtained from CHCl3–MeOH (9.5:0.5) was a mixture of two components, which were separated by column chromatography using the solvent system CHCl3–MeOH (9.2:0.8) to afford compounds 2 (30 mg) and 1 (25 mg) from the top and the tail fractions, respectively (). Fraction D obtained from CHCl3–MeOH (9.3:0.7) was a mixture of two components, which were separated by column chromatography using the solvent system CHCl3–MeOH (9.0:1.0) to afford compounds 7 (18 mg) and 8 (15 mg) from the top and the tail fractions, respectively.
Compounds 2–8 were identified through comparison of their physical and spectral data with those reported in the literatureCitation5–Citation10.
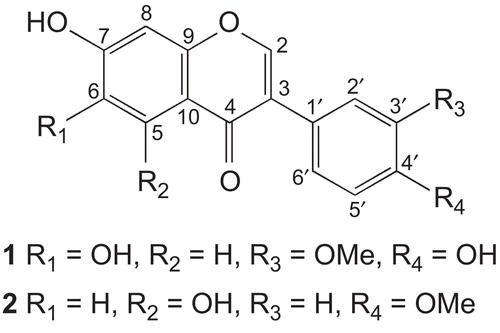
Conferin (1) White solid (25 mg), M.p. 218–219°C. UV (MeOH) λmax (log ε): 258 (2.45), 288 (2.75) nm. IR (KBr) cm−1: 3450, 1665 and 1580. EIMS m/z (rel. int.): 300 (100), 285 (27), 257 (28), 229 (36), 154 (20), 148 (15), 133 (26). HREIMS: m/z 300.0625, calcd. for C16H12O6 300.0633. 1H-NMR (400 MHz, pyridine-d5) δ: 3.80 (3H, s, OMe-3’), 7.09 (1H, d, J = 8.4 Hz, H-5’), 7.25 (1H, s, H-8), 7.38 (1H, dd, J = 8.4, 2.0 Hz, H-6’), 7.89 (1H, d, J = 2.0 Hz, H-2’), 8.17 (1H, s, H-2), 8.23 (1H, s, H-5). 13C-NMR (100 MHz, pyridine-d5) δ: 56.5 (OMe), 152.4 (C-2), 124.1 (C-3), 175.5 (C-4), 109.4 (C-5), 146.5 (C-6), 154.3 (C-7), 103.7 (C-8), 152.1 (C-9), 118.1 (C-10), 125.8 (C-1’), 118.5 (C-2’), 149.2 (C-3’), 147.5 (C-4’), 112.8 (C-5’), 121.0 (C-6’).
Antioxidant assay
The free radical scavenging activity was measured using 1,1-diphenyl-2-picryl-hydrazyl (DPPH)Citation11. A solution of DPPH 0.3 mM was prepared in ethanol. Five microliters of each sample of a different concentration (62.5–500 μg) were mixed with 95 μL of DPPH solution in ethanol. The mixture was dispersed in 96-well plates and incubated at 37°C for 30 min. The absorbance at 515 nm was measured by microtiter plate reader (SpectraMax Plus 384; Molecular Devices, Sunnyvale, CA, USA) and percent radical scavenging activity was determined in comparison with the methanol treated control. Butylated hydroxyanisole (BHA) was used as standard.
where Ac is the absorbance of the control (dimethylsulfoxide (DMSO) treated) and As is the absorbance of the sample
Determination of total reducing ability
Total reducing capability of these compounds was estimated according to the method of OyaizuCitation12 with some modification. Different concentrations of samples (5–100 μg/mL) in methanol were mixed with phosphate buffer (250 μL, 0.2 M, pH 6.6). Then 250 μL potassium ferricyanide (K3[Fe(CN)6]) (1%) was added. The mixture was incubated at 50°C for 20 min. After incubation, 250 μL trichloroacetic acid (10%) was added to the mixture which was centrifuged for 10 min; the upper layer of the solution (250 μL) was separated in another set of test tubes and mixed with an equal volume of deionized water (250 μL). Then 50 μL of ferric chloride (FeCl3) (0.1%) was added and the absorbance was measured at 700 nm using a spectrophotometer (Specord 200; Analytik Jena, Germany). A higher absorbance of the reaction mixture indicated a higher reducing power.
Lipoxygenase inhibition assay
All chemicals required for the assay were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Lipoxygenase inhibiting activity was measured by modifying the spectrophotometric method developed by TappelCitation13. A mixture of test compound (10 μL; 1 mM) in MeOH, type-1B lipoxygenase (EC 1.13.11.12; from soyabean) (20 μL; 70 units) in 0.1 M aqueous phosphate buffer (pH 8.0) in a total volume of 160 μL was incubated for 10 min at 25°C. Then the reaction was initiated by the addition of a solution of linoleic acid as substrate (10 μL; 20 μM), resulting in the formation of (9Z,11E,13S)-13-hydroperoxyoctadeca-9,11-dienoate. The change in UV absorbance at 234 nm was followed over a period of 6 min. All reactions were performed in triplicate, and analyzed with a 96-well plate reader (SpectraMax Plus 384; Molecular Devices). The IC50 values were calculated using EZ-Fit enzyme-kinetics software (Perrella Scientific, Inc., Amherst, MA, USA).
Anti-inflammatory assay
Inflammation is a defensive reaction to injuryCitation14. The symptoms of inflammation (redness, edema, heat, pain, and disturbed tissue function) are the result of a complex pathophysiological processCitation15. Conventional non- steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, diclofenac, and naproxen are widely used for the treatment of pain and inflammation. The prolonged use of these drugs is associated with a substantial toxicity profile with side effects mainly affecting the gastrointestinal tract, heart, and kidneyCitation16. In inflammation, the role of both cyclooxygenase (COX) and lipoxygenase (LOX) is apparent. Therefore, the search for anti-inflammatory agents that retain the therapeutic efficacy of classical NSAIDs and have greater safety via equally blocking both the COX and the 5-LOX metabolic pathways is justifiedCitation17. Traditionally used plants could provide useful information in this regard.
In vivo anti-inflammatory assay
The anti-inflammatory potential of compounds 1 and 2 was evaluated in rats using the following chemicals: carrageenan and DMSO obtained from Sigma and diclofenac sodium from Novartis Pharmaceuticals. Wistar rats (120–180 g) of either sex were obtained from the animal house of the Agha Khan University of Karachi. Animals were kept under standard environmental conditions with free access to food and water. Rats were divided into four groups (six in each group). Group 1 served as control and received 0.9% saline while group 2 received diclofenac sodium orally. Group 3 received compound 1 in a concentration 30 mg/kg and group 4 received 30 mg/kg of compound 2. Acute edema was induced in the right hind paw of rats by injecting 0.1 mL of freshly prepared carrageenan 1% solution subcutaneously into the plantar region. Drugs were given 1 h before carrageenan challenge. Paw volume was measured using a plethysmometer at 0 and 4 h after carrageenan injection. The edema volume and percent inhibition in edema were calculated using the method of ChristopherCitation18.
Results and discussion
The ethyl acetate soluble fraction of the methanolic extract of the whole plant of Caragana conferta was subjected to a series of column chromatographic techniques to obtain compounds 1–8, and their structures were established by UV, IR, MS, and NMR techniques.
Conferin (1) was isolated as white amorphous solid which gave violet coloration with FeCl3. The molecular formula C16H12O6 was established by HREIMS showing a [M + H]+ peak at m/z 300.0625 (calcd. for C16H12O6 300.0633) having eleven degrees of unsaturation. The broadband and distortionless enhancement by polarization transfer (DEPT) 13C-NMR spectra of 1 showed 16 carbon signals, including one methyl, six methane, and nine quaternary carbons. The signal at δ 175.5 was assigned to the conjugated carbonyl, while the conjugated olefinic carbon resonated at δ 152.4Citation4. The methoxyl carbon was observed at δ 56.5, while oxygenated aromatic carbons gave signals at δ 154.3, 149.2, 147.5, and 146.5. In the 1H-NMR spectrum a singlet of conjugated olefinic proton was observed at δ 8.17. Thus, 1 was deduced to be an isoflavone. In HREIMS the retro-Diels–Alder fragments were observed at m/z 154 and 148, revealing the presence of two hydroxyl groups in ring A and one methoxyl and one hydroxyl group in ring B. Ring A showed two singlets in the 1H-NMR spectrum at δ 7.25 and δ 8.23, revealing the presence of hydroxyl groups at C-6 and C-7Citation19.
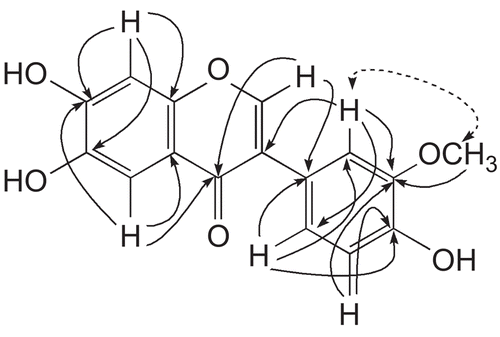
The protons of the 1,3,4-trisubstituted ring B were observed at δ 7.89 (1H, d, J = 2.0 Hz, H-2’), δ 7.09 (1H, d, J = 8.4 Hz, H-5’), and δ 7.38 (1H, dd, J = 8.4, 2.0 Hz, H-6’). The signal of the methoxyl group appeared at δ 3.80 (3H, s). The position of the methoxyl group was ascertained through HMBC, showing 3J correlation of methoxyl protons with C-3’ (δ 149.2). It was supported by the observation of a strong NOESY (nuclear Overhauser effect spectroscopy) correlation between the methoxyl protons and H-2’ (). All these data were in complete agreement to the assigned structure of conferin (1) as 6,7,4’-trihydroxy-3’-methoxy isoflavone.
Compounds 1 and 2 were evaluated for their antioxidant activity as shown in . Compound 1 reflected potent antioxidant ability while 2 was moderately active when compared with the standard BHA. This shows that the number and position of hydroxyl groups are apparently the controlling factors for antioxidant activity. Thus, compound 1 having a greater number of phenolic functionalities including two of them in ortho positions induces greater antioxidant activity than 2.
Table 1. IC50 (μM) values of compounds 1 and 2 in antioxidant assay and against lipoxygenase.
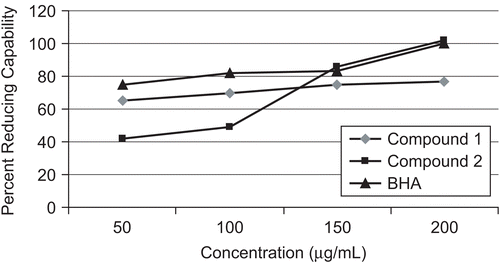
The reducing power of a compound may serve as a significant indicator of its potential antioxidant activityCitation20. The reductive capabilities of compounds are assessed by the extent of conversion of the Fe3+/ferricyanide complex to the Fe2+/ferrous form. The reducing power of compound 1 was observed at different concentrations (50, 100, 150, 200 μg/mL) and results were compared with BHA as shown in . Compound 1 showed 65, 70, 75, and 77% reducing capability while the percentage reducing capability of compound 2 was 42, 49, 83, and 102% at concentrations 50, 100, 150, and 200 μg/mL, respectively. This means that compound 1 exhibits powerful reducing ability as compared to compound 2 at the lowest concentration. It is apparent that the phenolic groups at C-6 and C-7, which are adjacent to each other, are responsible for the higher reducing ability of compound 1. Surprisingly, the reducing power of compound 2 rapidly increased with an increase in concentration, as shown in . It is suggested that due to the increase in concentration, some intramolecular changes may occur which cause the free hydroxyl group to become unavailable to react. It is also possible that compound 1 is more reactive toward itself than the reagent at a higher concentration while compound 2 is more reactive toward the reagent.
Determination of the lipoxygenase inhibition activity as shown in indicates that compound 1 is a moderate lipoxygenase inhibitor with an IC50 value of 55.2 μM while compound 2 has an IC50 value of 100 μM when compared with baicalein (22.6 μM). It has been found that lipoxygenase inhibitors play an important role in the treatment of a variety of disorders such as bronchial asthma and inflammationCitation21, and also have a profound influence on the development of several cancersCitation22; therefore, the search for clinically efficacious inhibitors is in demand in the global market. Lipoxygenases are therefore potential targets for rational drug design and the discovery of mechanism based inhibitors for the treatment of bronchial asthma, inflammation, and autoimmune diseases.
The anti-inflammatory activity of compounds 1 and 2 was determined in carrageenan induced paw edema of rats, and both were found to be significant when compared with group 2 which received diclofenac sodium (positive control) ().
Table 2. Anti-inflammatory potential of compounds 1 and 2 in carrageenan-induced paw edema of rats.
Acknowledgements
Declaration of interest: The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Ali SI, Qaiser M. Flora of Pakistan. Karachi: Ferozsons Publishers, 2001;100:98–102.
- Susumu K, Michio T, Kazutoshi M. Oligomeric stilbenes from Caragana chamlagu Lamark root. Chem Pharm Bull 1996;44:565–7.
- Khan R, Malik A, Adhikari A, Choudhary MI. Conferols A and B, new anti-inflammatory 4-hydroxyisoflavones from Caragana conferta. Chem Pharm Bull 2009;57:415–17.
- Khan R, Fatima I., Malik A. Caragin, new isoflavone from Caragana conferta. J Asian Nat Prod Res 2008;10:823–5.
- Fujinori H, Satoshi T, Junya M. Isoflavonoids produced by Iris pseudacorus leaves treated with cupric chloride. Phytochemistry 1991;30:157–63.
- Katherine NS. Carbon-13 nuclear magnetic resonance of biologically important aromatic acids. J Am Chem Soc 1972;94:8564–8.
- Seo S, Tomita, Tori K. Carbon-13 NMR spectra of urs-12-enes and applications to structural assignment of components of Isodon japonica hara tissue cultures.Tetrahedron Lett 1975;16:7–10.
- Masami K, Hiroyuki M, Mai N, Hironobu T. Oleanane-type triterpenes from Viburnum awabuki. Phytochemistry 1998;47:1337–41.
- Li HL, Jian XP, Jing FZ, Liang LI. A new lignan from Boschniakia himalaica. Chin Chem Lett 2004;15:43–5.
- Das B, Venkataiah B, Kashinatham A. Syringaresinol from Parthenium hysterophorus. Fitoterapia 1999;70:101–2.
- Gülçin İ, Alici, HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005;53:281–5.
- Oyaizu M. Studies of products browning reaction. Antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr 1986;44:307–15.
- Tappel AL. Methods of Enzymology. NewYork: Academic Press, 1962;5:539–42.
- Deplorte C, Backhouse N, Negrete R, Gallardo M.C. Anti-inflammatory and antipyretic activities of spartidienedione isolated from Psila spartioides. Int J Pharmacogn 1996;34:179–83.
- Hadjipavlou LD. Quantitative structure-activity relationship (QSAR) studies on non-steroidal anti-inflammatory drug (NSAIDs). Curr Med Chem 2000;7:375–88.
- Hawkey CJ, Skelly MM. Gastrointestinal safety of selective COX-2 inhibitors. Curr Pharm Des 2002;8:1077–89.
- Xavier L, Fabien J, Jacques D, Bernard P. New trends in dual 5-LOX/COX inhibition. Curr Med Chem 2002;9:941–62.
- Christopher JM. Carrageenan-induced paw edema in the rat and mouse. In: Winyard PG, Willoughby DA, eds. Inflammation Protocols. Totowa, NJ: Humana Press, 2003;225:115–21.
- Suraj PS, Yuji N, Tadahiro T. Chemical constituents of Nepalese propolis (II). Chem Pharm Bull 2007;55:926–9.
- Meir S, Kanner J, Akiri B. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 1995;43:1813–19.
- Steinhilber DA. 5-Lipoxygenase: a target for anti-inflammatory drugs revisited. Curr Med Chem 1999;6:71–5.
- Ding XY, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology 2001;4:291–9.