Abstract
A series of ternary copper(II) complexes have been derived using levofloxacin and five phenanthroline derivatives. Complexes were characterized using infrared spectroscopy, Thermogravimetric (TG)-analysis, fast atom bombardment mass spectroscopy and reflectance spectra. Synthesized complexes exhibit the only d-d band at ∼ 666 nm points toward a distorted square pyramidal geometry at metal centre with one unpaired electron responsible for paramagnetic behaviour of whole moiety. Binding behaviour of the complexes toward Herring Sperm DNA were determined using ultraviolet-Vis (UV-Vis) absorption titration and viscometric titration experiment, where as the cleavage efficacy of the complexes toward pUC19 DNA was determined by electrophoresis in presence of ethidium bromide. Complexes exhibit superoxide dismutase–like activity with their IC50 values ranging from 0.7917 to 1.7432 µM.
Introduction
Quinolones can act as antibacterial drugs that effectively inhibit DNA replication and are widely used in the treatment of many infections. The interaction of metal ions with diverse deprotonated quinolones have been thoroughly studiedCitation1. The recently released fluoroquinolone levofloxacin (LFLH) possess broad-spectrum antibacterial activity similar to that of earlier quinolones. However, it has enhanced activity against gram-positive and a typical organismsCitation2,Citation3. But, the optically active S(–)-isomer, LFLH is 2 fold more potent than the racemate and 8–28 fold more potent than its R(+)-isomer, ofloxacinCitation4.
The interaction of transition metal complexes with DNA has received considerable attention because they serve as potential models of biological systemsCitation5. Copper complex derived from 1,10-phenanthroline shows efficient DNA cleavage activity. The chemical attributes of metal complexes of phenanthroline are particularly attractive for developing new diagnostic and therapeutic agentsCitation6. Among the metal complexes so far investigated, those of polypyridyl phenanthroline bases have attracted great attention by virtue of its binding propensity to nucleic acid under the physiological conditionCitation7–9. Copper phenanthroline derivatives show an important biological activity. This includes chemical nuclease, antitumoural, antimycobacterial, antifungal, and antimicrobial activityCitation10.
Copper is a biologically relevant element and many enzymes that depends on copper for their activity have been identified. Copper(II) complexes are known to play a significant role in naturally occurring biological systems like [Cu,Zn–superoxide dismutase (SOD)] SOD. SOD can destroy the superoxide very rapidly, is a nature’s gift to organism to get rid from the burden of reactive superoxide radical. In fact a native SOD enzyme is found in many studies to exhibit protection in animal models of inflammatory diseasesCitation11. We present the synthesis and structural characterization of five ternary copper(II) complexes based on LFLH and five phenanthroline derivatives.
Experimental
Materials
Analytical grade of solvents, reagents, and chemicals were used throughout. Bayer AG (Wuppertal, Germany) generously supplied LFLH. Cupric chloride dihydrate was purchased from E. Merck (India) Ltd. Mumbai. 1,10-Phenanthroline, ethidium bromide (EtBr), bromophenol blue, agarose, and Luria Broth were purchased from Himedia (India). 2,9-Dimethyl-1,10-phenanthroline (A1), 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (A2), nicotinamide adenine dinucleotide reduced (NADH), nitro blue tetrazolium (NBT) and phenazin methosulphate (PMS) were purchased from Loba Chemie PVT. LTD (India).
Physical measurements
Elemental analyses (C, H, and N) of the synthesized complexes were performed with a model 240 Perkin Elmer elemental analyzer (MA, USA). Metallic content of the complex was determined after decomposing it under effect of acid mixture and titrating against EDTA solution volumetrically. Chlorine content of the complexes was carried out by modified Stepanow method. Room temperature magnetic measurement for the complexes was made using Gouy magnetic balance. The Gouy tube was calibrated using mercury(II)tetrathiocyanatocobaltate(II) a calibrant (χg = 16.44 × 10−6 cgs units at 20°C), Mumbai (India)Citation12. Thermogravimetric (TG) analyses data were obtained with a model 5000/2960 SDTA, TA instrument New castle (DE, USA). The electronic spectra were recorded on a UV-160A UV-Vis spectrophotometer, Shimadzu Kyoto (Japan). Infrared (IR) spectra were recorded on a Fourier transform–IR Shimadzu spectrophotometer as KBr pellets in the range 4000–400 cm−1. Fast atom bombardment (FAB)-mass spectrometry (MS) were recorded on Jeol SX 102/Da–600 mass spectrophotometer/Data system using Argon/Xenon (6 kV, 10 mA) as the FAB gas. The accelerating voltage was 10 kV and spectra were recorded at room temperature (MA, USA). Photo quantization of the gel after electrophoresis was done using AlphaDigiDoc™ RT. Version V.4.0.0 PC–Image software (CA, USA).
Preparation of phenanthroline derivative
4,5-Diazafluoren-9-one (A3), 1,10-phenanthroline-5,6-dione (A4) and 5-nitro-1,10-phenanthroline (A5) were prepared as per reported methodsCitation13–15.
Synthesis of ternary copper(II) complexes
A methanolic solution of CuCl2.2H2O (1.5 mmol) was added to a methanolic solution of NN donor ligand (1.5 mmol), followed by addition of a previously prepared solution of LFLH (1.5 mmol) in methanol in presence of CH3ONa (1.5 mmol). The pH was adjusted to ∼ 6.2 using dilute solution of CH3ONa. The resulting solution was refluxed for 1 h on a steam bath, followed by concentrating it to half of its volume. A fine amorphous product of green colour obtained was washed with ether/hexane and dried in vacuum desiccator.
Synthesized complexes at biological interphase
In-vitro antimicrobial behaviour
In-vitro antimicrobial tests were performed against Escherichia coli, Pseudomonas aeruginosa, Serratia marcescens, Bacillus subtilis and Staphylococcus aureus using 2-fold serial dilution technique in triplicateCitation16. Former three are gram-negative while later two are gram-positive organism. Microbes were subjected to test compounds at various concentration. The lowest concentration which inhibits the growth of microbes incubated at 37 ± 1°C for 24 h is termed as minimum inhibitory concentration (MIC; µM). All these operations were carefully performed under aseptic conditions.
DNA binding study by absorption titration
Hypochromism and bathochromism from intercalation mode of bindingCitation17, is due to strong stacking interaction between an aromatic chromophore and the DNA base pairCitation18. Selection of an appropriate absorbance peak was done by performing spectrophotometric wavelength scans of Cu(II) complexes. After addition of equivalent amount of DNA to reference cell, both were kept for incubation of 10 min at room temperature followed by absorption measurement. It was specifically done to enable direct comparison between the assays that was required to interpret the results obtained. The intrinsic binding constant, Kb was determined by making it subject in following equationCitation19.
where [DNA] is the concentration of DNA in base pairs, ϵa the apparent extinction coefficient is obtained by calculating Aobs./[complex] and ϵf corresponds to the extinction coefficient of the complex in its free form. The data were fitted to above equation where ϵb refers to the extinction coefficient of the complex in the fully bound form. When each set of data, fitted to the above equation, gave a straight line with a slope of 1/(ϵa – ϵf) and a y–intercept of 1/Kb(ϵb – ϵf). Kb was determined from the ratio of the slope to Intercept.
DNA binding study by hydrodynamic volume measurement
Ubbelohde viscometer immersed in a thermostatic bath maintained at 27 ± 0.1°C was used to measure the change in hydrodynamic volume with change in complex concentration. Digital stopwatch with least count of 0.01 sec. was engaged for flow times measurement with accuracy of ± 0.1 sec. Plot of (η/η0)1/3 versus [complex]/[DNA] is use to study the behaviour of binding, where η is the viscosity of DNA in presence of complex and η0 is the viscosity of DNA alone. Viscosity values were calculated from the observed flow time of DNA–containing solutions (t) corrected for that of the buffer alone (t0), η = (t – t0)Citation20.
DNA cleavage study by gel electrophoresis
Gel electrophoresis of plasmid DNA (pUC19 DNA) was carried out in Tris-acetate-EDTA (TAE) buffer (0.04 M TAE, pH 8, 0.001 M EDTA). Fifteen microlitre reaction mixture containing plasmid DNA in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) and 200 µM complex. Reactions were allowed to proceed for 24 h at 37°C. All reactions were quenched by addition of 5 µL loading buffer (0.25% bromophenol blue, 40% sucrose, 0.25% xylene cyanole, and 200 mM EDTA). The aliquots were loaded directly on to 1% agarose gel and electrophoresed at 50 V in 1X TAE buffer. Gel was stained with 0.5 µg/mL EtBr and was photographed on a UV illuminator. The percentage of each form of DNA was quantities using AlphaDigiDoc™ RT. Version V.4.0.0 PC–Image software.
Enzymatic behaviour
NBT/NADH/PMS system was used to study SOD-like behaviour of the complexes. The superoxide radial produce by 79 µM NADH, 30 µM PMS in phosphate buffer (pH = 7.8) was responsible for reduction of 75 μM NBT in system, and 0.25–3.0 µM tested compound are responsible for retardation in the reduction rate of NBT which was determined spectrophotometrically by monitoring the concentration of blue formazan form which absorbs at 560 nm. All measurements were carried out at room temperature. The % inhibition (η) of NBT reduction was calculated using following equationCitation21:
where k′ and k present the slopes of the straight line of absorbance values as a function of time in presence and absence of SOD mimic or a model compound, respectively. IC50 value of the complex was determined by plotting the graph of percentage inhibition of NBT reduction against increase in concentration of the complex. Concentration of the complex which causes 50% inhibition of NBT reduction is reported as IC50.
Results and discussion
Characterization of complexes
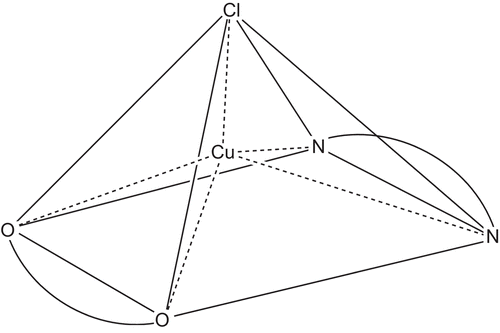
Several instrumental techniques like elemental analysis, magnetic measurement, TG-analysis, reflectance, IR, and FAB-MS were used to evaluate structure of the complexes. represents the sketch of proposed structure. Elemental analysis and magnetic moment data are in good agreement with proposed structure (). (Supplementary Figure S1).
Table 1. Analytical and physical data of the complexes.
IR spectroscopy
Major changes in IR spectra of ligands on complexation are comprised in and some important points are as follows:
Table 2. Characteristic IR bands (4000–400 cm−1) of LFLH and their complexes.
Band at 1634 and 1316 cm−1 in case of LFLH corresponds to ν(COO)assy and ν(COO)sym respectively, which on complexation with metal ion shifts about 1580 and 1347 cm−1 respectivelyCitation22.
Unidentate nature for the carboxylato group of LFLH is proved by frequency of separation (Δν = νCOOassy – νCOOsym) which is about 233 cm−1 Citation23.
Deprotonation of the hydroxyl group of LFLH was confirmed by deduction of band at 3519 cm−1 from spectra due to hydrogen bondingCitation24.
Coordination via pyridone oxygen atom of LFLH was suggested from the shift of its band from 1728 cm−1 to about 1625 cm−1 Citation25, these data are further supported by ν(M–O) which appear at ∼ 520 cm−1 Citation26.
NN donating nature of ligand was confirmed from band appearing at ∼ 538 cm−1 Citation27.
Thermogravimetric analyses
TG-analysis data confirms that there are five molecule of water of crystallization which are librated during 75–120°C. No loss in weight during 120–210°C points toward absence of coordinated water molecules. Residue remaining at the end of heating i.e. after 720°C is in full competition with expected weight for metal oxideCitation28.
Magnetic and electronic behaviour
The magnetic moment measurement was found about 1.79 Bohr Magneton (B.M.) which is very close toward spin only value i.e. 1.73 B.M. expected for one unpaired electron hence it confirms the copper(II) ion in form of d9 systemCitation29.
There are two possibilities of geometry, if copper(II) ion is surrounded by five donor atoms. First is trigonal bipyramidal, which shows the pattern of λmax > 800 nm along with shoulder at ∼ 660 nmCitation30 and second is square pyramidal, where only a broad band about 660 nm is observedCitation31 (Supplementary Figure S2). The spectra clearly kick out the first possibility and strongly directs toward distorted square pyramidal geometry for Cu(II)-d9 system.
FAB-MS
Peaks at 136, 137, 154, 289, and 307 m/z value appearing in the mass spectra are due to m-nitro benzyl alcohol. represents the FAB-MS of complex 1, i.e. [Cu(LFL)(A1)Cl] 5H2O. The doublet at 668 and 670 corresponds to (M) and (M + 2) of the complex 1 associated with two protons in absence of crystallization water; which is due to one chlorine and metal. Doublet at 459 and 461 is assigned to fragment with one chloride associated with one proton. Whereas the doublet at 306 and 308 due to fragment with single chlorine atom associated with no proton. Several other fragments at 424, 362, 271, and 209 m/z value are observed, attributed to fragments associated with different numbers of H+ ions. The proposed fragmentations are shown in Supplementary Figure S3.
Synthesized complexes at biological interphase
In-vitro antimicrobial behaviour
comprises the data for antibacterial efficacy of complexes, ligands, and LFLH against two gram(+) and three gram(−) microorganisms. It is clear from the data that all complexes are active compare to LFLH and phenanthroline derivatives but complex 5 proved to most active for all the bacterial species employed.
Table 3. Antimicrobial activities of LFLH, phenanthrolines and their complexes in terms of minimum inhibitory concentration (MIC; µM).
DNA binding studies by absorption titration
It is a general observation that the binding of intercalative molecules to DNA is accompanied by a red shift and hypochromism in the absorption spectraCitation32. The extent of spectral change is related to the strength of binding. The absorption spectra of the complex in absence and presence of DNA is illustrated in . In order to compare quantitatively the binding strength of the complexes, the intrinsic binding constant Kb was obtained by monitoring the changes in absorbance for complexes with increasing concentration of DNA. From the Kb value () and red shift it is clear that complexes bind via intercalation mode.
Table 4. Binding constant (Kb) and 50% inhibitory concentration (IC50) values of the complexes.
Figure 3. Absorption spectral traces on addition of Herring Sperm DNA to the solution of complex 1 after incubating it for 10 min at room temperature in phosphate buffer at 7.2 pH. (shown by arrow). Inset: plot of [DNA]/(ϵa-ϵf) versus [DNA] for absorption titration of Herring Sperm DNA with complex 1.
![Figure 3. Absorption spectral traces on addition of Herring Sperm DNA to the solution of complex 1 after incubating it for 10 min at room temperature in phosphate buffer at 7.2 pH. (shown by arrow). Inset: plot of [DNA]/(ϵa-ϵf) versus [DNA] for absorption titration of Herring Sperm DNA with complex 1.](/cms/asset/130eb04a-a0a0-4b88-b3ec-de3fc604be6a/ienz_a_506874_f0003_b.gif)
DNA binding study by hydrodynamic volume measurement
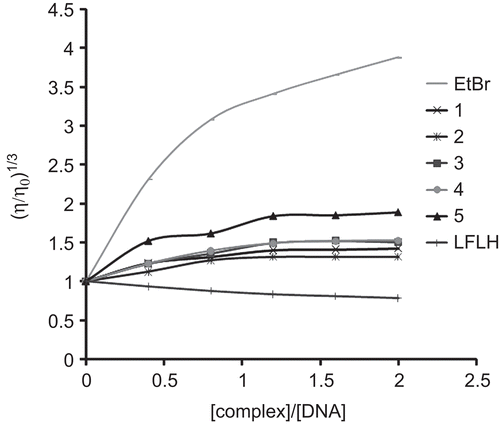
The binding modes of the Cu(II) complexes were further investigated by viscosity measurements, which are sensitive to DNA length change, regarded as the least ambiguous and the most critical tests of binding in solution. A classical intercalative mode demands that the DNA helix must lengthen as base pairs are separated to accommodate the binding ligand, leads to the increase in DNA viscosityCitation33. The effects of complexes, EtBr and LFLH on the viscosity of rod-like DNA are shown in . EtBr increases the relative specific viscosity by lengthening the DNA double helix through the intercalation mode. On increasing the amounts of complexes, the relative viscosity of DNA increases steadily, this is similar to the behaviour of EtBr. The increased degree of viscosity, which may depend on its affinity to DNA follows the order of EtBr > 5 > 4 > 3 > 1 > 2. In contrast, LFLH binds via partial, non-classical intercalation of ligand on bending (or kinking) the DNA helix and reduce its effective length and, concomitantly, its viscosityCitation34.
DNA cleavage study
Agarose gel electrophoresis is used as a base for monitoring plasmid DNA cleavage reaction. Circular plasmid DNA when subjected to electrophoresis, relatively fast migration is observed for the intact supercoil form ( SC; Form I). The scission on one strand (nicking) will relax supercoil to generate a slower-moving open circular form (OC; Form II), and if both strands are cleaved, a linear form (LC; Form III) that migrates between Form I and Form II will be generatedCitation35. Data of the cleavage study obtain from are presented in . Difference in DNA cleavage efficiency of complexes was due to the difference in binding affinity of complexes to DNA.
Table 5. Percentage of all the three forms of plasmid separated on agarose gel, when subjection to different synthesized complexes at 200 µM concentration.
Figure 5. Gel electrophoresis diagram showing the cleavage of SC pUC19 DNA(300 µg/mL) with as series of copper(II) complex (200 µM) in a final volume of 15 µL, incubated at 37°C, using 1% agarose gel, at 50 mV for 1.5 h. Lane 1, DNA control; Lane 2, DNA + CuCl2·2H2O; Lane 3, DNA + LFLH; Lane 4, DNA + [Cu(LFL)(A1)Cl].5H2O; Lane 5, DNA + [Cu(LFL)(A2)Cl].5H2O; Lane 6, DNA + [Cu(LFL)(A3)Cl].5H2O; Lane 7, DNA + [Cu(LFL)(A4)Cl].5H2O; Lane 8, DNA + [Cu(LFL)(A5)Cl].5H2O.
![Figure 5. Gel electrophoresis diagram showing the cleavage of SC pUC19 DNA(300 µg/mL) with as series of copper(II) complex (200 µM) in a final volume of 15 µL, incubated at 37°C, using 1% agarose gel, at 50 mV for 1.5 h. Lane 1, DNA control; Lane 2, DNA + CuCl2·2H2O; Lane 3, DNA + LFLH; Lane 4, DNA + [Cu(LFL)(A1)Cl].5H2O; Lane 5, DNA + [Cu(LFL)(A2)Cl].5H2O; Lane 6, DNA + [Cu(LFL)(A3)Cl].5H2O; Lane 7, DNA + [Cu(LFL)(A4)Cl].5H2O; Lane 8, DNA + [Cu(LFL)(A5)Cl].5H2O.](/cms/asset/522c5795-888e-4405-b7c8-bcf4d3e80508/ienz_a_506874_f0005_b.gif)
Enzymatic behaviour; SOD-like activity
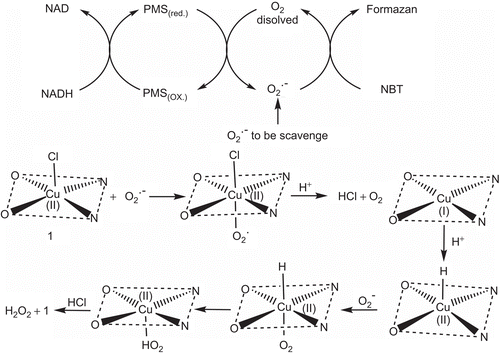
Measured IC50 values for SOD-like activity at biological pH ranges from 0.7917 to 1.7432 µM () (Supplementary Figure S4). The complexes show different extent of superoxide scavenging ability. The higher IC50 can be accredited to the vacant coordination site facilitating the binding of superoxide anion, electrons of aromatic ligands that stabilize Cu–O2− interaction and not only to the partial dissociation of complex in solution. Difference in electron withdrawing group on phenanthroline can also be taken under consideration for difference in affinity of complexes toward reactive specie. Cotton and Wilkinson suggested the mechanism for scavenging of superoxide radical which move forward via unstable octahedral adduct under influence of Jahn–Teller effectCitation36–38, and there is a possibility of rapid interconversion between Cu(II) and Cu(I) via electron transfer between copper and reactive oxygen radical anion following the principle of electroneutralityCitation39. The proposed mechanism for generation of reactive oxygen species and its dismutation is shown in
Conclusion
Data of magnetic behaviour and electronic spectral measurement points towards the d9 system with distorted square pyramidal geometry. Taking a while toward the data from MIC, absorption titration, viscosity measurement, DNA cleavage, and SOD; increasing order of the activity of the synthesized complexes is 2 < 1 < 3 < 4 < 5. MIC values of complexes are much better compare to ligands, drug, and metal salt, which can be studied under chelation theory. From the absorption titration and viscosity data intercalative mode of binding of complexes is proved. Also the antioxidant activity of complex 5 is found to be the highest among all.
Declaration of interest
Authors thank The Head, Department of Chemistry, Sardar Patel University, India for making it convenient to work in laboratory and U.G.C. for providing financial support under ‘UGC Research Fellowship in Science for Meritorious Students’ scheme.
Supplementary Material
Download PDF (681.4 KB)References
- Efthimiadou EK, Katsaros N, Karaliota A, Psomas G. Mononuclear copper(II) complexes with quinolones and nitrogen-donor heterocyclic ligands: synthesis, characterization, biological activity and interaction with DNA. Inorg Chim Acta 2007;360:4093–4102.
- Emami S, Shafiee A, Foroumadi A. Structural features of new quinolones and relationship to antibacterial activity against gram-positive bacteria. Mini Rev Med Chem 2006;6:375–386.
- Hooper DC. Mode of action of fluoroquinolones. Drugs 1999;58(Suppl 2):6–10.
- Foroumadi A, Emami S, Mansouri S, Javidnia A, Saeid-Adeli N, Shirazi FH, Shafiee A. Synthesis and antibacterial activity of levofloxacin derivatives with certain bulky residues on piperazine ring. Eur J Med Chem 2007;42:985–992.
- Chan S, Wong WT. 10. Ruthenium 1992. Coord Chem Rev 1995;138:219–296.
- Chen CY, Chen QZ, Wang XF, Liu MS, Chen YF. Synthesis, characterization, DNA binding properties, and biological activities of a mixed ligand copper(II) complex of ofloxacin. Trans Met Chem 2009;34:757–763.
- Chetana PR, Rao R, Roy M, Patra AK. New ternary copper(II) complexes of l-alanine and heterocyclic bases: DNA binding and oxidative DNA cleavage activity. Inorg Chim Acta 2009;362:4692–4698.
- Lu LP, Zhu ML, Yang P. Crystal structure and nuclease activity of mono(1,10-phenanthroline) copper complex. J Inorg Biochem 2003;95:31–36.
- Patra AK, Roy S, Chakravarty AR. Synthesis, crystal structures, DNA binding and cleavage activity of l-glutamine copper(II) complexes of heterocyclic bases. Inorg Chim Acta 2009;362:1591–1599.
- Oliver MB, Raso ÁG, Terrón Á,Molins E, Prieto MJ, Moreno V, Serra JM, Lladó V, López I, Gutiérrez A, Escribá PV. Ternary copper(II) complexes with hippurate derivatives and 1,10-phenanthroline: synthesis and biological activity. Inorg Chim Acta 2009;362:4744–4753.
- Patel RN, Shukla KK, Singh A, Choudhary M, Chauhan UK, Dwivedi S. Copper(II) complexes as superoxide dismutase mimics: synthesis, characterization, crystal structure and bioactivity of copper(II) complexes. Inorg Chim Acta 2009;362:4891–4898.
- Henderson LJ Jr., Fronczek FR, Cherry WR. Selective perturbation of ligand field excited states in polypyridine ruthenium(II) complexes. J Am Chem Soc 1984;106:5876–5879.
- Hiort C, Lincoln P, Norden B. DNA binding of.DELTA.-and.LAMBDA.-[Ru(phen)2DPPZ]2+. J Am Chem Soc 1993;115:3448–3454.
- Smith GF, Cagle FW Jr. The improved synthesis of 5-nitro-1,10-phenanthroline. J Org Chem 1947;12:781–784.
- Pelczar MJ, Reid RD, Chan ECS. Microbiology. 4th edn. New Delhi, India: Tata McGraw–Hill, 1979.
- Trommel JS, Marzilli LG. Synthesis and DNA binding of novel water-soluble cationic methylcobalt porphyrins. Inorg Chem 2001;40:4374–4383.
- Mudasir S, Yoshioka N, Inoue H. DNA binding of iron(II) mixed-ligand complexes containing 1,10-phenanthroline and 4,7-diphenyl-1,10-phenanthroline. J Inorg Biochem 1999;77:239–247.
- Wolfe A, Shimer GH Jr., Meehan T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987;26:6392–6396.
- Ihmels H, Otto D. Intercalation of organic dye molecules into double-stranded DNA-general principles and recent developments. Top Curr Chem 2005;258:161–204.
- Basili S, Bergen A, Dall’Acqua F, Faccio A, Granzhan A, Ihmels H, Moro S, Viola G. Relationship between the structure and the DNA binding properties of diazoniapolycyclic duplex- and triplex-DNA binders: efficiency, selectivity, and binding mode. Biochemistry 2007;46:12721–12736.
- Le X, Liao S, Liu X, Feng X. Synthesis, structure and SOD-like activity of a ternary cu(II) complex with 1,10-phenanthroline and l-valinate. J Coord Chem 2006;59:985–995.
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogel’s Textbook of Practical Organic Chemistry. 5th edn. Harlow: Longman, 2004.
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enzyme Inhib Med Chem 2005;20:303–307.
- Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 4th edn. New York: A Wiley Interscience Publication, 1986.
- Patel SH, Pansuriya PB, Chhasatia MR, Parekh HM, Patel MN. Coordination chain polymeric assemblies of trivalent lanthanides with multidentate schiff base synthetic, spectral investigation and thermal aspects. J Therm Anal Cal 2008;91:413–414.
- Turel I, Leban I, Bukovec N. Crystal structure and characterization of the bismuth(III) compound with quinolone family member (ciprofloxacin). Antibacterial study. J Inorg Biochem 1997;66:241–245.
- Freedman HH. Intramolecular H-bonds. I. A spectroscopic study of the hydrogen bond between hydroxyl and nitrogen. J Am Chem Soc 1961;83:2900–2905.
- Figgis BN, Lewis J. In: Lewis J, Wilkins RG, eds. Modern Coordination Chemistry: Principles and Methods. The magnetochemistry of complex compounds. New York: Interscience, 1960; pp. 400–454.
- Melnik M. Mono-, bi-, tetra- and polynuclear copper(II) halogenocarboxylates. Coord Chem Rev 1981;36:1–44.
- Wang LY, Chen QY, Huang J, Wang K, Feng CJ, Gen ZR. Synthesis, characterization, and bioactivities of copper complexes with n-substituted di(picolyl)amines. Trans Met Chem 2009;34:337–345.
- Iskander MF, EL-Sayed L, Salem NMH, Warner R, Haase WJ. Synthesis, characterization and magnetochemical studies of dicopper(II) complexes derived from bis(n- salicylidene)dicarboxylic acid dihydrazides. J Coord Chem 2005;58:125–139.
- Zhang QL, Liu JH, Liu JZ, Zhang PX, Ren XZ, Liu Y, Huang Y, Ji LN. DNA-binding and photoactivated enantiospecific cleavage of chiral polypyridyl ruthenium(II) complexes. J Inorg Biochem 2004;98:1405–1412.
- Satyanaryana S, Daborusak JC, Chaires JB. Tris(phenanthroline)ruthenium(II) enantiomer interactions with DNA: mode and specificity of binding. Biochem 1993;32:2573–2584.
- Liu JG, Ye BH, Li H, Zhen QX, Ji LN, Fu YH. Polypyridyl ruthenium(II) complexes containing intramolecular hydrogen-bond ligand: syntheses, characterization, and DNA-binding properties. J Inorg Biochem 1999;76:265–271.
- Sitlani S, Long EC, Pyle AM, Barton JK. DNA photocleavage by phenanthrenequinone diimine complexes of rhodium(III): shape-selective recognition and reaction. J Am Chem Soc 1992;114:2303–2312.
- Cotton F, Wilkinson G. Advanced Inorganic Chemistry. New York: A Wiley Interscience Publication, 1972.
- Fee JA. In: Siegel H, ed. Metal Ions in Biological Systems. Vol. 13. New York: Marcel Dekker, 1981.
- Huheey JE. Inorganic Chemistry Principles of Structure and Reactivity. 3rd edn. New York: Harper and Row, 1983.
- Ramadan AM, EI-Naggar MM. Synthesis, characterization and demonstration of superoxide dismutase-like activity of copper(II) chloride, bromide, nitrate, thiocyanate, sulphate, and perchlorate complexes with 2-methyl-amino pyridine. J Inorg Biochem 1996;63:143–153.

![Figure 2. Fast atom bombardment (FAB)-mass spectrum of complex 1, i.e. [Cu(LFL)(A1)Cl]·5H2O recorded on Jeol SX 102/Da–600 mass spectrophotometer/Data system using Argon/Xenon (6 kV, 10 mA) as the FAB gas at accelerating voltage of 10 kV.](/cms/asset/31150527-3a63-41d8-be35-a00ea2a5911d/ienz_a_506874_f0002_b.gif)