Abstract
In this study, some novel inhibitors were synthesised from the further stage reactions of 4-benzoyl-1-(4-nitrophenyl)-5-phenyl-1H-pyrazole-3-carbonyl chloride with 5-amino-1,3,4-thiadiazole-2-sulphonamide 1 (inhibitor 1). They were characterised by elemental and spectral (1H NMR, 13C NMR, IR) analyses. Human carbonic anhydrase isoenzymes (hCA-I and hCA-II) were purified from erythrocyte cells by affinity chromatography. The inhibitory effects of inhibitor 1, acetazolamide (2) and the 11 newly synthesised amides (8–18) on the hydratase and esterase activities of these isoenzymes (hCA-I and hCA-II) were studied in vitro. In relation to these activities, the inhibition equilibrium constants (Ki) were determined. The Ki values for the new compounds (8–18) were observed to be well below that of the parent compound inhibitor 1 and were also compared to 2 under the same experimental conditions. The comparison of the newly synthesised amides to inhibitor 1 and to 2 indicated that the new derivatives preferentially inhibited hCA-II and were more potent inhibitors of hCA-II than the parent inhibitor 1 and 2.
Introduction
The sulphonamides constitute an important class of drugs which show many pharmacologic activities such as antibacterial, antihypertensive, antiviral, anti-thyroid, diuretic, hypoglycaemic and anti-tumour activity. Furthermore the sulphonamides are the best-known inhibitors of the carbonic anhydrase enzyme, currently used for the treatment of glaucoma in clinical medicine [Citation1–20].
Glaucoma is a group of diseases characterised by a gradual loss of the visual field due to an elevation in intraocular pressure (IOP) and is the second leading cause of blindness worldwide [Citation14,Citation15]. Since carbonic anhydrase inhibitors have been shown to reduce intraocular pressure exclusively by lowering the aqueous humour flow, these compounds have been used for the treatment of glaucoma for years [Citation16,Citation17].
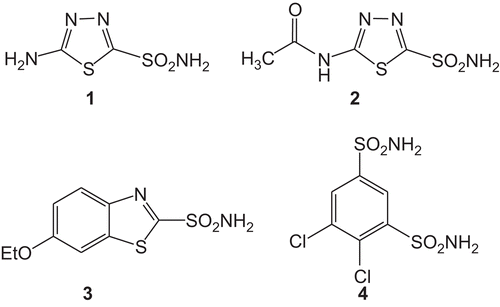
5-amino-1,3,4-thiadiazole-2-sulphonamide (1) has several biological activities and antibacterial properties [Citation21]. Several derivatives of this drug have been synthesised and used in the treatment of glaucoma and one of them is acetazolamide (2). Compound 2 is used systematically (i.e. an oral medication) and reduces intraocular pressure by lowering fluid formation in the eye. A number of side effects of this drug are however experienced such as numbness and tingling in the fingers and toes, taste alterations, blurred vision, kidney stones and an increase in urination. Ethoxzolamide (3) and dichlorophenamide (4) are two other carbonic anydrase inhibitors, formulated for topical ophthalmic use (see for the structures) [Citation22,Citation23]. Nevertheless, people taking these two drugs also experience side effects as stinging, burning, blurred vision, upset stomach, dry eye, headache or dizziness [Citation22,Citation24–26]. The purpose of the present study was to synthesise and investigate some new inhibitors of carbonic anydrase isoenzymes with a potential use in the treatment of glaucoma.
Materials and methods
General
The chemical compounds used in this research were of analytical purity and the solvents were purified by using the appropriate purifying agents and distillation. All melting points were recorded on a Barnstead Electrothermal 9200 apparatus and were uncorrected. The IR spectrum data of the compounds were determined by a Mattson 1000 FT-IR using KBr pellets. 1H NMR and 13C NMR spectra were evaluated by Bruker DPX-400, (400 MHz) and High Performance Digital FT-NMR (100 MHz) spectrometers. At the end of the each experiment TLC was performed using DC Alufolien Kiesegel 60F/254 Merck and Camag TLC devices. Elemental analyses were carried out on a Leco CHNS-932 instrument.
Synthesis of organic compounds
1-(4-Aminophenyl)-4-benzoyl-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazole-2-yl)-1H-pyrazole-3-carboxamide (7)
The starting compound (7) containing aromatic primary amine function was prepared as described previously [Citation2] and crystallised from methanol. 465 mg, 85%; mp 183°C; IR (ν, cm−1): 3312–3216 (NH), 3026 (Ar CH), 1665 (C=O), 1596–1426 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 6.51 (m, 2H, Ar NH2), 8.37 (m, 2H, SO2NH2), 7.8–6.82 (m, 14H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 190.58 (benzoyl C=O), 165.36 (amide C=O), 161.44 and 160.44 (thiadiazole C–2 and C–5), 152.2, 148.8, 144.57, 143.36, 137.58, 134.1, 130.48, 130.13, 129.64, 129.06, 127.65, 127.4, 127.3, 122.91, 114.4; Anal. Calcd. for C25H19N7O4S2: C, 55.04; H, 3.51; N, 17.97; S, 11.75. Found: C, 55.32; H, 3.59; N, 17.75; S, 11.6.
General procedure for the synthesis of compounds 8–17
Sodium acetate (3 g) was dissolved in 10 mL H2O and 2 mL HCl. Aromatic amine (1 mmol) and alcohol were added to the mixture until it dissolved. The temperature was adjusted to 0–5°C. A solution of NaNO2 (83 mg, 1.2 mmol) in 10 mL H2O was added to the mixture slowly and the process of diazotisation was performed. The aromatic and β-diketone (1 mmol) compounds were dissolved in ethanol and added dropwise to the diazonium salt which had been prepared previously. Finally, the coloured precipitate was filtered and purified from the ethanol.
4-Benzoyl-1-(4-((2-hydroxynaphthalen-1-yl)diazenyl)phenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (8)
This was produced according to the method in the general procedure; 567 mg, 81%; mp 284–285°C; IR (ν, cm−1): 3388 (OH), 3200 (NH), 3027 (Ar CH), 1686 (C=O), 1597–1448 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 15.7 (s, 1H, Ar–OH), 14.19 (br, s, 1H, CONH), 8.39 (s, 2H, SO2NH2), 8.56–6.69 (m, 20H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 190.55 (benzoyl C=O), 165.35 (amide C=O), 161.44 and 160.35 (thiadiazole C–2 and C–5), 144.74 (=C–OH), 144.47, 143.22, 141.61, 137.69, 137.13, 134.1, 133.09, 130.37, 130.34, 130.32, 130.21, 130.12, 129.75, 129.66, 129.48, 129.09, 128.42, 127.88, 127.47, 126.81, 125, 123.12, 122.13, 119.22; Anal. Calcd. for C35H24N8O5S2: C, 59.99; H, 3.45; N, 15.99; S, 9.15. Found: C, 59.82; H, 3.49; N, 16.03; S, 9.11.
4-Benzoyl-1-(4-((4-hydroxyphenyl)diazenyl)phenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (9)
This was produced according to the method in the general procedure; 429 mg, 66%; mp 175–177°C; IR (ν, cm−1): 3375 (OH), 3195 (NH), 3028 (Ar CH), 1679 (C=O), 1595–1447 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 13.2 (s, 1H, CONH), 8.55–7.83 (br, s, 1H, Ar–OH), 7.79 (s, 2H, SO2NH2), 7.5–6.89 (m, 18H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 195.63 (benzoyl C=O), 169.18 (amide C=O), 165.56 and 165.35 (thiadiazole C–2 and C–5), 148.96 (=C–OH), 148.61, 143.49, 142.48, 138.15, 138.11, 134.57, 134.29, 134.15, 133.9, 133.54, 133.43, 133.28, 132.5, 130.25, 130.21, 127.58, 121.53, 120.89; Anal. Calcd. for C31H22N8O5S2: C, 57.22; H, 3.41; N, 17.22; S, 9.86. Found: C, 57.11; H, 3.45; N, 17.21; S, 9.92.
4-Benzoyl-1-(4-iodophenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (10)
The diazonium salt solution of the aromatic amine compound was prepared according to the method in the general procedure. The KI (166 mg, 1 mmol) was dissolved in ethanol and cooled to 0–5°C and added dropwise to the diazonium salt solution which was prepared previously. The precipitated yellow product was filtered and purified from ethanol. 400 mg, 61%; mp 160–162°C; IR (ν, cm−1): 3320 (NH), 3005 (Ar CH), 1672 (C=O), 1595–1447 (Ar C=C and C=N); 1H NMR (400 MHz, CDCl3) δ (ppm): 12.15 (br, s, 1H, CONH), 7.77 (s, 2H, SO2NH2), 7.61–6.75 (m, 14H, ArH); 13C NMR (100 MHz, CDCl3) δ (ppm): 190.5 (benzoyl C=O), 165.45 (amide C=O), 161.43 and 160.38 (thiadiazol C–2 and C–5), 97.09 (ArC–I), 144.48, 143.25, 143.06, 139.7, 139.61, 137.63, 134.07, 130.26, 129.98, 129.83, 129.63, 129.07, 128.92, 122.5; Anal. Calcd. for C25H17IN6O4S2: C, 45.74; H, 2.61; N, 12.80; S, 9.77. Found: C, 45.63; H, 2.64; N, 12.82; S, 9.81.
4-benzoyl-1-(4-(2-(2,4-dioxopentan-3-ylidene)hydrazinyl)phenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (11)
433 mg, 66%; mp 271–273°C; IR (ν, cm−1): 3170 (NH), 3028 (Ar CH), 2971 (aliphatic CH), 1686 (C=O), 1596–1445 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 13.81 (s, 1H, CONH), 8.36 (s, 2H, SO2NH2), 2.48 and 2.41 (s, 6H, 2CH3), 7.82–7.24 (m, 14H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 197.49 and 196.87 (acetyl C=O), 190.56 (benzoyl C=O), 165.34 (amide C=O), 161.42 and 160.36 (thiadiazole C–2 and C–5), 31.71 and 26.87 (CH3), 144.49, 143.06, 142.59, 137.68, 135.31, 134.79, 134.06, 130.29, 130.02, 129.63, 129.08, 129.03, 127.85, 127.54, 122.91, 116.91; Anal. Calcd. for C30H24N8O6S2: C, 54.87; H, 3.68; N, 17.06; S, 9.77. Found: C, 54.80; H, 3.69; N, 17.08; S, 9.75.
4-Benzoyl-1-(4-(2-(1,3-dioxo-1-phenylbutan-2-ylidene)hydrazinyl)phenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (12)
445 mg, 62%; mp 187–190 °C; IR (ν, cm−1): 3190 (NH), 3005 (Ar CH), 2971 (aliphatic CH), 1674 (C=O), 1597–1447 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 13.86 (br, s, 1H, CONH), 11.31 (s, 1H, Ar–NH−N=C), 8.35 (s, 2H, SO2NH2), 1.92 (s, 3H, CH3), 7.81–7.11 (m, 19H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 196.6 (acetyl C=O), 195.43 and 190.66 (benzoyl C=O), 165.11 (amide C=O), 161.74 and 160.7 (thiadiazole C–2 and C–5), 25.42 (CH3), 144.38, 143.85, 140.27, 137.74, 136.01, 134.91, 134.01, 133.45, 130.58, 130.26, 129.9, 129.6, 129.52, 129.25, 129.06, 128.99, 128.4, 127.96, 127.48, 122.86, 122,72, 115.15; Anal. Calcd. for C35H26N8O6S2: C, 58.49; H, 3.65; N, 15.59; S, 8.92. Found: C, 58.38; H, 3.68; N, 15.63; S, 8.96.
4-Benzoyl-1-(4-(2-(1,3-dioxo-1,3-diphenylpropan-2-ylidene)hydrazinyl)phenyl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (13)
656 mg, 84%; mp 181–182°C; IR (ν, cm−1): 3180 (NH), 3027 (Ar CH), 1656 (C=O), 1596–1448 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 13.7 (br, s, 1H, CONH), 11.7 (s, 1H, Ar–NH–N=C), 8.35 (s, 2H, SO2NH2), 8.2–7.2 (m, 24H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 194.17, 191.01 and 190.59 (benzoyl C=O), 165.31 (amide C=O), 161.44 and 160.38 (thiadiazole C–2 and C–5), 144.39, 143.69, 142.89, 138.5, 137.68, 137.31, 136.44, 135.04, 134.62, 134.06, 133.78, 133.52, 133.02, 130.57, 130.27, 129.95, 129.61, 129.53, 129.33, 129.12, 128.99, 128.67, 127.9, 127.46, 122.79, 115,51; Anal. Calcd. for C40H28N8O6S2: C, 61.53; H, 3.61; N, 14.35; S, 8.21. Found: C, 61.45; H, 3.64; N, 14.35; S, 8.22.
Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-1,3,4-thiadiazol-2-ylcarbamoyl)-1H-pyrazol-1-yl)phenyl)hydrazono)-3-oxo-3-phenylpropanoate (14)
434 mg, 58%; mp 133–135°C; IR (ν, cm−1): 3197 (NH), 3027 (Ar CH), 2971 (aliphatic CH), 1664 (C=O), 1597–1448 (Ar C=C and C=N); 1H NMR (400 MHz, CDCl3) δ (ppm): 12.62 (s, 1H, CONH), 8.14 (s, 2H, SO2NH2), 4.36 (q, 2H, OCH2), 1.32(t, 3H, CH3), 7.78–7.08 (m, 19H, ArH); 13C NMR (100 MHz, CDCl3) δ (ppm): 191.39 and 189.46 (benzoyl C=O), 164.63 (ester C=O), 163.59 (amide C=O), 161.67 and 158.64 (thiadiazole C–2 and C–5), 61.72 (CH2), 13.99 (CH3), 144.65, 142.09, 141.85, 137.17, 137.08, 134.73, 134.04, 133.71, 132.87, 130.33, 129.8, 129.68, 128.81, 128.69, 128.53, 128.45, 128.17, 127.91, 127.27, 126.47, 122.59, 115,51; Anal. Calcd. for C36H28N8O7S2: C, 57.74; H, 3.77; N, 14.96; S, 8.56. Found: C, 57.61; H, 3.79; N, 14.99; S, 8.54.
Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-1,3,4-thiadiazol-2-ylcarbamoyl)-1H-pyrazol-1-yl)phenyl)hydrazono)-3-oxobutanoate (15)
439 mg, 64%; mp 193–195°C; IR (ν, cm−1): 3212 (NH), 3018 (Ar CH), 2971 (aliphatic CH), 1664 (C=O), 1600–1427 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 14.02 (br, s, 1H, CONH), 11.73 (s, 1H, Ar–NH–N=C), 8.33 (s, 2H, SO2NH2), 4.32 (q, 2H, OCH2), 2.51 (s, 3H, COCH3) 1.28(t, 3H, CH3), 7.79–7.08 (m, 14H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 194.29 (acetyl C=O), 190.72 (benzoyl C=O), 164.86 (ester C=O), 162.86 (amide C=O), 162.15 and 160.23 (thiadiazole C–2 and C–5), 61.79 (CH2), 25.79 (COCH3), 14.32 (CH3), 154.77, 144.29, 143.31, 137.81, 137.62, 133.96, 133.19, 130.25, 129.88, 129.59, 129.25, 129.04, 128, 127.51, 122.72, 115,67; Anal. Calcd. for C31H26N8O7S2: C, 54.22; H, 3.82; N, 16.32; S, 9.34. Found: C, 54.15; H, 3.82; N, 16.30; S, 9.37.
Diethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-1,3,4-thiadiazol-2-ylcarbamoyl)-1H-pyrazol-1-yl)phenyl)hydrazono)malonate (16)
358 mg, 50%; mp 170–172°C; IR (ν, cm−1): 3184 (NH), 3019 (Ar CH), 2970 (aliphatic CH), 1740 (C=O), 1610–1429 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 12 (br, s, 1H, CONH), 8.3 (s, 2H, SO2NH2), 4.34–4.12 (q, 4H, 2OCH2), 1.28(t, 6H, 2CH3), 7.79–6.55 (m, 14H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 190.79 (benzoyl C=O), 172.50 (ester C=O), 164.59 (amide C=O), 162.7 and 161.25 (thiadiazole C–2 and C–5), 61.89 and 61.31 (CH2), 14.56 and 14.32 (CH3), 149.85, 144.17, 138.93, 137.86, 133.92, 130.24, 129.89, 129.59, 129.37, 129.03, 128.96, 128.81, 128.04, 127.26, 126.22, 122.86, 122.27, 113,67; Anal. Calcd. for C32H28N8O8S2: C, 53.62; H, 3.94; N, 15.63; S, 8.95. Found: C, 53.49; H, 3.97; N, 15.65; S, 8.92.
tert-Butyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-1,3,4-thiadiazol-2-ylcarbamoyl)-1H-pyrazol-1-yl)phenyl)hydrazono)-3-oxobutanoate (17)
366 mg, 51%; mp 206–207°C; IR (ν, cm−1): 3190 (NH), 3025 (Ar CH), 2974 (aliphatic CH), 1673 (C=O), 1600–1426 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 14.18 (s, 1H, CONH), 11.92 (s, 1H, Ar–NH–N=C), 8.2 (s, 2H, SO2NH2), 2.37 (s, 3H, CH3), 1.53 (s, 9H, C(CH3)3), 7.76–6.55 (m, 14H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 193.88 (acetyl C=O), 190.46 (benzoyl C=O), 165.26 (ester C=O), 162.03 (amide C=O), 161.38 and 160.06 (thiadiazole C–2 and C–5), 83.29 (OC(CH3)3), 28.32 (OC(CH3)3), 26.66 (CH3), 144.11, 142.95, 142.81, 137.64, 133.74, 130.02, 129.47, 128.81, 128.78, 128.73, 127.74, 126.93, 126.87, 122.83, 115.79, 113.8; Anal. Calcd. for C33H30N8O7S2: C, 55.45; H, 4.23; N, 15.68; S, 8.97. Found: C, 55.32; H, 4.23; N, 15.71; S, 9.02.
4-Benzoyl-1-(2-methyl-1-(2-oxopropyl)-1H-indol-5-yl)-5-phenyl-N-(5-sulphamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-carboxamide (18)
The aromatic amine compound (1 mmol, 545 mg) was dissolved in 15 mL DMF and chloroacetone (0.182 mL) was added to the mixture. The reaction was maintained at 60°C for three days in a condenser with a CaCl2 headpiece. After the evaporation of the solvent of the dark coloured mixture in a rotary evaporator, the remainder composed of a dense liquid was added to water and mixed. The granulated product was filtered and purified from ethanol. 537 mg, 84%; mp 158–160°C; IR (ν, cm−1): 3240 (NH), 3059 (Ar CH), 2922 (aliphatic CH), 1689 (C=O), 1598–1448 (Ar C=C and C=N); 1H NMR (400 MHz, DMSO–d6) δ (ppm): 13.78 (br, s, 1H, CONH), 8.37 (s, 2H, SO2NH2), 6.64 (s, 1H, indole CH), 2.89 (s, 2H, CH2), 2.51 and 2.24 (s, 6H, 2CH3), 8.08–7.28 (m, 13H, ArH); 13C NMR (100 MHz, DMSO–d6) δ (ppm): 203.14 (acetyl C=O), 190.52 (benzoyl C=O), 165.33 (amide C=O), 162.79 and 161.41 (thiadiazole C–2 and C–5), 48.81 (CH2), 36.27 and 31.25 (CH3), 146.62, 144.48, 143.15, 137.75, 137.64, 134.1, 130.29, 130.21, 130.05, 129.92, 129.71, 129.63, 129.07, 128.93, 128.04, 127.33, 126.58, 123.76, 123.07; Anal. Calcd. for C31H25N7O5S2: C, 58.20; H, 3.94; N, 15.33; S, 10.02. Found: C, 58.07; H, 3.98; N, 15.35; S, 10.03.
Purification of isoenzymes hCA-I and II from human erythrocytes
In order to purify the hCA-I and II isoenzymes, first human blood was centrifuged at 1500 rpm for 20 min and after the removal of the plasma, the erythrocytes were washed with an isotonic solution (0.9% NaCl). After that, the erythrocytes were lysed with 1.5 volume of ice-cold water. The lysate was centrifuged at 20 000 rpm for 30 min to remove the cell membranes and non-lysed cells. The pH of the supernatant was adjusted to 8.7 with tris and loaded onto an affinity column containing Sepharose-4B-L-tyrosine-p-aminobenzene sulphonamide as the binding group. After extensive washing with 25 mM tris-HCl/22 mM Na2SO4 (pH 8.7), the hCA-I and II isoenzymes were eluted with 1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6) [Citation27,Citation28]. The amount of purified protein was estimated by the Bradford method [Citation29] and SDS-PAGE was carried out to determine whether the elute contained the enzyme [Citation30].
Hydratase activity assay
Carbonic anhydrase (CA) activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Citation31]. The CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation ((t0 − tc)/tc) where t0 and tc were the times for pH change of the nonenzymatic and the enzymatic reactions, respectively. The IC50 values (the concentration of inhibitor producing a 50% inhibition of CA activity) were obtained in vitro for the synthesised compounds (8–18), with 1 and 2 as the control compounds.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV-vis) according to the method described in the literature [Citation32,Citation33]. The enzymatic reaction, in a total volume of 3 mL, contained 1.4 mL of 0.05 M tris–SO4 buffer (pH 7.4), 1 mL of 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without any enzyme solution. The IC50 values were obtained in vitro for the synthesised compounds (8–18), with 1 and 2 as the control compounds.
Determination of Ki values
The Ki values were determined as described in the literature [Citation34–38]. According to this study, in its first part, the IC50 values were obtained as in vitro. IC50 values of the inhibitors (for the synthesised compounds 8–18, with 1 and 2 as the control compounds) were assayed by the hydrolysis of 4-nitrophenylacetate on the esterase activities of the CA isoenzymes in the presence of various inhibitor concentrations. The absorbance was determined at 348 nm after 3 min [Citation34]. Regression analysis graphs were drawn by the plotting inhibitor concentrations versus the enzyme activity by using the Microsoft Excel Program. In the second part of the study, the concentrations of the inhibitors which gave results of 30%, 50% and 70% inhibition on the isoenzymes were determined at five different substrate concentrations. At each of these inhibitor concentrations (30%, 50% and 70%), the enzyme activity was then measured in the presence of the various substrate concentrations given above and the data were linearised with a Lineweaver–Burk plot in order to obtain the Ki value.
Results and Discussion
The treatment of glaucoma primarily aims at decreasing the intraocular pressure, which may lead to optic nerve damage and subsequently to vision loss. Several of the carbonic anhydrase inhibitors currently in use act by lowering the intraocular pressure by decreasing the aqueous humor production [Citation22]. However, all medications, whether topical or oral, have some benefits as well as some side effects. Because of the ocular side effects after administration of the sulphonamide derivatives, the development of new carbonic anhydrase inhibitors as candidate drugs with fewer side effects will increasingly become valuable for the treatment of glaucoma. In this study, 11 derivatives of sulphonamides were synthesised and their effects on purified human carbonic anhydrase isoenzymes (hCA-I and hCA-II) were also investigated.
Chemistry
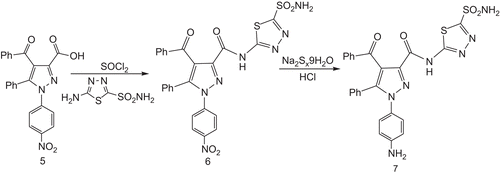
In order to synthesise the new compounds, firstly the activated pyrazole carboxylic acid was reacted with inhibitor 1 and a new amide derivative (6) was obtained (). After that, using this newly synthesised amide derivative, our target inhibitor was prepared by reducing the NO2 group to the NH2 group (7) [Citation39,Citation40].
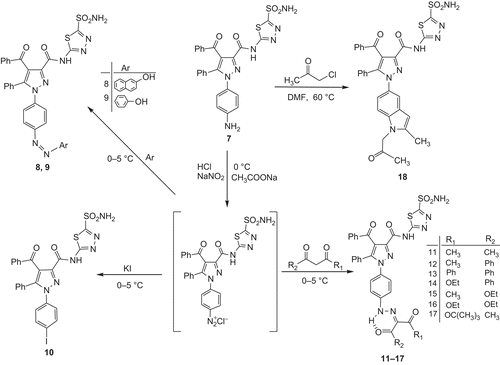
In our work, the diazotisation method was generally used to synthesise some new derivatives of compound 7 which was an aromatic primer amine. As described previously, the diazonium salt of the compound was prepared [Citation2] and then the coupling and replacement reactions were performed. During the reactions, the temperature was controlled (0–5°C). In our experiments, different pH ranges were tested and the best yield was observed in the range of pH = 3.5−4. The products were mostly coloured. The purity of each product was checked by TLC which was performed for each of the reactions. The structures of the compounds were characterised by spectral data (see experimental). Compound 7 gave azo compounds by the diazo coupling reaction with aromatic compounds such as phenol or β–naphthol. Compound 8 was produced in 81% yield and the derivatives of compound 9 were produced in a 66% yield. Compound 10 was produced in 61% yield by interference of the diazonium compound with KI. For the transfer of ion of I¯, known as a strong nucleophile, KI was sufficient without any need for a catalyser.
Some of the hydrazo derivatives (11−17) were produced in high yields by reaction of compound 7 with aliphatic 1,3–dicarbonile compounds which included active C–H protons [Citation2,Citation41]. When the results of the 1H NMR spectra were examined, it was obvious that the signals of compounds 11–17 at δ= 11.92–11.31 ppm intervals resulted from the hydrogen atoms in covalent linkage with nitrogene. Thus, these compounds including aliphatic active C–H groups prefer keto-hydrazo (−NH−N=C) tautomeric structures after coupling.
Compound 18 was produced in 84% yield by reaction of compound 7 with chloroacetone in DMF at 60°C for 72 h [Citation42]. Syntheses of compounds 8–18 from compound 7 are shown in .
In vitro inhibition studies
The effects of these new inhibitors on human carbonic anhydrase I (hCA-I) and II (hCA-II) purified from red blood cells were investigated. The CA isozymes were purified to homogeneity by affinity chromatography.
The inhibition effects of the parent compounds (1 and 2) and the newly synthesised compounds (8−18) as the control compounds on the hCA-I and hCA-II enzymes were studied by the hydratase and esterase activity methods, the IC50 values were determined and then the Ki values were determined for each compound. According to the in vitro studies, the average IC50 values for the hydratase activity of the newly synthesised compounds (9–18) ranged from 0.164 to 1.32 μM for hCA-I and from 0.08 to 1.3 μM for hCA-II lower than the IC50 values of 1 (3.35 and 2.75 μM for hCA-I and hCA-II respectively) and of 2 (4.2 and 4.02 μM for hCA-I and hCA-II respectively). The IC50 values obtained from the esterase activities of the synthesised compounds (9–12, 14–17) ranged from 0.23 to 1 μM for hCA–I. The IC50 values of the studied compounds for esterase activity (8–18) ranged from 0.03 to 2.1 μM for hCA–II. The IC50 values of these compounds were lower than 1 and 2 (2.8 and 3.5 μM for hCA–I and 7.4 and 7.9 μM for hCA–II respectively). Consequently the novel compounds have significant Kİ values. The Kİ values of some of the compounds (9–17) ranged from 0.095 to 1.532 for hCA-I. The Kİ values of some of the compounds (8–12, 14–17) were also ranged from 0.018 to 0.848 for hCA-II ().
Table 1. IC50 and Ki values of hCA-I and hCA-II isoenzyme hydratase and esterase activities obtained after the inhibition experiments.
Generally our synthesised compounds showed remarkable inhibition effects on hCA-I and hCA-II and showing higher inhibition than 1 and 2. Hence these compounds may be candidates for the treatment of glaucoma.
Declaration of interest
This research was funded by TUBITAK (The Foundation of Scientific and Technological Research of Turkey, 106T180) (Ankara, Turkey).
References
- Supuran CT, Casini A, Mastrolorenzo A, Scozzafava A. COX–2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini Rev Med Chem 2004;4:625–632.
- Kasımoğulları R, Bülbül M, Günhan H, Güleryüz H. Effects of new 5-amino-1,3,4-thiadiazole-2-sulfonamide derivatives on human carbonic anhydrase isozymes. Bioorg Med Chem 2009;17:3295–3301.
- Bülbül M, Kasımoğulları R, Küfrevioğlu Öİ. Amide derivatives with pyrazole carboxylic acids of 5-amino-1,3,4-thiadiazole 2-sulfonamide as new carbonic anhydrase inhibitors: Synthesis and investigation of inhibitory effects. J Enzyme Inhib Med Chem 2008;23:895–900.
- Owa T, Nagasu T. Novel sulphonamide derivatives for the treatment of cancer. Expert Opin Ther Pat 2000;10:1725–1740.
- Robinson C, Hartman RF, Rose SD. Emollient, humectant, and fluorescent alpha, beta-unsaturated thiol esters for long-acting skin applications. Bioorg Chem 2008;36:265–270.
- Sova M, Kovac A, Turk S, Hrast M, Blanot D, Gobec S. Phosphorylated hydroxyethylamines as novel inhibitors of the bacterial cell wall biosynthesis enzymes MurC to MurF. Bioorg Chem 2009;37:217–222.
- Rega MF, Leone M, Jung D, Cotton NJH, Stebbins JL, Pellecchia M. Structure-based discovery of a new class of Bcl-x (L) antagonists. Bioorg Chem 2007;35:344–353.
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem 2003;10:925–953.
- Chohan ZH, Hassan M, Khan MK, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide-derived Schiff’s bases and their metal complexes. J Enzyme Inhib Med Chem 2005;20:183–188.
- Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A, Klebe G. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: New pharmacological opportunities due to related binding site recognition. J Med Chem 2004;47:550–557.
- Pastorekova S, Casini A, Scozzafava A, Vullo D, Pastorek J, Supuran CT. Carbonic anhydrase inhibitors: The first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:869–873.
- Casey JR, Morgan PE, Vullo D, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004;47:2337–2347.
- Brown JM, William WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437–447.
- Scozzafava A, Banciu MD, Popescu A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of isozymes I, II and IV by sulfamide and sulfamic acid derivatives. J Enzyme Inhib Med Chem 2000;15:443–453.
- Schuman JS. Antiglaucoma medications: A review of safety and tolerability issues related to their use. Clin Ther 2000;22:167–208.
- Wilkinson BL, Bornaghi LF, Houston TA, Innocenti A, Vullo D, Supuran CT, Poulsen SA. Inhibition of membrane-associated carbonic anhydrase isozymes IX, XII and XIV with a library of glycoconjugate benzenesulfonamides. Bioorg Med Chem Lett 2007;17:987–982.
- Santos MA, Marques S, Vullo D, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of cytosolic/tumor-associated isoforms I, II, and IX with iminodiacetic carboxylates/hydroxamates also incorporating benzenesulfonamide moieties. Bioorg Med Chem Lett 2007;17:1538–1543.
- Güzel Ö, Maresca A, Scozzafava A, Salman A, Balaban AT, Supuran CT. Carbonic anhydrase inhibitors. Synthesis of 2,4,6-trimethylpyridinium derivatives of 2-(hydrazinocarbonyl)-3-aryl-1H-indole-5-sulfonamides acting as potent inhibitors of the tumor-associated isoform IX and XII. Bioorg Med Chem Lett 2009;19:2931–2934.
- Carta F, Maresca A, Scozzafava A, Vullo D, Supuran CT. Carbonic anhydrase inhibitors. Diazenylbenzenesulfonamides are potent and selective inhibitors of the tumor-associated isozymes IX and XII over the cytosolic isoforms I and II. Bioorg Med Chem 2009;17:7093–7099.
- Güzel, Ö,Maresca, A, Scozzafava, A, Salman, A, Balaban, AT, Supuran, CT. Discovery of low nanomolar and subnanomolar inhibitors of the Mycobacterial beta-carbonic anhydrases Rv1284 and Rv3273. J Med Chem 2009;52:4063–4067.
- Akbas E, Berber I. Antibacterial and antifungal activities of new pyrazolo[3,4-d] pyridazin derivatives. Eur J Med Chem 2006;41:904.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discovery 2008;7:168–181.
- Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm 2007;338:21–26.
- Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Ret Eye Res 2000;19:87–112.
- Ingram CJ, Brubaker RF. Effect of brinzolamide and dorzolamide on aqueous humor flow in human eyes. Am J Ophthalmol 1999;128:292–296.
- Konowal A, Morrison JC, Brown SVL, Cooke DL, Maguire LJ, Verdier DV, Fraunfelder FT, Dennis RF, Epstein RJ. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol 1999;127:403–406.
- Arslan O, Nalbantoğlu B, Demir N, Özdemir H, Küfrevioğlu Öİ. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Turk J Med Sci 1996;26:163–166.
- Rickli EE, Ghazanfar SA, Gibbson BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–1078.
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Clevage of structural proteins during in assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Innocenti A, Scozzafava A, Parkkila S, Pucceti L, De Simone G, Supuran CT. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–2271.
- Landolfi C, Marchetti M, Ciocci G, Milanese C. Development and pharmacological characterization of a modified procedure for the measurement of carbonic anhydrase activity. J Pharmacol Toxicol Methods 1998;38:169–172.
- Bülbül M, Hisar O, Beydemir Ş, Ciftci M, Kürevioğlu Öİ. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhib Med Chem 2003;18:371–375.
- Ciftci M, Bülbül M, Gül M, Gümüştekin K, Dane Ş, Süleyman H. Effects of nicotine and vitamin E on carbonic anhydrase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem 2005;20:103–108.
- Hisar O, Beydemir Ş, Bülbül M, Yanık T. Kinetic properties of carbonic anhydrase purified from gills of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 2006;30:185–187.
- Winum JY, Cecchi A, Montero JL, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with boron-containing sulfonamides, sulfamides, and sulfamates: Toward agents for boron neutron capture therapy of hypoxic tumors. Bioorg Med Chem Lett 2005;15:3302–3306.
- Şener A, Kasımoğulları R, Sener MK, Bildirici İ, Akçamur Y. Studies on the reactions of cyclic oxalyl compounds with hydrazines or hydrazones: Synthesis and reactions of 4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxylic acid. J Heterocycl Chem 2002;39:869–875.
- Şener A, Kasımoğulları R, Sener MK, Genç H, Studies on reactions of cyclic oxalyl compounds with hydrazines or hydrazones. Synthesis and reactions of 4-benzoyl-1-(4-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxylic acid. Chem Heterocycl Comp 2004;8:1201–1208.
- Kaymakçıoğlu BK, Rollas S. Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Farmaco 2002;57:595–599.
- Secrist JA, Liu PS. Studies directed toward a total synthesis of nucleoside Q. Annulation of 2,6–diaminopyrimidin–4–one with alpha–halo carbonyls to form pyrrolo[2,3–d] pyrimidines. J Org Chem 1978;43:3937–3941.