Abstract
A series of metal complexes of cobalt(II), nickel(II), and copper(II) have been synthesized with newly derived biologically active ligands. These ligands were synthesized by the condensation of 2-amino-4-phenyl-1,3-thiazole with 8-formyl-7-hydroxy- 4-methylcoumarin. The probable structure of the complexes has been proposed on the basis of analytical and spectroscopic data (IR, UV-Vis, ESR, FAB-mass, and thermoanalytical). Electrochemical study of the complexes is also reported. Elemental analysis of the complexes confined them to stoichiometry of the type ML2.2H2O [M = Co(II), Ni(II), and Cu(II)]. The Schiff base and its metal(II) complexes have been screened for their antibacterial (Escherichia coli, Staphylococcus aureus, Staphylococcus pyogenes, and Pseudomonas aeruginosa) and antifungal activities (Aspergillus niger, Aspergillus flavus, and Cladosporium) by the MIC method. The brine shrimp bioassay was carried out to study their in vitro cytotoxic properties, and also the Schiff base and its metal(II) complexes have been studied for DNA cleavage.
Introduction
Schiff bases and their relevant tranisition metal complexes are still found to be of great interest in coordination chemistryCitation1–3. Various cobalt, nickel, and copper metal complexes are reported to have antimicrobial activitiesCitation4,Citation5. Moreover, the incorporation of transition metals into Schiff bases enhances the biological activity of the ligand and decreases the cytotoxic effects of both the metal ion and ligand on the hostCitation6. The interactions of transition metal complexes with DNA have been extensively studied for their usage as probes for DNA structure and their potential application in chemotherapyCitation7–9. An important DNA-related activity of transition metal complexes is that some complexes show the ability to cleave DNA. Very recently, Cu(II) complexes have been reported to be active in DNA strand scissionCitation10–12.
Thiazole and substituted thiazoles possess interesting biological activity, probably conferred on them by the strong aromaticity of their ring systemCitation13. They are also a very interesting class of compounds because of their wide application as antimicrobialCitation14, anti-inflammatoryCitation15, anti-degenerativeCitation16, and anti-human immunodeficiency virus (HIV)Citation17 activities. In addition to the thiazole derivatives, coumarins and their derivatives display not only important structural variety but also significant biologicalCitation18–22 and pharmacologicalCitation23,Citation24 properties. Many of these compounds possess antibacterialCitation23, antifungalCitation24, and insecticidalCitation21 activities, and also the hydroxycoumarins are typical phenolic compounds and therefore act as potent metal chelators and free radical scavengers. They are powerful chain-breaking antioxidantsCitation25. Metal complexes of coumarin derivatives have been extensively investigated and reported from our laboratoryCitation26,Citation27.
Recently, a number of attempts have been made to obtain Ni(II), Cu(II), and Zn(II) complexes with the thiazole-derived Schiff basesCitation28.
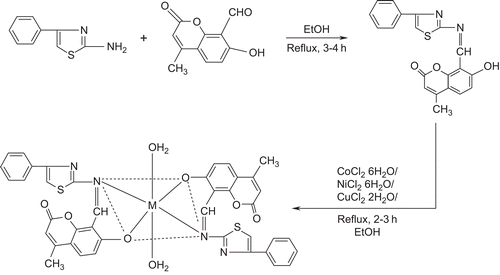
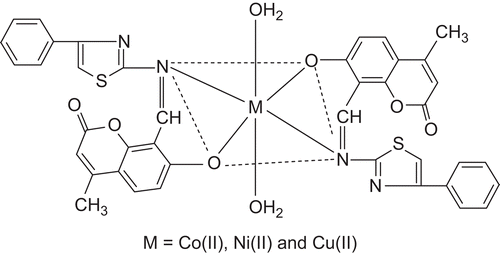
A survey of the literature reveals that no work has been carried out on the synthesis of metal complexes with the present Schiff base. This Schiff base has ON donor sites with varied coordination abilities. Because of the importance of thiazole and coumarin compounds in biological and medicinal fields, this attracted our attention and aroused our interest in elucidating the structure of Co(II), Ni(II), and Cu(II) complexes with this bioactive Schiff base. The synthesized metal complexes () have been characterized by analytical, spectral, thermal, and molar conductivity techniques. They have also been evaluated for their antibacterial and antifungal properties against various pathogenic bacterial strains using the minimum inhibitory concentration (MIC) method.
Experimental
Physical measurements
The infrared (IR) spectra of the ligand and its Co(II), Ni(II), and Cu(II) complexes were recorded on a Hitachi-270 IR spectrophotometer in the 4000–250 cm−1 region in KBr disks. The electronic spectra of the complexes were recorded in dimethylformamide (DMF) on a Varian Cary 50-Bio ultraviolet (UV) spectrophotometer in the region 200–1100 nm. The 1H nuclear magnetic resonance (NMR) spectrum of the ligand was recorded in dimethylsulfoxide (DMSO)-d6 on a Bruker 300 MHz spectrometer at room temperature using tetramethylsilane (TMS) as an internal reference. Fast atom bombardment (FAB) mass spectra were recorded on a Jeol SX 102/DA-6000 mass spectrometer/data system using argon/xenon (6 kV, 10 mA) as the FAB gas. The accelerating voltage was 10 kV, and the spectra were recorded at room temperature with m-nitrobenzyl alcohol used as the matrix. The mass spectrometer was operated in the +ve ion mode. Electron spin resonance (ESR) spectra of Cu(II) complexes were recorded on a Varian E-112 ESR spectrometer. The electrochemistry of Cu(II) complexes was determined on a CHI1110A-electrochemical (HCH Instruments) analyzer. Thermogravimetric analysis data were measured from room temperature to 1000°C at a heating rate of 10°C/min. The data were obtained using a PerkinElmer Diamond TG/DTA instrument. Molar conductivity measurements were recorded using an Elico-CM-82 T conductivity bridge with a cell having cell constant 0.51, and magnetic moments of the complexes were determined using a Faraday balance. All the chemicals used were of reagent grade. 7-Hydroxy-4-methyl-coumarin was obtained from Across Chemical Company, 8-formyl-7-hydroxy-4-methyl-coumarin was prepared as described in the literatureCitation29, and 2-amino-4-phenyl-1,3-thiazole was prepared as described in the literatureCitation30.
Synthesis of Schiff base
A mixture of 2-amino-4-phenyl-1,3-thiazole (0.002 mol, 25 mL) and 8-formyl-7-hydroxy-4-methylcoumarin (0.002 mol, 25 mL) in 1:1 molar proportions was boiled under reflux for 2–3 h in an alcoholic medium containing a few drops of concentrated HCl. The product was separated, filtered, washed with alcohol, and recrystallized from EtOH.
Synthesis of Co(II), Ni(II), and Cu(II) complexes
An alcoholic solution (25 mL) of the Schiff base (2 mmol) was refluxed with 1 mmol of metal(II) chloride (Co(II), Ni(II), or Cu(II)) in ethanol (25 mL) on a steam bath for 1 h. Then, to the reaction mixture, 2 mmol of sodium acetate was added and reflux was continued for 3 h. The separated complex was filtered, washed thoroughly with water, ethanol, and ether, and finally dried under vacuum over fused CaCl2.
Biological study
In vitro antibacterial and antifungal assay
The synthesized Schiff base and its Co(II), Ni(II), and Cu(II) complexes were screened in vitro for their biological activity using four bacteria, namely Escherichia coli, Staphylococcus aureus, Staphylococcus pyogenes, and Pseudomonas aeruginosa and three fungi, namely Aspergillus niger, Aspergillus flavus, and Cladosporium by the reported methodCitation31. A stock solution (1 mg/mL) of the test chemical was prepared by dissolving 10 mg of the test compound in 10 mL of N,N-dimethyl formamide (DMF) solvent. Control for each dilution was prepared by diluting 10 mL of solvent instead of stock solution with sterilized distilled water.
The bacteria were subcultured in agar medium. Petri dishes were incubated for 24 h at 37°C. Standard antibacterial drug (gentamicin) was also screened under similar conditions for comparison. The fungi were subcultured in potato dextrose agar medium. Standard antifungal drug (fluconazole) was used for comparison. Petri dishes were incubated for 48 h at 37°C. Wells were dug in the agar medium using a sterile metallic borer. Activity was determined by measuring the diameter of the zone showing complete inhibition (mm). Growth inhibition was compared with the standard drugs. In order to clarify any effect of DMF on the biological screening, separate studies were carried out with solutions of DMF alone and they showed no activity against any microbial strain.
Minimum inhibitory concentration (MIC)
Compounds showing promising antibacterial/antifungal activity were selected for minimum inhibitory concentration studies.
In vitro cytotoxicity
The synthesized Schiff base and its Co(II), Ni(II), and Cu(II) complexes were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyaer et al.Citation32. Brine shrimp (Artemia salina Leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm) filled with artificial seawater, which was prepared with a commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened, while the minor compartment was open to ordinary light.
After 2 days, nauplii were collected by a pipette from the light side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMF. From this, stock solutions of 100, 50, and 10 µg/mL were transferred to nine vials (three for each dilution were used for each test sample, and LD50 is the mean of three values) and one vial was kept as the control, having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After 2 days, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution), and the volume was adjusted with seawater to 5 mL per vial. After 24 h the number of survivors was counted. Data were analyzed using a Finney computer program to determine the LD50 valuesCitation33.
DNA cleavage experiment
Preparation of culture media
Nutrient broth (peptone, 10; yeast extract, 5; NaCl, 10 (g/L)) was used for culturing E. coli and potato dextrose broth (potato, 250; dextrose, 20 (g/L)) was used for the culture of A. niger. Amounts of 50 mL of media were prepared and autoclaved for 15 min at 121°C under 15 lb pressure. The autoclaved media were inoculated with the seed culture, and E. coli was incubated for 24 h and A. niger for 48 h at 37°C.
Isolation of DNA
Fresh bacterial culture (1.5 mL) was centrifuged to obtain a pellet, which was then dissolved in 0.5 mL of lysis buffer (100 mM Tris pH 8.0, 50 mM ethylenediaminetetraacetic acid (EDTA), 10% sodium dodecyl sulfate (SDS)). To this, 0.5 mL of saturated phenol was added and the mixture incubated at 55°C for 10 min. Then it was centrifuged at 10,000 rpm for 10 min. Next, an equal volume of chloroform/isoamyl alcohol (24:1) and one-twentieth volume of 3 M sodium acetate (pH 4.8) were added to the supernatant and centrifuged at 10,000 rpm for 10 min. To this supernatant, three volumes of chilled absolute alcohol were added. The precipitated DNA was separated by centrifugation. The pellet was dried and dissolved in TE buffer (10 mM Tris pH 8.0, 1 mM EDTA) and stored in cold conditions.
Agarose gel electrophoresis
Cleavage products were analyzed by the agarose gel electrophoresis method. Test samples (1 mg/mL) were prepared in DMF. The samples (25 μg) were added to the isolated DNA of E. coli and A. niger and incubated for 2 h at 37°C. Then, 20 μL of DNA sample (mixed with bromophenol blue dye at 1:1 ratio) were loaded carefully into the electrophoresis chamber wells along with a standard DNA marker containing TAE buffer (4.84 g Tris base, pH 8.0, 0.5 M EDTA/1 L) and finally loaded on agarose gel. A constant 50 V of electricity was passed for 30 min. The gel was removed and stained with 10.0 μg/mL ethidium bromide for 10–15 min, and the bands observed under UV transillumination and photographed to determine the extent of DNA cleavage. Results were compared with the standard DNA marker.
Results and discussion
All the cobalt(II), nickel(II), and copper(II) complexes were colored, stable in air, and non-hygroscopic solids. These complexes were soluble in DMF and DMSO. Elemental analysis showed that the cobalt(II), nickel(II), and copper(II) complexes have a 1:2 stoichiometry of the type ML2.2H2O. Molar conductance values in a concentration of solution of 10−3 M were too low to account for any dissociation of the complexes in DMF. Hence, these complexes are non-electrolytes in DMF (). The metal contents were estimated gravimetrically by the standard methodCitation34. Carbon, hydrogen, and nitrogen were estimated using a C, H, N analyzer. The results are listed in .
Table 1. Elemental analysis of Schiff base and its Co(II), Ni(II), and Cu(II) complexes with magnetic and molar conductance data.
Infrared spectra
Results for selected IR spectra of the Schiff base and its metal complexes along with their tentative assignments are presented in .
Table 2. Important infrared frequencies (in cm−1) of Schiff base and its Co(II), Ni(II), and Cu(II) complexes.
The IR spectrum of the Schiff base exhibited a broad, weak band with fine structure in the region 2674 cm−1, attributed to the intramolecular H-bonded -OH. The medium to high intensity band at 1645 cm−1 was assigned to ν(C=N) of the azomethine group. That confirms the presence of the 8-formyl-7-hydroxy-4-methylcoumarin moiety. The medium intensity band at 865 cm−1 observed in the free ligand was ascribed to ν(C-S-C) stretching vibrationCitation35.
A high intensity band was present in the 1290–1285 cm−1 region with an additional band around 1520–1510 cm−1 and was assigned to phenolic ν(C-O) vibration. The band located at 1740 cm−1 in the Schiff base was attributed to ν(C=O)Citation36.
In the case of Co(II), Ni(II), and Cu(II) complexes we observed the following changes.
A broad weak band with fine structure in the 2674 cm−1 region, assigned to the H-bonded -OH in the Schiff base, had disappeared in all the complexes. The high intensity band due to phenolic C-O appearing in the region 1290–1285 cm−1 in the Schiff base appeared as a medium to high intensity band in the 1380–1370 cm−1 region in the complexes. These observations support the formation of M-O bonds via deprotonation. It confirms that H-atoms of -OH groups were replaced by the metal ion. The bands at 1520–1510 cm−1 in the Schiff base appeared in the 1530–1515 cm−1 region in the complexes, suggesting the behavior of the phenolic oxygen atoms as monodentate. The medium intensity band at around 1645 cm−1, due to ν(C=N) in the Schiff base, showed a lower position shifted by 20–30 cm−1 in the complexes. The lower shift indicated that the C=N group of the Schiff base was coordinated to the metal ion through nitrogen. The band located at 1740 cm−1 due to ν(C=O) in the Schiff base was unaffected in the complexes, indicating that the lactone oxygen was not involved in the coordination. The medium intensity band at 865 cm−1 due to ν(C-S-C) in the Schiff base was unaffected in the complexes, indicating that the sulfur atom was not involved in the coordination. A broad band in the region 3440–3420 cm−1 indicated the presence of a coordinated water moleculeCitation37 and two weaker bands in the regions 800–750 and 720–700 cm−1 were due to ν(-OH) rocking and wagging modes of vibration, respectivelyCitation38. The complexes showed medium intensity bands in the regions 530–545 cm−1 and 430–455 cm−1 assigned to ν(M-O) and ν(M-N) modes.
Thus, the IR spectral data provide strong evidence for complexation of the potentially tetradentate ligand to the metal(II) ions.
1H NMR spectroscopy
The 1H NMR spectrum of the Schiff base exhibited singlets at 10.44, 8.62, and multiplets at 7.2–7.5 ppm due to phenolic OH, -CH=N, and aromatic protons, respectively. A singlet around 2.84 ppm was due to methyl protons.
13C NMR spectroscopy
The 13C NMR spectrum of the Schiff base exhibited signals at 117.4, 119.2, 120.4, 128.1, 135.0, and 149.2 ppm, corresponding to aromatic carbons. Resonance at 11.6 ppm was due to aliphatic (-CH3) carbon, and the azomethine (-HC=N) carbon showed a peak at 159.2 ppm.
Electronic spectral studies
The electronic spectrum of the cobalt(II) complex exhibited absorption bands in the regions 8000–10,000 cm−1 and 18,000–20,000 cm−1 corresponding to ν1 and ν3 transitions, respectively, and these were attributed to the transitions 4T1g (F) → 4T2g (F) (ν1) and 4T1g (F) → 4T1g (P) (ν3), respectivelyCitation39,Citation40. In the present Co(II) complex, absorption bands at 9500 and 19,000 cm−1 corresponded to ν1 and ν3 transitions, respectively. These bands are characteristic of the high-spin octahedral cobalt(II) complex. However, the ν2 band was not observed because of its proximity to the strong ν3 transition. The position of the ν2 band can be calculated by using the relationship given in the literatureCitation41,Citation42 ().
Table 3. Ligand field parameters of Co(II) and Ni(II) complexes.
The Ni(II) complex () exhibited three bands at 10,416, 16,459, and 26,452 cm−1 attributed to the 3A2g → 3T2g (ν1), 3A2g → 3T1g (F) (ν2), and 3A2g → 3T1g (P) (ν3) transitions, respectively, which indicates octahedral geometry around the Ni(II) ionCitation39.
The electronic spectrum of the Cu(II) complex showed an absorption band in the region 14,540–14,780 cm−1, attributed to the 2Tg ← 2Eg transition indicative of distorted octahedral geometryCitation43,Citation44.
Magnetic studies
The magnetic moments obtained at room temperature are listed in . The Co(II) complex showed a magnetic moment of 4.92 BM. This value is within the expected range of 4.7–5.2 BMCitation45,Citation46 for octahedral complexes. Hence, the Co(II) complex has an octahedral configuration. The Ni(II) complex showed a magnetic moment of 3.38 BM. It is reported that the octahedral Ni(II) complex exhibits a magnetic moment in the range 2.5–3.5 BMCitation46,Citation47. The Cu(II) complex showed a magnetic moment of 1.74 BM, which is slightly higher than the spin-only value, expected for one unpaired electron, which offers the possibility of an octahedral geometryCitation48. The magnetic moment values suggest an octahedral geometry around the metal(II) ions.
ESR spectral studies
The ESR spectrum of Cu(II) complex was studied, and the g| and g⊥ values were found to be 2.05 and 2.08, respectively. The gav value was calculated to be 2.07. The Cu(II) complex showed a reversed axial (compressed octahedral) type spectrum with g| < g⊥. The trend, g| < g⊥, showed that the electron was delocalized in the dz2 orbital of the ground state of Cu(II). In this case (g| < g⊥), distortion occurred by compressionCitation49. The parameter G, determined as G = (g| – 2)/(g⊥ – 2) is found to be much less than 4, suggesting considerable interaction in the solid stateCitation50.
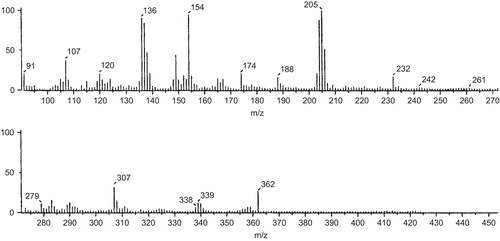
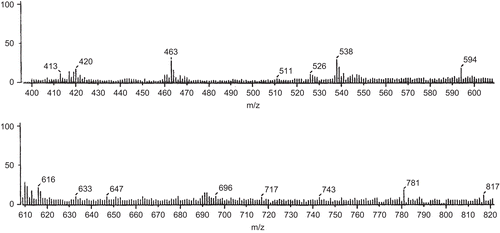
FAB-mass spectral studies of Schiff base and its Co(II) complex
The FAB-mass spectrum of the Schiff base was studied and is depicted in . The spectrum showed a molecular ion peak at m/z 362, which is equivalent to its molecular weight [L + H]+.
The FAB-mass spectrum of the Co(II) complex with the Schiff base was studied and is depicted in . The spectrum showed a molecular ion peak M+ at m/z 817, equivalent to its molecular weight [ML2.2H2O]+. The molecular ion by the loss of two water molecules gave a fragment ion peak at m/z 781.
All these fragments leading to the formation of the species [ML]+, which underwent demetallation to form the species [L + H]+, gave a fragment ion at m/z 360. All these fragmentation patterns were well observed in the FAB-mass spectrum.
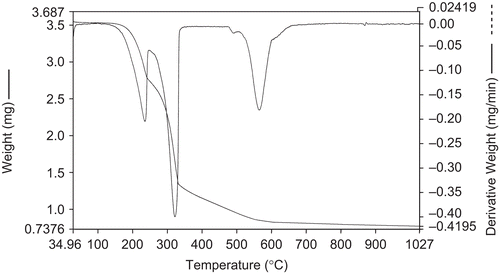
Thermogravimetric study
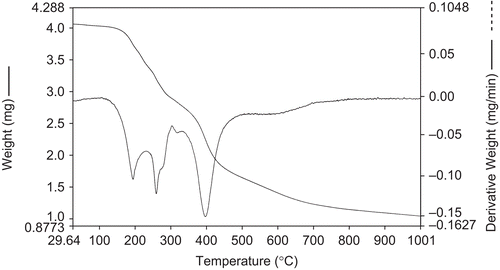
The nature of the proposed chemical change with temperature range is given in . The thermal decomposition of the respective Co(II), Ni(II), and Cu(II) complexes took place in three steps, as indicated by differential thermogravimetric (DTG) peaks around 175–198, 250–270, and 365–420°C of Co(II) and Ni(II) and around 205–230, 310–340, and 570–605°C of the Cu(II) complex, corresponding to the mass loss of two coordinated water moleculesCitation51, two thiazole moieties, and two coumarin moieties, respectively ( and ).
Table 4. Thermogravimetric data of Co(II), Ni(II), and Cu(II) complexes.
Electrochemistry
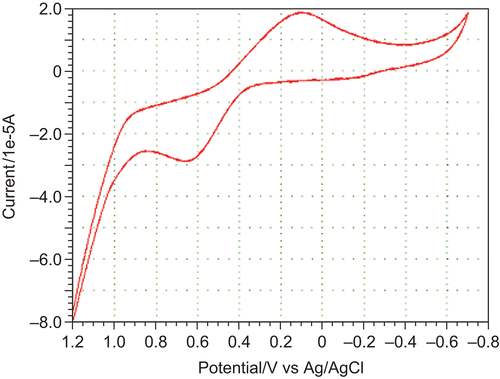
Electrochemical properties of the complexes were studied on a CHI1110A-electrochemical analyzer in N,N-dimethyl formamide (DMF) containing 0.05 M n-Bu4NClO4 as the supporting electrolyte. A cyclic voltammogram of Cu(II) displayed a reduction peak at Epc = 0.1023 V with a corresponding oxidation peak at Epa = 0.6472 V. The peak separation of this couple (ΔEp) was 0.5449 V at a scan rate of 0.1 V/s and increased with scan rate. The most significant feature of the Cu(II) complex was the Cu(II)/Cu(I) couple. The difference between forward and backward peak potentials can provide a rough evaluation of the degree of reversibility of the one-electron transfer reaction. Analyses of cyclovoltammetric responses with the scan rate varying from 50 to 250 mV/s gave evidence for quasi-reversible one-electron oxidation ().
The ratio of cathodic to anodic peak height was less than 1. However, the peak current increased with an increase of the square root of the scan rate. This established the electrode process as diffusion-controlledCitation52. The separation in peak potentials increased at higher scan rates. These characteristic features are consistent with the quasi-reversibility of the Cu(II)/Cu(I) couple.
Biological results
In vitro antibacterial and antifungal activity
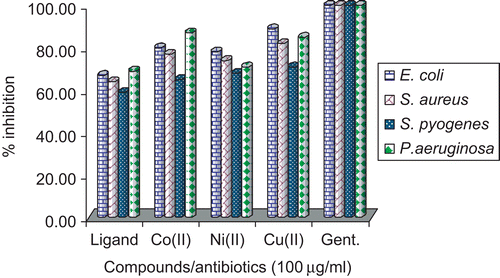
The microbial results are systematized in and and . The antibacterial and antifungal studies suggested that the Schiff base was biologically active, and its metal(II) complexes showed significantly enhanced antibacterial and antifungal activity. It is, however, knownCitation53,Citation54 that chelation tends to make Schiff bases act as more powerful and potent bacteriostatic agents, thus inhibiting the growth of bacteria and fungi more than the parent Schiff base. It is suspected that factors such as solubility, conductivity, dipole moment, and cell permeability mechanism (influenced by the presence of metal ions) may be the possible reasons for the increase in activity.
Table 5. Antibacterial results for Schiff base and its Co(II), Ni(II), and Cu(II) complexes.
In the case of bacteriological studies it was observed that the ligand exhibited moderate activity toward E. coli, P. aeruginosa, and S. aureus and low activity toward S. pyogenes. Among the Co(II), Ni(II), and Cu(II) complexes, Cu(II) and Co(II) showed high activity toward E. coli and P. aeruginosa. The Ni(II) complex showed moderate activity.
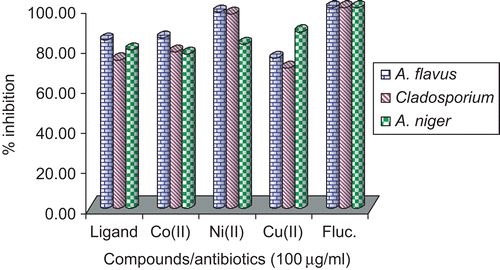
In the study of antifungal activity it was observed that the Schiff base showed high activity against A. flavus and A. niger and less toward Cladosporium. In the case of the Co(II) complex, it exhibited high activity against A. niger and Cladosporium and moderate activity toward A. niger; the result for A. flavus was interesting in that it showed high activity (almost equal to standard) in lower concentration, and the same was observed for the Cu(II) complex toward A. flavus and A. niger, with moderate activity toward Cladosporium.
Although the ligand showed good antifungal activity against A. niger, A. flavus, and Cladosporium, it was evident from the data that this activity was significantly increased on coordination with the metal ions. This enhancement in the activity may be rationalized mainly on the basis that their structures possess an additional C=N bond. It has been suggested that ligands with nitrogen and oxygen donor systems inhibit enzyme activity, since the enzymes which require these groups for their activity appear to be especially more susceptible to deactivation by metal ions on coordination. Moreover, coordination reduces the polarityCitation32 of the metal ion mainly because of the partial sharing of its positive charge with the donor groupsCitation50 within the chelate ring system formed during coordination. This process, in turn, increases the lipophilic nature of the central metal atom, which favors its permeation more efficiently through the lipid layer of the micro-organismCitation54, thus destroying it more aggressively. A minimum inhibitory concentration value of 5 μg/mL was shown by Cu(II) and Co(II) complexes against E. coli, S. aureus, P. aeruginosa, A. flavus, and A. niger and the Schiff base against E. coli, P. aeruginosa, A. niger, and Cladosporium. In all other cases, the compounds exhibited MICs ranging from 5 to 100 μg/mL against all the microbial strains, as given in .
Table 6. Minimum inhibitory concentration (MIC, μg/mL) results for Schiff base and its Co(II), Ni(II), and Cu(II) complexes.
In vitro cytotoxicity results
It is evident from the data recorded in that only Co(II) and Cu(II) complexes displayed cytotoxic activity with LD50 = 8.439 × 10−4 and 9.564 × 10−4 M/mL, respectively, against Artemia salina, while the Schiff base and Ni(II) complex were almost inactive for this assay.
Table 7. Brine shrimp bioassay data for Schiff base and its Co(II), Ni(II), and Cu(II) complexes.
Electrophoretic analysis
The Schiff base and the Co(II), Ni(II), and Cu(II) complexes were studied for their DNA cleavage activity by the agarose gel electrophoresis method, and the results are presented in .
Figure 8. Agarose gel electrophoresis. M, standard molecular weight marker; C-E.coli, control DNA of E. coli; lanes 1–4, E. coli DNA treated with Schiff base, Cu(II), Co(II), and Ni(II) complexes, respectively; lanes 5–8, A. niger DNA treated with Schiff base, Cu(II), Co(II), and Ni(II) complexes, respectively; C-niger, control DNA of A. niger.
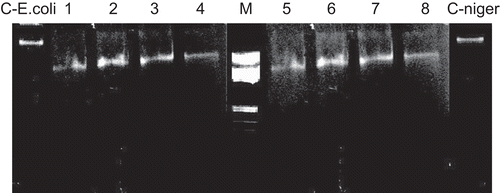
The gel after electrophoresis clearly revealed that both the Schiff base and the complexes acted on DNA, as there was a molecular weight difference between the control and the treated DNA samples. The difference was observed in the bands (lanes 1–8) compared to the control DNA of E. coli and A. niger. This shows that the control DNA alone did not demonstrate any apparent cleavage whereas the complexes did. However, the nature of reactive intermediates involved in DNA cleavage by the complexes is not clear. The results indicate the important role of metal in these isolated DNA cleavage reactions. As the compounds were observed to cleave DNA, it can be concluded that the compounds inhibited growth of the pathogenic organism by cleaving the genome.
Conclusion
The synthesized Schiff base acts as a tetradentate ligand through the coordination of azomethine nitrogen and phenolic oxygen atoms to the metal ion. The bonding of ligand to metal ion was confirmed by the analytical, IR, electronic, magnetic, ESR, and FAB-mass studies.
Electrochemical study of the Cu(II) complex could provide the degree of reversibility of the one-electron transfer reaction and it has quasi-reversible character. Among the Co(II), Ni(II), and Cu(II) complexes, Co(II) and Cu(II) showed high activity toward E. coli, S. pyogenes, and P. aeruginosa and the Co(II) complex showed high activity (almost equal to the standard) in lower concentration toward A. flavus; the same was observed for the Cu(II) complex toward A. flavus and A. niger, with moderate activity toward Cladosporium; the Ni(II) complex showed moderate activity. From DNA cleavage study it can be concluded that the compounds inhibit growth of the pathogenic organism by cleaving the genome.
All these observations together lead us to propose the structure shown in , in which the complex has stoichiometry of the type ML2.2H2O (M = Co(II), Ni(II), and Cu(II)).
Acknowledgements
The authors are grateful to the Chairman, Department of Chemistry, Karnatak University, Dharwad for the facilities.
Declaration of interest: One of the authors (G.B.B.) is grateful to Karnatak University, Dharwad for the grant of a University Research Studentship.
References
- Hills A, Hughes DL, Leigh GJ, Sanders JR. Reactions of vanadium(IV) halide complexes containing Schiff-base ligands with hydrazines; preparation and structure of [N,N’-ethylenebis(salicylideneiminato)]bis-(phenylhydrazine)vanadium(III) iodide. J. Chem. Soc. Dalton Trans. 1991:325–329.
- Knoch R, Wilk A, Wannowius KJ, Reinen D, Elias H. Spectroscopic and kinetic investigation of bis(N-alkylsalicylaldiminato)copper(II) complexes: a study on the existence of planar. dblharw. tetrahedral configuration equilibria. Inorg. Chem. 1990; 29:3799–3805.
- Bottino FA, Finocchiaro P, Libbertini E. Synthesis and characterization of schiff base complexes with zinc halides. J. Coord. Chem. 1988;16(4):341–345.
- El-Ayaan U, Abdel-Aziz AAM. Synthesis, antimicrobial activity and molecular modeling of cobalt and nickel complexes containing the bulky ligand: bis[N-(2,6-diisopropylphenyl)imino] acenaphthene Eur. J. Med. Chem. 2005;40(12):1214–1221.
- Sonmez M, Berber I, Akbas E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. Eur. J. Med. Chem. 2006;41(1):101–105.
- kTravnice Z, Malon M, Sindelar Z, Dolezal K, Rolcik J, Krystof V, Strnad M, Marek J. Preparation, physicochemical properties and biological activity of copper(II) complexes with 6-(2-chlorobenzylamino)purine (HL1) or 6-(3-chlorobenzylamino)purine (HL2). The single-crystal X-ray structure of [Cu(H+L2)2Cl3]Cl·2H2O. J. Inorg. Biochem. 2001;84(1-2):23–32.
- Yanping Lia, Yanbo Wua, Jing Zhaoa, Pin Yang DNA-binding and cleavage studies of novel binuclear copper(II) complex with 1,1'-dimethyl-2,2'-biimidazole ligand. J. Inorg. Biochem. 2007; 101(2):283–290.
- Xiang-Li Wang, Hui Chao, Hong Li, Xian-Lan Hong, Liang-Nian Ji, Xiao Yuan Li. Synthesis, crystal structure and DNA cleavage activities of copper(II) complexes with asymmetric tridentate ligands. J. Inorg. Biochem. 2004; 98(3):423–429.
- Cowan JA. Chemical nucleases Curr. Opin. Chem. Biol. 2001;5(6):634–642.
- Liu J, Zhang T, Lu T, Qu L, Zhou H, Zhang Q, Ji L. DNA-binding and cleavage studies of macrocyclic copper(II) complexes. J. inorg. Biochem. 2002;91:269.
- Vaidyanathen VG, Nair BU. Oxidative cleavage of DNA by tridentate copper (II) . J. Inorg. Biochem. 2003;93:271.
- Reddy PR, Rao KS, Satyanarayanak B. Synthesis and DNA cleavage properties of ternary Cu(II) complexes containing histamine and amino acids Tetrahedron Lett. 2006;47:7311.
- Kornis G, Comprehensive Heterocyclic Chemistry Katritzky AR, Ed. Pregamon press. 1984; (48)6.
- Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of Benzothiazole-derived Schiff bases with antibacterial activity. J. Enz. Inhib. Med. Chem. 2003;18(3):259–263.
- Geronikaki A, Hadjipavlou-Litina D, Amourgianou M. Novel thiazolyl, thiazolinyl and benzothiazolyl Schiff bases as possible lipoxygenase’s inhibitors and anti-inflammatory agents. IL Farmaco. 2003; 58(7):489–495.
- Panico AM, Geronikaki A, Mgonzo R, Cardile V, Gentile B, Doytchinova I. Aminothiazole derivatives with antidegenerative activity on cartilage. Bioorg. Med. Chem. 2003;11(13):2983–2989.
- Maass GIU, Koenig B, Leser U, Mueller B, Goody R, fatt BP. Viral resistance to the thiazolo-iso-indolinones, a new class of nonnucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37(12):2612–2617.
- Murray RDH. Prog. Chem. Org. Nat. Prod. 1991;58:83.
- Musajo I, Rodighero G. The Skin photosensitizing furocoumarins. Experientia. 1962;18:153.
- Motgomery GW, Martin GB, Bars LJ, Pelletier J. Gonadotropin release in ovariectomized ewes fed different amounts of coumestrol. J. Reprod. Fertil. 1985;73:457–463.
- Fukami H, Nakijama M, Jacobson M, Crosby DG, Dekker M, Naturally occurring insecticides New York Eds. 1971: 71.
- Hoult JRS,Payd M, Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996;27:713.
- Campo AD, Fazzi PL. Riv 1st Steroterap Ital. 1958;33:389.
- Kumari SS, Rao KRMS, Rao NVS. Proc Ind. Acad. Sci. 1973;77:149.
- Irena Kostova, Synthetic and Natural Coumarins as Cytotoxic Agents. Curr. Med. Chem –Anti-Cancer Agents, 2005;5:29–46.
- Bagihalli GB, Badami PS, Patil SA. Synthesis, spectral characterization and in vitro biological studies of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. J. Enzyme. Inhib. Med. Chem. 2009;24(2):381–394.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activity of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2009;43:2639–2649.
- Chohan ZH. Ni (II), Cu (II) and Zn (II) Metal Chelates With Some Thiazole Derived Schiff-Bases: Their Synthesis, Characterization and Bactericidal Properties. Metal-Based Drugs, 1999;2:75–80.
- Spath E, Pailer M. Uber naturlive Coumarine. Chem. Ber. 1935;68:940.
- Carroll King L, Robert J. Hlavacek, The Reaction of Ketones with Iodine and Thiourea. J. Am. Chem. Soc. 1950;72:3722–3725.
- Sadana AK, Miraza Y, Aneja KR, Prakash O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003;38:533–536.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents, Planta Medica, 1982;45:31.
- Bauer AW, Kirby WM, Sherris JC. Antibiotic susceptibility testing by a standardized single disk method. Am, Turck M. J. Clin. Pathol. 1966;45:493.
- Vogel AI, A Text Book of Quantitative Inorganic Analyses, ELBS Longman’s Green and Co. Ltd., London, 3rd Edn 1962.
- Mohapatra S, Rao C, J. Indian Chem. Soc. 1980;57:262.
- Rehman SU, Chohan ZH, Gulnaz1 F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J. Enz. Inhib. Med. Chem.2005;20:333–340.
- Singh G, Singh PA, Singh K, Singh DP, Handa RN, Dubey SN. Proc. Nat. Acad. Sci. Ind. 2002;72A:87–94.
- Shukla PR, Singh VK, Jaiswal AM, Narain J. Studies on some Schiff base complexes of Cu(II), Ni(II) and Co(II) derived from salicylaldehyde, Aminophenols and Anthranilic Acid. J Ind Chem Soc. 1983;60:321–324.
- Lever ABP. Inorganic electronic spectroscopy Elsevier Amsterdam.1984.
- Khalil SME. Dioxouranium(VI) Schiff base complexes as ligands towards Cu(II) and Ni(II) ionsChem. Papers. 2000;54(1):12–18.
- Lever ABP. Inorganic Electronic Spectroscopy Elsevier. Amsterdam.1968.
- Saha N, Datta KM. Cobalt(II) complexes with 5(3)-methylpyrazole-3(5)-carbohydrazide Ind. J. Chem. 1982;21A:180–182.
- Raman N, Thangaraja C, Raja SJ. Mixed ligand transition metal(II) complexes of Knoevenagel condensate-β-ketoesters with 1,2-diaminobenzene: Synthesis, structural characterization, electrochemical behaviour and antimicrobial study. Ind. J. Chem. 2005; 44A:693.
- Reddy KR, Reddy KM, Mahendra KN. Synthesis, characterization, antibacterial and anthelmentic activities of copper(II) complexes with benzofuran Schiff bases. Ind. J. Chem. 2006; 45A:377–381.
- Badiger BM, Patil SA, Kudari SM, Kulkarni VH. Magnetic and spectral properties of cobalt(II) and copper(II) complexes with 3-substituted-4-(5'-substituted-salicylidene/2'-hydroxy-Acetophenoneimine)-1,2,4-triazole. Rev. Roum. Chim. 1986;31:849.
- Cotton FA, Wilkinson G. advanced Inorganic Chemistry 5th Edn. Wiley. New York.1988.
- Figgis BN. Introduction to ligand fields Interscience New york. 1966: 220.
- Patel BK, Patel MM. 2,4-Dihydroxybenzaldehyde oxime-formaldehyde polymer as a polymeric ligand. Ind. J. Chem. 1990;29:90–92.
- Rossenberg RC, Root AC, Bernstein PK, Gray HB. Spectral studies of copper(II) carboxypeptidase A and related model complexes. J. Am. Chem. Soc. 1975;97:2092–2096.
- Hathaway BT. Struct. Bonding. 1973;14:60.
- Nikolaev AV, Longvinenko VA, Mychina CI. Thermal Analysis, Academic Press, New York. 1969.
- Bard AJ, Izatt LR, (Eds) Electrochemical methods: fundamentals and Applications, 2nd ed., Wiley: New York.2001.
- Chohan ZH, Supuran CT, Scozzafava A. synthesis and antibacterial activity of cobalt(II), copper(II), nickel(II) and zinc(II) complexes of kefzol. J. Enz. Inhib. Med. Chem. 2004;19(1):79–84.
- Chohan ZH, Praveen M. Synthesis, characterization, coordination and antibacterial properties of novel asymmetric 1,11-disubstituted ferrocene-derived Schiff base ligands and their Co(II), Cu(II), Ni(II) and Zn(II) complexes. Appl. Organomet. Chem. 2001;15:617–625.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica. 1982;45: