Abstract
Novel 1,3,4-oxadizole derivatives containing the 1,4-dioxane ring system were synthesised starting from 2,3-dihydro-1,4-benzodioxane-2-carbohydrazide. The synthesised compounds were evaluated for antihepatotoxic activity against CCl4-induced hepatotoxicity in rats. Some compounds demonstrated a significant antihepatotoxic activity comparable to the standard drug Silymarin.
Introduction
The liver is an organ of paramount importance as it plays an essential role in maintaining the biological equilibrium of vertebrates. Traditional drugs used in the treatment of liver diseases are sometimes inadequate to cater for the needs of a large population. In spite of tremendous strides in modern medicine, there are few drugs available for the treatment of liver disorders. Many natural products of herbal origin are in use for the treatment of liver ailments [Citation1–4]. The drugs available in the modern systems of medicine are mainly corticosteroids and immunosuppressive agents, these bring about only symptomatic relief and in most cases have no influence on the disease process. Further, their use is associated with the risk of relapses and the danger of side effects.
Benzodioxane represents a series of synthetic and natural compounds of considerable medicinal importance. Compounds containing dioxane ring systems exhibit a variety of biological activities such as antihepatotoxic [Citation5,Citation6], α-adrenergic blocking agents [Citation7], anti-inflammatory [Citation8], and D2 antagonist/5-HT1A partial agonist activity [Citation9].
The compound silymarin isolated from seeds of Silybum marianum commonly known as “milk thistle” has been found to be a potent antihepatotoxic agent against a variety of toxicants. Silymarin has been found to be a mixture of three isomers of flavonolignan i.e. silybin, silychristin and silydianin. Silybin is the most potent component containing the 1,4-dioxan ring system, whereas the other isomers namely silychristin and silydianin do not possess the 1,4-dioxan ring system and hence do not display any significant antihepatotoxic activity. We have therefore concluded that the 1,4-dioxane ring system plays an important role in exhibiting antihepatotoxic activity and if compounds are prepared containing the 1,4-dioxane ring, they will exhibit the antihepatotoxic activity. Thus we have prepared some new 1,3,4-oxadizole derivatives containing the 1,4-dioxane ring system namely, 2-(substituted-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole derivatives and evaluated them for antihepatotoxic activity against CCl4 induced hepatotoxicity in rats. Among them, compounds 3Ai, 3Avii, 3Axiv and 3Axvii were found to show significant antihepatotoxic activity as comparable to standard drug silymarin.
Materials and methods
Chemistry
The IR spectra were recorded on a Brucker spectrometer (Central Instrumental Facility (CIF), Hamdard University, New Delhi). The mass spectra were recorded on a Bruker daltronics high resolution mass spectrometer, the 1H NMR (300 MHz) was recorded on a Bruker DPX 300 spectrometer in CD3OD and DMSO-d6 using TMS as the internal standard reference and the chemical shifts were in δ (ppm). Elemental analyses were performed on Elementar Vario EL III, Carlo Erba 1108 (Central Instrumental Facility (CIF), Hamdard University, New Delhi). The melting points were determined by capillary method.
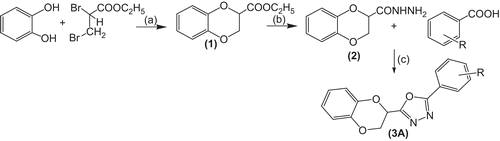
Synthesis of ethyl-l,4-benzodioxane-2-carboxylate (1)
Anhydrous potassium carbonate (50 g) was added in portions to a stirred solution of 55 g of catechol in 200 ml of dry acetone followed by the dropwise addition of 34.5 g of ethyl 2,3-dibromopropionate. Another 50 g of potassium carbonate and 34.5 g of the dibromoester were added similarly and this was repeated twice more using a total of 200 g of potassium carbonate and 137.5 g of ester. Stirring and refluxing was continued for another 15 h. The reaction mixture was then filtered and the solid was washed several times with acetone. The filtrate was concentrated to about 75 ml and the residue was diluted with 50 ml of cold water. The oily layer was separated from the aqueous layer and the latter was extracted repeatedly with ether. The combined oily layer and ether extracts were washed with water, dried over magnesium sulphate and evaporated. The dark residue was distilled at 96–97°C (0.1 mm) to yield 38 g of ester 1 as colourless semisolid. 1H NMR (300 MHz, DMSO-d6): δ 1.23 ( 3H, t, J= 7.1 Hz, CH3-12), 4.2 (2H, q, J = 7.1, 5.7 Hz, CH2-12), 4.3 (2H, d, J = 2.7, CH2-3), 4.77 (1H, t, J = 2.7, CH-2), 6.84 (4H, m, Ar-H); FTIR cm−1: 3052 (=C-H, aromatic), 1772 (C=O), 1653 (C=C), 1292 (C-O, ester)
Synthesis of 2,3-dihydro-1,4-benzodioxane-2-carbohydrazide (2)
To a solution of ethyl-1,4-benzodioxane-2-carboxylate (0.01 mol) in ethanol (20 ml), hydrazine hydrate (0.01 mol) was added and the reaction mixture was refluxed. The progress of the reaction was monitored by TLC. After the completion of the reaction (usually 16 h), the excess solvent was removed under reduced pressure. The reaction mixture was poured over crushed ice. The solid thus separated was filtered, dried and crystallised with methanol to give a white powder; mp: 110–112°C; Yield: 80%; 1H NMR (300 MHz, DMSO-d6): δ 3.91 (2H, br-s, NH2-13), 4.24 (1H, dd, J = 6, 11.4 Hz, Ha-3), 4.46 ( 1H, dd, J = 6, 11.4 Hz, Hb-3), 4.78(1H, d, J = 6, CH2), 6.91 (4H, m, Ar-H), 7.78 (1H, s, NH-12); FTIR (KBr) cm−1: 3052 (=C-H, aromatic), 1772 (C=O), 1673 (C=C), 1259 (-NH2), 1195 (-NH), 758 (C=C); Anal Calcd. for C9H10N2O3: C, 55.67; H, 5.19; N, 14.43; O, 24.72. Found: C: 55.37; H, 5.02; N, 14.67; O, 24.73.
Synthesis of 2-(phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Ai)
A solution of 0.01 mole of 2,3-dihydro-1,4-benzodioxane-2-carbohydrazide, 0.01 mole benzoic acid and 5 ml of POCl3 was refluxed with stirring for 6–7 h. The reaction mixture was cooled and poured over crushed ice. The precipitate thus obtained was filtered, washed with sodium bicarbonate, dried and recrystallised with benzene: methanol. 1H NMR (300 MHz, DMSO-d6): δ 4.33 (2H, m, unresolved doublet, CH2-3), 5.02 (1H, brs, unresolved doublet, CH-2), 6.88–7.67 (4H, m, Ar-H, ring A), 7.87 (5H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3162 (=C-H, aromatic), 1678 (C=C), 1492 (C=N), 1078 (C-O-C). HR-MS (m/z): 281.197 [MH]+ (Calcd. for C16H12N2O3, 280.2782); Anal Calcd. fo C16H12N2O3: C, 68.56; H, 4.32; N, 9.99; O, 17.13; Found: C, 68.46; H, 4.42; N, 10.05; O, 17.12.
2-(2-Bromo-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Aii)
1H NMR (300 MHz, DMSO-d6): δ 4.24 (2H, m, unresolved doublet, CH2-3), 5.15 (1H, brs, unresolved doublet, CH2-2), 6.67–7.91 (4H, m, Ar-H, ring A), 7.65 (5H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3069 (=C-H, aromatic), 1670 (C=C), 1485 (C=N), 1067 (C-O-C), 756 (C-Br); Anal Calcd. for C16H11BrN2O3: C, 53.5; H, 3.09; N, 7.8; O, 13.36; Found: C, 53.43; H, 3.19; N, 7.67; O, 13.43.
2-(3-Bromo-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Aiii)
1H NMR (300 MHz, DMSO-d6): δ 4.26 (2H, m, unresolved doublet, CH2-3), 5.41 ( 1H, brs, unresolved doublet, CH2-2), 6.58–7.23 (4H, m, Ar-H, ring A), 7.56 (5H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3106 (=C-H, aromatic), 1654 (C=C), 1498 (C=N), 1053 (C-O-C), 768 (C-Br); Anal Calcd. for C16H11BrN2O3:C, 53.5; H, 3.09; N, 7.8; O, 13.36: Found: C, 53.45; H, 3.08; N, 7.84; O, 13.43.
2-(4-Bromo-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl) -1,3,4-oxadiazole (3Aiv)
1H NMR (300 MHz, DMSO-d6): δ 4.35 (1H, dd, J = 5.4, 9.9 Hz, CH2-3, H-α), 4.62 (1H, dd, J = 3.3, 3.2 Hz, CH2-3, H-β), 5.97 (1H, brs, unresolved doublet CH-2), 6.87–7.19 (4H, m, Ar-H, ring A), 7.47–8.02 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3156 (=C-H, aromatic), 1687 (C=C), 1493 (C=N), 1043 (C-O-C), 746 (C-Br); HRMS (m/z): 359.1955 [M]+ (Calcd for C16H11BrN2O3, 359.1742). Anal Calcd. for C16H11BrN2O3: C; 53.5; H, 3.09; Br, 22.25; N, 7.8; O, 13.36. Found: C; 53.48; H, 3.15; N, 7.78; O, 13.26.
2-(2-Chloro-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Av)
1H NMR (300 MHz, DMSO-d6): δ 4.92 (2H, m (unresolved doublet), CH2-3), 5.62 (1H, brs, unresolved doublet, CH-2), 6.74–7.82 (4H, m, Ar-H, ring A), 7.02–7.39 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3197(=C-H, aromatic), 1648 (C=C), 1489 (C=N), 1028 (C-O-C), 745 (C-Cl). Anal Calcd. for C16H11ClN2O3:C, 61.06; H, 3.52; N, 8.9; O, 15.25; Found C, 61.12; H, 3.45; N, 8.87; O, 15.29.
2-(3-Chloro-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Avi)
1H NMR (300 MHz, DMSO-d6): δ 4.54 (2H, m, unresolved doublet, CH-3), 5.22 ( 1H, brs, unresolved doublet, CH-2), 6.88–7.57 (4H, m, Ar-H, ring A), 7.23–7.45 (5H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3057 (=C-H, aromatic), 1643 (C=C), 1468 (C=N), 1023 (C-O-C), 768 (C-Cl). Anal Calcd. for C16H11ClN2O3: C, 61.06; H, 3.52; Cl, 11.26; N, 8.9; O, 15.25; Found C, 61.03; H, 3.48;N, 8.78: O, 15.3.
2-(4-Chloro-phenyl)-5-(2, 3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Avii)
1H NMR (300 MHz, DMSO-d6): δ 4.25 (2H, m, unresolved doublet, CH-3), 5.02 ( 1H, brs, unresolved doublet, CH2-2), 6.88–7.67 (4H, m, Ar-H, ring A), 7.87 (5H, m, Ar-H, ring A); FTIR (KBr) cm−1: 3158 (=C-H, aromatic), 1642 (C=C), 1475 (C=N), 1016 (C-O-C), 743 (C-Cl) Anal Calcd. for C16H11ClN2O3: C, 61.06; H, 3.52; Cl, 11.26; N, 8.9; O, 15.25. Found: C, 60.98; H, 3.48; N, 8.85; O, 15.3.
2-(2, 4-Dichloro-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Aviii)
1H NMR (300 MHz, DMSO-d6): δ 4.35 (2H, m, unresolved doublet, CH2-3), 5.91 ( 1H, brs, unresolved doublet, CH-2), 6.88–7.07 (4H, m, Ar-H, ring A), 7.73–7.92 (3H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3050 (=C-H, aromatic), 1693 (C=C), 1478 (C=N), 1070 (C-O-C), 827, 734 (C-Cl). Anal Calcd. for C16H10Cl2N2O3: C, 55.04; H, 2.89; Cl, 20.31; N, 8.02; O, 13.75. Found: C, 54.94; H, 2.75; Cl, 20.28; N, 8.53; O, 13.65.
2-(2-Methyl-phenyl)–5-(2,3-dihydro-1,4benzodioxane-2-yl)-1,3,4-oxadiazole (3Aix)
1H NMR (300 MHz, DMSO-d6): δ 2.35 (3H, s, Ar-CH3), 4.52 (2H, m, unresolved doublet, CH2-3), 5.17 ( 1H, brs, unresolved doublet, CH-2), 6.78–7.57 (4H, m, Ar-H, ring-A), 7.12–7.46 (4H, m, Ar-H, ring-B); FTIR (KBr) cm−1: 3048 (=C-H, aromatic), 2970 (Ar-CH3), 1638 (C=C), 1474 (C=N), 1025 (C-O-C); Anal Calcd. for C17H14N2O3:C, 69.38; H, 4.79; N, 9.52; O, 16.31. Found: C, 69.25; H, 4.72; N, 9.54; O, 16.34.
2-(3-Methyl-phenyl)–5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3 4-oxadiazole (3Ax)
1H NMR (300 MHz, DMSO-d6): δ 2.42 (3H, s, Ar-CH3), 4.41 (1H, dd, J = 5.4, 12.3 Hz, CH2-3, H-α), 4.62 (1H, dd, J = 2.1, Hz, CH2-3, H-β), 5.18 (1H, brs, unresolved doublet CH-2) 6.88–7.01 (4H, m, Ar-H, ring A), 7.25–7.97 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3197 (=C-H, aromatic), 2950 (Ar-CH3), 1687 (C=C), 1490 (C=N), 1076 (C-O-C); Anal Calcd. for C17H14N2O3: C, 69.38; H, 4.79; N, 9.52; O, 16.31; Found: C, 69.46; H, 4.78; N, 9.49; O, 16.27.
2-(4-Methyl-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Axi)
1H NMR (300 MHz, DMSO-d6): δ 2.26 (3H, s, Ar-CH3), 4.27 (2H, m, unresolved doublet, CH2-3), 5.43 ( 1H, brs, unresolved doublet, CH-2), 6.68–7.37 (4H, m, Ar-H, ring A), 7.34–7.87 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3142 (=C-H, aromatic), 2850 (Ar-CH3), 1668 (C=C), 1475 (C=N), 1038 (C-O-C); Anal Calcd. for C17H14N2O3:C: 69.38; H, 4.79; N, 9.52; O, 16.31; Found: C: 69.42; H, 4.81; N, 9.48; O, 16.29.
2-(4-Hydroxy-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole(3Axii)
1H NMR (300 MHz, DMSO-d6): δ 10.24 (1H, s, ArOH), 4.37 (2H, m, unresolved doublet, CH2-3), 5.26 ( 1H, brs, unresolved doublet, CH-2), 6.88–7.67 (4H, m, Ar-H, ring A), 7.26–7.34 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3145 (=C-H, aromatic), 1646 (C=C), 1479 (C=N), 1023 (C-O-C); Anal Calcd. for C16H12N2O4: C, 64.86; H, 4.08; N, 9.46; O, 21.6; Found: C, 64.82; H, 4.25; N, 9.45; O, 21.56.
2- (3,4-Dihydroxy-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Axiii)
1H NMR (300 MHz, DMSO-d6): δ 4.39 (2H, m (unresolved doublet), CH2-3), 5.02 (1H, brs, unresolved doublet, CH-2), 6.88–7.05 (4H, m, Ar-H, ring A), 6.26–7.12(3H, m, Ar-H, ring B), 10.36 (2H, s, Ar-OH); FTIR (KBr) cm−1: 3042 (=C-H, aromatic), 1648 (C=C), 1469 (C=N), 1048 (C-O-C); Anal Calcd. for C16H11N2O5: C, 61.54; H, 3.87; N, 8.97; O, 25.62; Found: C, 61.58; H, 3.85; N, 8.89; O, 25.59.
2-(4-Methoxy-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Axiv)
1H NMR (300 MHz, DMSO-d6): δ3.84 (3H, s, Ar-OCH3), 4.62 (2H, m, unresolved doublet, CH2-3), 5.87 (1H, brs, unresolved doublet, CH-2), 6.91–7.16 (4H, m, Ar-H, ring A), 7.92–7.94 (4H, m, Ar-H, ring B); FTIR (KBr) cm−1: 3062 (=C-H, aromatic), 1611 (C=C), 1494 (C=N), 1180, 1017 (C-O-C); Anal Calcd. for C17H14N2O4: C, 65.80; H, 4.55; N, 9.03; O, 20.62; Found: C, 65.78; H, 4.58; N, 9.13; O, 20.69.
2-(3, 4-dimethoxy-phenyl)-5-(2, 3-dihydro- 1,4-benzodioxane-2-yl)-1, 3, 4-oxadiazole (3Axv)
1H NMR (300 MHz, DMSO-d6): δ 3.76 (2H, s, Ar-OCH3), 4.52 (2H, m, unresolved doublet, CH2-3), 5.35 ( 1H, brs, unresolved doublet, CH-2), 6.88–7.67 (4H, m, Ar-H, ring-A), 7.01–7.32 (3H, m, Ar-H, ring-B); FTIR (KBr) cm−1: 3067 (=C-H, aromatic), 1664 (C=C), 1469 (C=N), 1245, 1030, 1024 (C-O-C); Anal Calcd. for C18H16N2O5: C, 63.52; H, 4.74; N, 8.23; O, 23.51; Found: C, 63.48; H, 4.79; N, 8.26; O, 23.49.
2-(4-amino-phenyl)-5-(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazole (3Axvi)
1H NMR (300 MHz, DMSO-d6): δ 4.35 (2H, s, Ar-NH2), 4.61 (2H, m (unresolved doublet), CH2-3), 5.25 (1H, brs, unresolved doublet, CH-2), 6.73–7.21 (4H, m, Ar-H, ring-A), 7.66–8.15 (4H, m, Ar-H, ring-B); FTIR (KBr) cm−1: 3072 (=C-H, aromatic), 1648 (C=C), 1449 (C=N), 1320 (C-N), 1036 (C-O-C); Anal Calcd. for C16H13N3O3: C, 65.08; H, 4.44; N, 14.23; O, 16.25; Found: C, 65.1; H, 4.45; N, 14.24; O, 16.21.
Testing the antihepatotoxic activity of the synthesised compounds
Animals
Male albino rats weighing 150–200 g were used for the study. The animals were housed in clean metabolic cages and maintained at a controlled temperature (23 ± 2°C). They were fed with a standard pellet diet and had water ad libitum. The animals were maintained at 25°C to 28°C with 40–70% RH and 12 h light/dark cycles and were fasted for 12 hours prior to the experiment. The protocol was approved by the Institutional Animal Ethical Committee constituted by Jamia Hamdard for such a purpose.
Adult rats of either sex weighing 150–200 g were divided into eight groups each consisting of six animals (). Group I received liquid paraffin only (1.5 ml/kg, orally) and served as control. Rats of the remaining seven groups received suspension of carbon tetrachloride (CCl4) in liquid paraffin (1:1, v/v, 1.5ml of CCl4/kg, per oral.) to induce hepatic damage 24 h before the start of treatment. Group III received the CCl4 suspension, in addition to silymarin (10 mg/kg, po) daily. Groups IV-VIII received the synthesised compounds 3Ai, 3Avii, 3Axii, 3Axiv, 3Axvii (10 mg/kg, po, for each compound) orally every day in addition to the CCl4 suspension for 8 days. Blood was withdrawn through the retro-orbital plexus of the rats on the 8th day. Serum was separated from the blood of each rat by centrifugation for estimation of glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) [Citation10], alkaline phosphatase (ALP) [Citation11], and total protein [Citation12]. The rats were sacrificed and the livers rapidly exercised immediately after sacrifice. The liver was fixed in formalin (10%), serially sectioned and microscopically examined after staining with hematoxylin and eosin.
Table 1. The chemical structures, melting points and percentage yields for the synthesised compounds in scheme 1.
Statistical analysis
The data obtained were analysed by one-way ANOVA followed by Dunnett’s test. The level of significance was set at P < 0.05.
Results and Discussion
Chemistry
The synthetic route used to prepare the starting materials and the title compounds have been outlined in . The starting material ethyl-l,4-benzodioxane-2-carboxylate (1) was prepared by a reaction between catechol and ethyl-2,3-dibromopropionate in dry acetone in the presence of anhydrous potassium carbonate, which on treatment with hydrazine hydrate afforded the corresponding hydrazide (2). The reaction of the hydrazide (2) with substituted aryl carboxylic acids in phosphorus oxychloride (POCl3) afforded the cyclised products; 2-(substituted-phenyl)-5 -(2,3-dihydro-1,4-benzodioxane-2-yl)-1,3,4-oxadiazoles (3Ai–3Axvii). The synthesised compounds were characterised by IR, 1H-NMR, mass spectroscopic data and elemental analysis.
Antihepatotoxic activity of the synthesised compounds
The CCl4-induced hepatotoxicity is mediated by the primary and secondary bond formation of the reactive species to critical cellular molecules such as DNA, lipid, proteins or carbohydrates. It has been well established that the hepatotoxicity by CCl4 is due to the enzymatic activation to release the CCl3. radical in the free state, which in turn disrupts the structure and function of the lipid and protein macromolecules in the membrane of the cell organelles. Hence, elevated levels of serum enzymes are indicative of cellular leakage and the loss of the functional integrity of the cell membrane due to the toxicity produced by CCl4. A significant rise in the serum enzymatic concentration, namely SGOT and SGPT, could be taken as an index of liver damage. It generally induces the deposition of fat in the liver and plays a significant role in inducing triacyl glycerol accumulation, depletion of GSH, increased lipid oxidation, membrane damage, depression of protein synthesis and the loss of enzyme activity. Being cytoplasmic in location, the damage marker enzymes SGOT, SGPT are released in serum. It has been shown that protective agents exert their action against CCl4 mediated lipid peroxidation, either through a decreased production of free radical derivatives or due to the antioxidant activity of the protective agent itself.
As shown in , the activities of the liver enzymes serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate oxaloacetate transaminase (SGPT), alkaline phosphatase (ALP) were markedly increased, whereas total proteins (TP) were decreased in the CCl4-treated rats in comparison with the normal values. Administration of silymarin (standard drug) and the synthesised compounds at a dose level of 10mg/kg body weight, prevented the CCl4-induced elevation of SGOT, SGPT, ALP, as well as preventing the decrease in total protein. Silymarin (10mg/kg) significantly decreased the level of SGOT, SGPT, ALP and increased the level in total protein. The histopathological studies also showed a significant recovery of the hepatocytes of the liver in both the standard drug and compound treated animals (), which again correlated with the results of the biochemical parameters. The results of the liver histopathological studies have been presented in , these showed hepatocyte swelling and necrosis in the CCl4-treated rats in comparison with the normal control rats. Administration of the synthesised compounds exhibited a significant protection of the hepatocytes against injury and showed normalisation of the tissues as neither fatty accumulation nor necrosis was observed. The central vein appeared clearly indicating a potent antihepatotoxic activity.
Table 2. The effect of the synthesised compound on serum enzymatic activity in CCl4 induced liver damage in rats.
Table 3. Histopathological changes in the livers of the Wistar rats.
Acknowledgements
The authors would like to thank the Head, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India for providing necessary research facilities. We would also like to thank Showkat Ahmad Shah, In charge, Animal House facility, Jamia Hamdard, New Delhi for providing experimental animals for carrying out the antihepatotoxic activity.
Declaration of interest
The authors would like to thank University Grant Commission, New Delhi, for approving the major research project for financial assistance.
References
- Venkateswaran S, Pari L, Viswanathan P, Menon VP. Protective effect of Livex, a herbal formulation against erythromycin estolate-induced hepatotoxicity in rats. Journal of Ethnopharmacol 1997;57:161–167.
- Latha U, Rajesh MG, Latha MS. Hepatoprotective effect of an ayurvedic medicine. Indian Drugs 1999;36:470–473.
- Mitra SK, Seshadri SJ, Venkataranganna MV, Gopumadhavan S, Venkatesh Udupa U, Sarma DNK. Effect of HD-03-a herbal formulation in galactosamine-induced hepatopathy in rats. Indian Journal of Physiol Pharmacol 2000;44:82–86.
- Dhuley JN, Naik SR. Protective effect of Rhinax, a herbal formulation against CCl4-induced liver injury and survival in rats. Journal of Ethnopharmacol 1997;56:159–164.
- Ahmed B, Khan SA, Alam T. Synthesis and antihepatotoxic activity of some heterocyclic compounds containing the 1,4-dioxane ring system. Pharmazie 2003;58:173–176.
- Khan SA, Ahmed B, Alam T. Synthesis and antihepatotoxic activity of some new chalcones containing 1,4 - dioxane ring system. Pak J Pharm Sci 2006;19: 290–294.
- Chapleo CB, Myers PL, Butler CM, Doxey JC, Roach AG, Smith CFC. α-Adrenoreceptor reagents. Synthesis of some 1,4-benzodioxans as selective presynaptic α 2-adrenoreceptor antagonists and potential antidepressants. J Med Chem 1983;26:823–831
- Vázquez, MT, Rosell G, Pujol MD. Synthesis and anti-inflammatory activity of rac-2-(2, 3-dihydro-1,4-benzodioxin) propionic acid and its R and S enantiomers. European J Med Chem 1997;32:529–534
- Birch AM, Bardley PA, Gill JC, Kerrigan F, Needham PL. N-Substituted (2,3-dihydro-1,4-benzodioxin-2-yl) methylanine derivatives as d2 antagonists/5-ht1a partial agonists with potential as atypical antipsychotic agents. J Med Chem 1999;42:3342–3355.
- Reitman S, Frankel S. In vitro determination of transaminase activity in serum. American Journal of Clin Path 1957;28:56–63.
- Kind P R N, King E J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino antipyrine. Journal of Clin Path 1954; 7: 322–326.
- Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with Folin-phenol reagent. J Biol Chem 1951;193:265–275.