Abstract
A new series of macrocyclic complexes of type [M(TML)X]X2, where M = Cr(III), Fe(III), TML is tetradentate macrocyclic ligand, and X = Cl−, NO3−, CH3COO−, have been synthesized by condensation of isatin and ethylenediamine in the presence of metal salt. The complexes were synthesized by both conventional and microwave methods. The complexes have been characterized with the help of elemental analysis, conductance measurement, magnetic measurement, and infrared, far infrared, and electronic spectral studies. Molar conductance values indicate them to be 1:2 electrolytes. Electronic spectra along with magnetic moments suggest five-coordinate square pyramidal geometry for these complexes. The complexes were also tested for their in vitro antibacterial activity. Some of the complexes showed satisfactory antibacterial activitiy.
| Abbreviations: | ||
| MIC, | = | minimum inhibitory concentration; |
| MTCC, | = | microbial type culture collection; |
| MHA, | = | Müller Hinton agar; |
| CFU, | = | colony forming unit; |
| B.M., | = | Bohr magneton; |
| DMF, | = | N,N-dimethylformamide; |
| DMSO, | = | dimethylsulfoxide; |
| BHI, | = | brain heart infusion. |
Introduction
The study of macrocyclic complexes is becoming a growing class of research. The chemistry of macrocyclic complexes has received much attention in recent years on account of its various applications in bioinorganic chemistry. The field of macrocyclic complexes has undergone enormous growth due to the synthesis of a variety of macrocycles which behave as coordinating agents for metal ions. Nitrogen containing macrocycles have a strong tendency to form stable complexes with transition metals. The condensation reaction between diketones and primary diamines in the presence of metal ions has played a vital role in the development of tetraazamacrocyclic complexesCitation1–4. Macrocyclic nickel complexes find use in DNA recognition and oxidationCitation5, while macrocyclic copper complexes find use in DNA binding and cleavageCitation6. Some macrocyclic complexes have been reported as showing antibacterial, antifungal, and anti-inflammatory activitiesCitation7,Citation8. Macrocyclic metal chelating agents (e.g. DOTA) are useful to detect tumor lesionsCitation9. Isatin derivatives have been reported to exhibit antibacterial, antifungal, and anti-human immunodeficiency virus (HIV) activitiesCitation10. Prompted by these, in the present article, macrocyclic complexes of Cr(III) and Fe(III) derived from isatin and ethylenediamine synthesized by both the conventional method and microwave irradiation are discussed. The complexes have been characterized by various physicochemical techniques such as infrared (IR), far IR, electronic spectroscopy, magnetic susceptibility, elemental analysis, and molar conductance.
Experimental
Chemistry
All the reported macrocyclic complexes were prepared by both template and microwave methods.
Conventional method
To a hot, well stirred MeOH solution (∼50 cm3) of ethylenediamine (10 mmol) was added trivalent chromium or iron salt (5 mmol) dissolved in a minimum quantity of MeOH (20 cm3). The resulting solution was refluxed for 0.5 h. After that, isatin (10 mmol) dissolved in ∼20 cm3 MeOH was added to the refluxing mixture, and refluxing continued for 6–8 h. The mixture was concentrated to half of its volume and kept in a desiccator for 2 days. The complexes were then filtered, washed with MeOH, Me2CO, and Et2O, and dried in vacuo. The complexes were soluble in DMF and DMSO, but were insoluble in common organic solvents and water. They were found to be thermally stable up to ∼250°C and then decomposed.
Microwave irradiation method
A mixture of ethylenediamine (10 mmol) in 5 cm3 of MeOH and trivalent chromium and iron salt (5 mmol) dissolved in 5 cm3 of MeOH was subjected to microwave irradiation at an output of 300 W for a specified time of 0.5–1 min. After that, isatin (10 mmol) dissolved in 10 cm3 of MeOH was added to the reaction mixture. Irradiation of the reaction mixture was continued under the specified conditions for the next 1–2 min. The contents were cooled and kept in desiccators. The complexes thus obtained were washed with MeOH, Me2CO, and Et2O and then dried in vacuo.
Synthesis of the complexes by conventional and microwave irradiation methods may be shown by the following scheme:
where M = Cr(III) and Fe(III), X = Cl−, NO3−, CH3COO−, and TML is tetradentate macrocyclic ligand.
In vitro antibacterial activity (biological assay)
The synthesized macrocyclic complexes were tested for in vitro antibacterial activity against some bacterial strains using the method of spot-on-lawn on Müller Hinton agarCitation11.
Test pathogens
Four test pathogenic bacterial strains, viz Bacillus cereus (MTCC 1272), Salmonella typhi (MTCC 733), Escherichia coli (MTCC 739), and Staphylococcus aureus (MTCC 1144), were considered for determination of the MIC (minimum inhibitory concentration) of the selected complexes.
Culture conditions
The test pathogens were subcultured aerobically using brain heart infusion agar (HiMedia, Mumbai, India) at 37°C/24 h. Working cultures were stored at 4°C in brain heart infusion (BHI) broth (HiMedia, Mumbai, India), while stock cultures were maintained at −70°C in BHI broth containing 15% (v/v) glycerol (Qualigens, Mumbai, India). Organism was grown overnight in 10 mL BHI broth and centrifuged at 5000g for 10 min, and the pellet was suspended in 10 mL of phosphate buffered saline (PBS, pH 7.2). Optical density at 545 nm (OD-545) was adjusted to obtain 108 CFU/mL, followed by plating of serial dilutions onto plate count agar (HiMedia, Mumbai, India).
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is the lowest concentration of antimicrobial agent that prevents the development of viable growth after overnight incubation. The antimicrobial activity of the compounds was evaluated using the spot-on-lawn method on Müller Hinton agar (MHA; HiMedia, Mumbai, India). Soft agar was prepared by adding 0.75% agar in Müller Hinton broth (HiMedia, Mumbai, India). Soft agar was inoculated with 1% of 108 CFU/mL of the test pathogen and 10 mL was overlaid on MHA. From a ×1000 solution of compound (1 mg/mL of DMSO), ×1, ×2, ×4, ×8, ×16, ×32, ×64, and ×128 solutions were prepared. Dilutions of standard antibiotics (linezolid and cefaclor) were also prepared in the same manner. Five microliters of the appropriate dilution was spotted on the soft agar and incubated at 37°C for 24 h. Zones of inhibition of the compounds were considered after subtraction of the inhibition zone of DMSO. Negative control (with no compound) was also observed.
Analyses
The microanalysis of C, H, and N was recorded on an Elementar Vario EL III (Carlo Erba 1108) analyzer. These analyses were carried out at CDRI, Lucknow. Melting points were determined using capillaries in an electrical melting point apparatus. The metal contents were estimated using standard methods.
Physical measurements
Electronic spectra of the metal complexes were recorded in the region 1100–200 nm on a Hitachi U-2000 spectrophotometer. IR spectra were recorded on a Beckman IR-20 spectrophotometer in KBr/Nujol mull in the range 4000–200 cm−1. Magnetic moment studies were carried out at SAIF, IIT, Roorkee, on a vibrating sample magnetometer (model PAR 155). The conductivity was measured on a digital conductivity meter (HPG System, G-3001).
Results and discussion
Chemistry
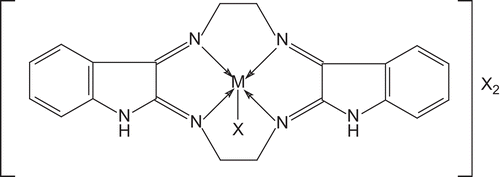
The analytical data suggest the formula of the macrocyclic complexes as: [M(C20H18N6)X]X2, where M = Cr(III), Fe(III) and X = Cl−, NO3−, CH3COO−. The test for anions was positive before and after decomposing the complexes with concentrated HNO3, indicating their presence outside and inside the coordination sphere. Conductivity measurements in DMSO indicated them to be 1:2 electrolye in nature (150–180 ohm−1 cm2 mol−1)Citation12. All compounds gave satisfactory elemental analysis results as shown in .
Table 1. Analytical data of trivalent chromium and iron complexes derived from isatin and ethylenediamine.
Several attempts to grow crystals of complexes in different solvents or mixtures of solvents for the purpose of X-ray crystallography were unsuccessful.
Infrared spectra
A pair of bands at ∼3230 cm−1 and ∼3260 cm−1 was observed in the spectrum of ethylenediamine corresponding to ν(NH2), but was found to be absent from the spectra of all complexes. A strong peak was observed at ∼1700 cm−1 in the spectrum of isatin, which may be attributed to the >C=O group. This peak was found to be absent from the spectra of all complexes, which indicates the absence of >C=O groups of the isatin moiety in the complexes. This confirms the condensation of carbonyl groups of isatin and amino groups of ethylenediamineCitation13,Citation14. This fact is also supported by the appearance of a new strong absorption band in the region ∼1590–1615 cm−1, which may be attributed to ν(C=N)Citation15,Citation16. These results provide strong evidence for the formation of the macrocyclic frameCitation17. The lower values of ν(C=N) indicate coordination of azomethine nitrogens to metalCitation18. The bands present at ∼1000–1350 cm−1 may be assigned to ν(C-N) vibration. The bands presents at ∼2900–3130 cm−1 may be assigned to various ν(C-H) vibrations.
Far infrared spectra
The far infrared spectra showed bands in the region ∼420–460 cm−1 corresponding to ν(M-N) vibrations in all complexesCitation19–21. The presence of bands in all complexes in the region ∼420–460 cm−1 originates from the ν(M-N) azomethine vibration modes and gives an idea about coordination of the azomethine nitrogenCitation22. The bands present at ∼300–310 cm−1 may be assigned as being due to ν(M-Cl) vibrationsCitation19,Citation21. The bands present at ∼210–250 cm−1 in all nitrato complexes are assignable to ν(M-O) vibrations of the nitrato groupCitation19.
Magnetic measurements and electronic spectra
Chromium complexes Magnetic moments of chromium complexes were found in the range of 4.20–4.60 B.M. The electronic spectra of chromium complexes showed bands at ∼9100–9260, 13,400–13,550, 17,400–18,300, 27,500–27,800, and 34,850 cm−1. However, these spectral bands cannot be interpreted in terms of a four- or six-coordinated environment around the metal atom. In turn, the spectra are comparable to those of five-coordinated Cr(III) complexes, whose structure has been confirmed with the help of X-ray measurementsCitation23. Thus, keeping in view the analytical data and electrolytic nature of these complexes, a five-coordinated square pyramidal geometry may be assigned to these complexes. Thus, assuming the symmetry C4V for these complexesCitation24, the various spectral bands may be assigned as: 4B1 → 4Ea, 4B1 → 4B2, 4B1 → 4A2, and 4B1 → 4Eb.
Iron complexes The magnetic moments of iron complexes lay in the range 5.85–5.90 B.M. The electronic spectra of trivalent iron complexes showed various bands at 9850–9900, 15,300–15,500, and 27,500–27,600cm−1, and these bands do not suggest an octahedral or tetrahedral geometry around the metal atom. The spectral bands are consistent with the range of spectral bands reported for five-coordinate square pyramidal iron(III) complexesCitation25. Assuming C4V symmetry for these complexes, the various bands can be assigned as: dxy → dxz, dyz and dxy → dz2. Any attempt to make an accurate assignment is difficult due to interactions of the metal–ligand pi-bond systems lifting the degeneracy of the dxz and dyz pair.
Biological assay results
Six chemically synthesized macrocyclic complexes were tested for their in vitro antibacterial activity against four test bacteria, Bacillus cereus (MTCC 1272), Salmonella typhi (MTCC 733), Escherichia coli (MTCC 739), and Staphylococcus aureus (MTCC 1144). MIC values of standard antibiotics, i.e. linezolid and cefaclor, were also registered against these bacteria. Linezolid showed MICs of 4, 4, 16, and 32 μg/mL against Bacillus cereus, Salmonella typhi, Escherichia coli, and Staphylococcus aureus, respectively, while cefaclor showed MICs of 8, 2, 8, and 16 μg/mL against Bacillus cereus, Salmonella typhi, Escherichia coli, and Staphylococcus aureus, respectively. However, all the complexes showed MICs greater than those of the standard antibiotics. Some complexes showed poor or no antibacterial activity. The MIC values registered by the synthesized complexes are given in .
Table 2. Minimum inhibitory concentration (MIC) shown by complexes against test bacteria using agar dilution assay.
Conclusions
Chemistry
Based on elemental analysis, conductivity, magnetic, electronic, and IR spectral studies the structure as shown in may be proposed for the reported complexes. Further, it was found that microwave irradiation required a shorter reaction time and less solvent and gave higher product yield, as compared to the conventional method ().
Table 3. Comparative data of trivalent chromium and iron complexes for conventional and microwave methods.
Biological activity
It has been suggested that chelation/coordination reduces the polarity of the metal ion mainly because of partial sharing of its positive charge with the donor group within the whole chelate ring system. This process of chelation thus increases the lipophilic nature of the central metal atom, which in turn favors its permeation through the lipid layer of the membrane, thus causing the metal complex to cross the bacterial membrane more effectively and hence increasing the activity of the complexes. It has also been observed that some moieties such as an azomethine linkage or a heteroaromatic nucleus introduced into such compounds exhibit extensive biological activities that may be responsible for the increase in hydrophobic character and liposolubility of the molecules in crossing the cell membrane of the microorganism, enhancing the biological utilization ratio and activity of the complexesCitation26,Citation27.
Acknowledgments
Thanks are also due to the authorities of NIT, Kurukshetra for providing necessary facilities. We are highly thankful to Jitender Singh for carrying out studies of the biological activities of the synthesized macrocyclic complexes.
Declaration of interest: One of the authors (D.P.S.) thanks the University Grants Commission, New Delhi for financial support in the form of a Major Research Project.
References
- Lindoy LF, ed. The Chemistry of Macrocyclic Ligand Complexes. Cambridge: Cambridge University Press, 1989.
- Constable EC, ed. Coordination Chemistry of Macrocyclic Compounds. Oxford: Oxford University Press, 1999.
- Singh DP, Kumar R, Malik V, Tyagi P. Synthesis and characterization of complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) with macrocycle 3,4,11,12-tetraoxo-1,2,5,6,9,10,13,14-octaazacyclohexadeca-6,8,14,16-tetraene and their biological screening. Transition Met Chem 2007;32:1051–5.
- Singh DP, Kumar R, Kamboj M, Jain. Synthesis, spectral, and antibacterial studies of 16-membered tetraazamacrocyclic complexes. J Coord Chem 2009:62:2995–3002
- Muller JG, Chen X, Dadiz AC, Rokita SE, Burrows CJ. Macrocyclic nickel complexes in DNA recognition and oxidation. Pure Appl Chem 1993;65:545–50.
- Liu J, Lu TB, Deng H, Ji LN, Qu LH, Zhou H. Synthesis, DNA-binding and cleavage studies of macrocyclic copper(II) complexes. Transition Met Chem 2003;28:116–21.
- Keypour H, Khanmohammadi H, Wainwright KP, Taylor MR. Synthesis, crystal structure, NMR and ab initio molecular-orbital studies of some magnesium(II) macrocyclic Schiff-base complexes, with two 2-aminoethyl pendant arms. Inorg Chim Acta 2004;357:1283–91.
- Singh DP, Kumar R, Malik V, Tyagi P. Template synthesis, spectroscopic studies and biological activities of macrocyclic complexes derived from thiocarbohydrazide and glyoxal. J Enzyme Inhib Med Chem 2007;22:177–82.
- Kosmas C, Snook D, Gooden CS, Courtenay-Luck NS, McCall MJ, Claude F, et al. Development of humoral immune responses against a macrocyclic chelating agent (DOTA) in cancer patients receiving radioimmunoconjugates for imaging and therapy. Cancer Res 1992;52:904–11.
- Ferrari MB, Pelizzi C, Pelosi G, Rodriguez MC. Preparation, characterization and x-ray structures of 1-methylisatin 3-thiosemicarbazone copper, nickel and cobalt complexes. Polyhedron 2002;21:2593.
- Singh DP, Kumar R, Singh J. Biologically active trivalent metal macrocyclic complexes derived from oxalyldihydrazide and benzil: synthesis and spectroscopic approach. Eur J Med Chem 2009;44:1731–6.
- Kumar R, Singh R. Chromium(III) complexes with different chromospheres macrocyclic ligand: synthesis and spectroscopic studies. Turk J Chem 2006;30:77–87.
- Prasad RN, Mathur M. CrIII, FeIII, CoII, NiII, CuII and ZnII complexes of 26- and 28-membered tetraazamacrocycles. J Indian Chem Soc 2006;83:1208–13.
- Zeng Q, Sun J, Gou S, Zhou K, Fang J, Chen H. Synthesis and spectroscopic studies of dinuclear copper(II) complexes with new pendant-armed macrocyclic ligands. Transition Met Chem 1998;23:371–3.
- Singh AK, Singh R, Saxena P. Macrocyclic metal complexes: synthesis and characterization of 14- and 16- membered tetraaza macrocyclic complexes of transition metals. Transition Met Chem 2004;29:867–9.
- Gupta LK, Chandra S. Physicochemical and biological characterization of transition metal complexes with a nitrogen donor tetra-dentate novel macrocyclic ligand. Transition Met Chem 2006;31:368–73.
- Mohamed AK, Islam KS, Hasan SS, Shakir M. Metal ion directed synthesis of 14-16 membered tetraimine macrocyclic complexes. Transition Met Chem 1999;24:198–201.
- Lodeiro C, Basitida R, Bertolo E, Macias A, Rodriguez R. Synthesis and characterization of four novel NxOy-Schiff base macrocyclic ligands and their metal complexes. Transition Met Chem 2003;28:388–94.
- Shakir M, Islam KS, Mohamed AK, Shagufta M, Hasan SS. Macrocyclic complexes of transition metals with divalent polyaza units. Transition Met Chem 1999;24:577–80.
- Aqra FMAM. New macrocyclic complexes containing amide, imine and secondary amine functions. Transition Met Chem 1999;24:337–9.
- Chandra S, Kumar R. Synthesis and spectral studies on mononuclear complexes of chromium(III) and manganese(II) with 12-membered tetradentate N2O2, N2S2 and N4 donor macrocyclic ligand. Transition Met Chem 2004;29:269–75.
- Rana VB, Singh DP, Singh P, Teotia MP. Trivalent chromium, manganese, iron and cobalt chelates of a tetradentate N6 macrocyclic ligand. Transition Met Chem 1982;7:174–7.
- Wood JS. Stereochemical electronic structural aspects of five coordination. Prog Inorg Chem 1972;16:227.
- Singh DP, Rana VB. Binuclear chromium (III), manganese(III), iron(III) and cobalt(III) complexes bridged by diaminopyridine. Polyhedron 1995;14:2901–6.
- Lever ABP. Inorganic Electronic Spectroscopy. Amsterdam: Elsevier, 1984.
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enzyme Inhib Med Chem 2006;21:749–55.
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff bases derived from fluorobenzaldehyde and triazoles. J Enzyme Inhib Med Chem 2006;21:557–62.