Abstract
In this study, a series of novel 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole (6a–g) and 1,3,4-oxadiazole (7a–g, 8) were synthesized from N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide derivatives of benzothiazole class. Antimicrobial properties of the title compound derivatives were investigated against one Gram (+) bacteria (Staphylococcus aureus), three Gram (−) bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) and five fungi (Candida albicans, Aspergillus niger, Aspergillus flavus, Monascus purpureus and Penicillium citrinum) using serial plate dilution method. The investigation of antibacterial and antifungal screening data revealed that all the tested compounds showed moderate to good inhibition at 12.5–100 µg/mL in DMSO. It has been observed that triazolo-thiadiazole derivatives are found to be more active than 1,3,4-oxadiazole derivatives against all pathogenic bacterial and fungal strains.
Introduction
Antimicrobials reduce or completely block the growth and multiplication of bacteria. This has made them unique for the control of deadly infectious diseases caused by a variety of pathogens. They have transformed our ability to treat infectious diseases such as pneumonia, meningitis, tuberculosis, malaria and AIDSCitation1. Although deaths from bacterial and fungal infections have dropped in the developed world, these are still major causes of death in the developing worldCitation2.
According to WHO, each year 1.4 million children died of gut infections and diarrhoea caused by gram-negative bacteria like Pseudomonas, Salmonella, Shigellae and gram positive rods like Corynebacterium diptheriaeCitation2.Decades of antibiotic use have resulted in the development of widespread resistance to commonly prescribed antibacterial agentsCitation3. Therefore, there is a need to develop new, potent, fast-acting antimicrobial drugs with low toxicity. In the design of new compounds, development of hybrid molecules through the combination of different pharmacophores in one structure may lead to compounds with increased antimicrobial activityCitation4.
Despite numerous attempts to develop new structural prototype in the search for more effective antimicrobials, benzothiazole still remain as one of the most versatile class of compounds against microbes and therefore, are useful substructures for further molecular exploration. Benzothiazole’s literature is enriched with progressive findings of the moiety in respect of anticonvulsant and antimicrobial activitiesCitation5. Benzothiazole and its derivatives constitute the most versatile and valuable source of antimicrobial compounds. They appear to transcend the chemotherapeutic boundaries of other antiparasitic drugs with a spectrum of activity that includes the majority of fungi, bacteria, protozoa and helminthic species.
Several benzothiazole derivatives were also associated with antitumorCitation6, antimicrobialCitation7,Citation8, anticonvulsantCitation9, and anti-inflammatoryCitation7,Citation10 activities. In addition, triazolo-thiadiazoles nucleus constitutes the active part of several biologically active compounds, including antibacterialCitation11, antifungalCitation12, antitumorCitation13, anti-inflammatoryCitation14, analgesicCitation15 and so on. Moreover, 1,3,4-oxadiazole were also reported to possess significant antimicrobialCitation16, anti-inflammatoryCitation17 and analgesic activitiesCitation18.
A triazolo-thiadiazole system may be viewed as a cyclic analogue of two very important components-thiosemicarbazide Citation19,Citation20 and biguanide Citation21, which often display diverse biological activities. Inspired by these observations a composite system was investigated, which combine these two biolabile components in a ring together to give a compact and planar structure, and screened for their biological activities.
The prime objective for the current study is to develop novel derivatives of benzothiazole moiety and finally screen them against different microbial strains (bacteria and fungi) at variable concentrations. The rationale for the study includes the designing of the derivatives having some common structural features that are important for the compound to exhibit an antimicrobial activity that includes the following:Citation22–24
A lipohilic bicyclic aromatic ring system.
Another bulky lipophilic group (e.g. phenyl, tert-butyl) as a side chain.
Two lipophilic domain linked by a spacer of appropriate length with polar centre at defined position, for example, Naftifine, Butenafine, Terbinafine, Debacarb, Penicillins and Cephalosporins.
In view of the above mentioned facts and in continuation of our interest in the synthesis of heterocycles containing benzothiazole moiety, to identify new candidates that may be of value in designing new, potent, selective and less toxic antimicrobial agents, we report herein the synthesis and antimicrobial evaluation of some novel structure hybrids incorporating both the benzothiazole moiety with either the triazolo-thiadiazole or oxadiazole ring systems through different linkages. This combination was suggested in an attempt to investigate the influence of such hybridization and structure variation on the anticipated biological activities, hoping to add some synergistic biological significance to the target molecules. The substitution pattern of triazolo-thiadiazole and oxadiazole rings was carefully selected so as to confer different electronic environment to the molecules.
Hence, to discover new and useful agents for treatment of microbial diseases, we have replaced the hydrazide group of N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide with additional heterocycles, which have been found to possess an interesting profile of antimicrobial activity. The heterocycles reported here are triazolo-thiadiazole and 1,3,4-oxadiazole. Thus, the synthesized compounds were investigated against one Gram (+) bacteria (Staphylococcus aureus), three Gram (−) bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) and five fungi (Candida albicans, Aspergillus niger, Aspergillus flavus, Monascus purpureus and Penicillium citrinum) using serial plate dilution method.
Experimental
Chemistry
Chemicals were purchased from Merck Chemical Company, S.D. Fine (India) and Qualigens (India). Melting points were determined in open capillary tubes in a Hicon melting point apparatus and are uncorrected. IR (KBr) spectra were recorded on a Nicolet, 5PC FTIR spectrometer (νmax in cm−1) and 1H NMR spectra were recorded in CDCl3/DM SO-d6 on a Bruker DRX-300 (300 MHz FT NMR) spectrometer using tetramethylsilane (TMS) as internal reference. Chemical shift (δ) are expressed in parts per million (ppm); coupling constants (J) are reported in hertz and refer to apparent peak multiplicities, which may not necessarily be true coupling constants. Mass spectra were recorded using Jeol SR-102 (FAB) mass spectrometer. The purity of various synthesized compounds was checked by TLC and elemental analysis. Spectral data (1H NMR, IR and mass) of the synthesized compounds were in full agreement with the proposed structures.
General procedure for the synthesis of 6-chloro-1,3-benzothiazole-2-amines (1)
A mixture of aniline (0.01 mol) and potassium thiocyanate (0.01 mol) in glacial acetic acid (10%) was cooled and stirred. To this solution bromine (0.01 mol) was added dropwise at such a rate as to keep the temperature below 10°C throughout the addition. Stirring was continued for an additional 3 h and the separated hydrochloride salt was filtered, washed with acetic acid and dried. It was dissolved in hot water and neutralized with aqueous ammonia solution (25%), filtered, washed with water and dried, recrystallized with benzene to obtain 6-chloro-1,3-benzothiazole-2-amines.
1. IR (KBr) νmax (cm−1): 817 (C-Cl), 1570 (C = N), 3480 (NH); 1H NMR (300 MHz) (DMSO-d6) δ (ppm) 6.12 (2H, s, NH2), 6.61–6.64 (3H, J = 9 Hz, m, Ar-H).
General procedure for the synthesis of 1-(6-chloro-1,3-benzothiazol-2-yl) urea (2)
To the solution of sodium cyanate in minimum quantity of water, glacial acetic acid (5 mL) was added. This solution was heated with 2-amino-6-chloro-benzothiazole 1 (0.01 mol) in alcohol till the contents of mixture become turbid and volume remained half of the original volume. The contents were added to ice cool water. The solid obtained was filtered off and dried.
2. IR (KBr) νmax (cm−1): 830 (C-Cl), 1560 (C = N), 1628 (C = O), 3310 (NH); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 6.34 (2H, s, NH2), 6.68–6.70 (3H, J = 6 Hz, m, Ar-H), 8.10 (1H, s, NHC = O).
General procedure for the synthesis of N-(6-chlorobenzo[d]thiazol-2-yl)hydrazine carboxamide (3)
To the warm hydrazine hydrate solution of compound 2 in alcohol, conc. NaOH was added and refluxed for 6 h. Reaction mixture was poured into crushed ice and solid obtained was filtered off and dried. The solid collected out was recrystallized from suitable solvent to get the compound N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide.
(3). IR (KBr) νmax (cm−1): 657 (C-S-C), 817 (C-Cl), 1588 (C = N), 1660 (C = O), 3300 (NH); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.28 (1H, s, NHNH2), 7.70–7.74 (3H, J = 12 Hz, m, Ar-H), 9.12 (1H, s, NHC = O).
General procedure for the synthesis of potassium dithiocarbazinate (4)
Potassium hydroxide (0.03 mol) was dissolved in absolute ethanol (50 mL). The solution was cooled in ice bath and acid hydrazide (3; 0.02 mol) was added with stirring. To this carbon disulphide (0.025 mol) was added in small portions with constant stirring. The reaction mixture was agitated continuously for 12 h at room temperature. The precipitated potassium dithiocarbazinate was collected by filtration, washed with anhydrous ether (100 mL) and dried in vacuum. The potassium salt thus obtained was in quantitative yield and was used in the next step without further purification.
General procedure for the synthesis of 4-amino-5-(6-chlorobenzo[d]thiazol-2-ylamino)-4H-1,2,4-triazole-3-thiol (5)
A suspension of potassium dithiocarbazinate (4; 0.02 mol) in water (50 mL) and hydrazine hydrate (99%,0.04 mol) was refluxed for 18–20 h with occasional shaking. The colour of the reaction mixture changed to green, with evolution of hydrogen sulphide gas. A homogenous reaction mixture was obtained during the reaction process. The reaction mixture was cooled to room temperature and diluted with water (20 mL). On acidification with acetic acid, the required triazole was precipitated out. It was filtered, washed thoroughly with cold water, dried and recrystallized from ethanol. Purity of the compound was checked by TLC using silica gel-G coated plates by using toluene: ethyl acetate:formic acid (T:E:F); (5:4:1) as solvent system, and observed in UV light.
5. IR (KBr) νmax (cm−1): 635 (C-S-C), 817 (C-Cl), 1528 (C = N), 2518 (SH), 3338 (NH2); 1H NMR (DMSO-d6) (300 MHz) δ (ppm): 5.15(s, 2H, NH2), 7.28–7.32 (3H, J = 12 Hz, Ar-H), 9.29 (1H, s, NH),13.18 (1H, br s, SH).
General procedure for the synthesis of 6-chloro-N-(6-substituted-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-3-yl)benzo[d] thiazol-2-amine (6a–g)
An equimolar mixture (0.10 mol) of 4-amino-5-(6-chlorobenzo[d]thiazol-2-ylamino)-4H-1,2,4-triazole-3-thiol (5) and aromatic acids in phosphorus oxychloride (10 mL) was refluxed for 5 h. The reaction mixture was cooled to room temperature and then gradually poured on to crushed ice with stirring. The mixture was allowed to stand overnight and the solid separated out was filtered, treated with dilute sodium hydroxide solution and washed thoroughly with cold water. The compound so obtained was dried and recrystallized from ethanol.
(6a). IR (KBr) νmax (cm−1): 610 (C-S-C benzothiazole), 674 (C-S-C triazolo-thiadiazole), 837 (C-Cl ), 1269 (N-N = C triazolo-thiadiazole), 1416 (C-N benzothiazole), 1518 (C = C aromatic), 3084 (C-H aromatic), 3314 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm): 7.41–7.44 (8H, J = 9 Hz, m, Ar-H), 8.06 (1H, s, NH); 13C NMR δ (ppm): 118.3,121.2, 125.8, 128.7, 129.2, 129.8, 130.9, 132.3, 133.5, 143.3, 151.3, 157.2, 167.6, 174.5; Mass (m/z): 384 (M+). Analysis for C16H9ClN6S2 (384.87); Calcd: C; 49.96, H; 2.40, N; 21.88, S; 16.69. Found: C; 49.93, H; 2.36, N; 21.84, S; 16.66.
(6b). IR (KBr) νmax (cm−1): 616 (C-S-C benzothiazole), 688 (C-S-C triazolo-thiadiazole), 821 (C-Cl), 1274 (N-N = C triazolo-thiadiazole), 1422 (C-N benzothiazole), 1524 (C = C aromatic), 3114 (C-H aromatic), 3318 (N-H);1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.36–7.40 (7H, J = 12 Hz, m, Ar-H), 8.01 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 127.3, 128.9, 129.3, 129.8, 130.1, 132.2, 132.3, 136.9, 151.3, 157.2, 167.6, 174.1, 174.5; Mass (m/z): 419 (M+). Analysis for C16H8Cl2N6S2 (419.31); Calcd: C; 45.86, H; 1.94, N; 20.07, S; 15.31. Found: C; 45.83, H; 1.92, N; 20.04, S; 15.29.
(6c). IR (KBr) νmax (cm−1): 614 (C-S-C benzothiazole), 686 (C-S-C triazolo-thiadiazole), 822 (C-Cl), 1275 (N-N = C triazolo-thiadiazole), 1424 (C-N benzothiazole), 1526 (C = C aromatic), 3112 (C-H aromatic), 3317 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.40–7.44 (6H, J = 12 Hz, m, Ar-H), 8.03 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 127.4, 129.8, 130.3, 130.9, 132.3, 133.6, 135.0, 135.7, 151.3, 157.2, 167.6, 174.1, 174.5; Mass (m/z): 453 (M+). Analysis for C16H17Cl3N6S2 (453.76); Calcd: C; 42.37, H; 1.58, N; 18.56, S; 14.15. Found: C; 42.35, H; 1.55, N; 18.52, S; 14.13.
(6d). IR (KBr) νmax (cm−1): 618 (C-S-C benzothiazole), 691 (C-S-C triazolo-thiadiazole), 829 (C-Cl), 1281 (N-N = C triazolo-thiadiazole), 1427 (C-N benzothiazole), 1531 (C = C aromatic), 3119 (C-H aromatic),3319 (N-H);1H NMR (DMSO-d6) (300 MHz) δ (ppm) 2.34(3H, S, CH3), 7.35–7.37 (6H, J = 6 Hz, m, Ar-H), 8.07 (1H, s, NH); 13C NMR δ (ppm): 18.7,118.3, 121.2, 125.8, 126.2, 127.4, 128.6,129.5, 129.8, 132.3, 136.9, 137.2, 151.3, 157.2, 167.6, 174.1, 174.5; Mass (m/z): 398 (M+). Analysis for C16H17Cl3N6S2 (398.89); Calcd: C; 51.21, H; 2.82, N; 21.10, S;16.12. Found: C; 51.19, H; 2.78, N; 21.07, S; 16.08.
(6e). IR (KBr) νmax (cm−1): 626 (C-S-C benzothiazole), 684 (C-S-C triazolo-thiadiazole), 817 (C-Cl), 1267 (N-N = C triazolo-thiadiazole), 1436 (C-N benzothiazole), 1527 (C = C aromatic), 3106 (C-H aromatic), 3324 (N-H);1H NMR (DMSO-d6) (300 MHz) δ (ppm) 2.51(3H, s, OCOCH3), 7.24–8.28 (7H, J = 12 Hz, m, Ar-H),8.10 (1H, s, NH); 13C NMR δ (ppm): 20.3,118.3, 121.2, 123.2, 125.8, 126.0, 127.9, 129.1, 129.4,129.8, 132.3, 151.1, 151.3, 157.2, 167.6, 169.0, 174.1, 174.5; Mass (m/z): 442 (M+). Analysis for C18H11ClN6O2S2 (442.90); Calcd: C; 48.84, H; 2.52, N; 18.99, S; 14.51. Found: C; 48.81, H; 2.50, N; 18.97, S; 14.48.
(6f). IR (KBr) νmax (cm−1): 626 (C-S-C benzothiazole), 692 (C-S-C triazolo-thiadiazole), 836 (C-Cl), 1274 (N-N = C triazolo-thiadiazole), 1434 (C-N benzothiazole), 1532 (C = C aromatic), 3123 (C-H aromatic), 3318 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.29–7.31 (m, J = 6 Hz,8H, Ar-H), 8.10 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 122.7, 124.6, 125.8, 129.7, 129.8, 132.3, 151.3, 155.2, 157.2, 167.6, 174.5; Mass (m/z): 400 (M+). Analysis for C16H9ClN6OS2 (400.87); C; 47.97, H; 2.30, N; 20.98, S; 16.04. Found: C; 47.94, H; 2.26, N; 20.96, S; 16.00.
(6g). IR (KBr) νmax (cm−1): 621 (C-S-C benzothiazole), 689 (C-S-C triazolo-thiadiazole), 830 (C-Cl), 1280 (N-N = C triazolo-thiadiazole), 1369 (NO2), 1429 (C-N benzothiazole), 1527 (C = C aromatic), 3118 (C-H aromatic), 3320 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.39–7.41 (7H, J = 6 Hz, m, Ar-H), 8.12 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 124.4, 125.8, 128.4,129.8, 132.3, 139.6, 143.3, 147.9, 151.3, 157.2, 167.6, 174.1, 174.5; Mass (m/z): 429 (M+). Analysis for C16H17Cl3N6S2 (429.86): C; 48.84, H; 2.52, N; 18.99, S; 14.51. Found: C; 48.81, H; 2.50, N; 18.97, S; 14.48.
General procedure for the synthesis of N-(6-Chlorobenzo[d]thiazol-2-yl)-5-phenyl-1,3,4-oxadiazol-2-amine (7a)
Compound 3 (0.001 mol) and appropriate aromatic acid (0.001 mol) was dissolved in phosphorus oxychloride and refluxed for 20 h. The reaction mixture was slowly poured over crushed ice and kept overnight. The solid thus separated out was filtered, washed with water, dried and recrystallized from ethanol.
(7a). IR (KBr) νmax (cm−1): 622 (C-S-C benzothiazole), 1454 (C-N benzothiazole), 1488 (C-O-C oxadiazole), 1518 (C = C aromatic), 3318 (N-H),. 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.24–7.28 (8H, J = 12 Hz, m, Ar-H), 8.10 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 126.1, 127.5, 128.7, 129.2, 129.8, 132.3, 151.3, 164.5, 169.3, 174.5; Mass (m/z): 328 (M+). Analysis for C15H9 ClN4OS (328.78): C; 54.82, H; 2.78, N; 17.08, S; 9.77. Found: C; 54.80, H; 2.76, N; 17.04, S; 9.75.
(7b). IR (KBr) νmax (cm−1): 624 (C-S-C benzothiazole), 821 (C-Cl ), 1456 (C-N benzothiazole), 1491 (C-O-C oxadiazole), 1512 (C = C aromatic), 3317 (N-H).1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.22–7.26 (7H, J = 12 Hz, m, Ar-H), 8.14 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 127.3, 128.9, 129.3, 129.8, 130.1, 132.2,132.3, 136.9, 151.3, 164.5, 169.3, 174.5; Mass m/z: 363 (M+). Analysis for C15H8 Cl2N4OS (363.22): C; 49.64, H; 2.24, N; 15.45, S; 8.85. Found: C; 49.60, H; 2.22, N; 15.42, S;8.83.
(7c). IR (KBr) νmax (cm−1): 628 (C-S-C benzothiazole), 826 (C-Cl), 1461 (C-N benzothiazole), 1497 (C-O-C oxadiazole), 1516 (C = C aromatic), 3321 (N-H). 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.26–7.30 (6H, J = 12 Hz, m, Ar-H), 8.20 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 128.8, 129.8, 130.2, 130.3, 130.7, 132.3, 132.9, 138.3, 151.3, 164.5, 169.3, 174.5; Mass (m/z): 397 (M+).Analysis for C15H7 Cl3N4OS (397.67): C; 45.32, H; 1.79, N; 14.11, S; 8.08. Found: C; 45.30, H; 1.77, N; 14.09, S; 8.06.
(7d). IR (KBr) νmax (cm−1): 632 (C-S-C benzothiazole), 812 (C-Cl), 1467 (C-N benzothiazole), 1496 (C-O-C oxadiazole), 1514 (C = C aromatic), 3331 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 2.21(3H, s, CH3), 7.64–7.68 (7H, J = 12 Hz, m, Ar-H), 8.12 (1H, s, NH); 13C NMR δ (ppm): 18.7, 118.3, 121.2, 125.8, 126.2, 127.4, 128.6, 129.5, 129.8, 132.3, 136.9, 137.2, 151.3, 164.5, 169.3, 174.5; Mass (m/z): 342 (M+).Analysis for C16H11 ClN4OS (342.80): C; 56.06, H; 3.23, N; 16.34, S; 9.35. Found: C; 56.10, H; 3.25, N; 16.36, S; 9.37.
(7e). IR (KBr) νmax (cm−1): 638 (C-S-C benzothiazole), 824 (C-Cl), 1468 (C-N benzothiazole), 1489 (C-O-C oxadiazole), 1517 (C = C aromatic), 3336 (N-H). 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 2.59 (3H, s, OCOCH3), 7.24–7.28 (7H, J = 12 Hz, m, Ar-H),8.16 (1H, s, NH); 13C NMR δ (ppm): 20.3, 117.8, 118.3, 121.2, 123.2, 125.8, 126.0, 129.1, 129.8, 132.3, 137.1, 151.1, 151.3, 164.5, 169.0, 169.3, 174.5; Mass (m/z): 386 (M+). Analysis for C17H11 ClN4O3S (386.81): C; 52.79, H; 2.87, N; 14.48, S; 8.29. Found: C; 52.81, H; 2.88, N; 14.51, S; 8.32.
(7f). IR (KBr) νmax (cm−1): 634 (C-S-C benzothiazole), 824 (C-Cl), 1464 (C-N benzothiazole), 1492 (C-O-C oxadiazole), 1518 (C = C aromatic), 3326 (N-H). 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.25–7.28 (8H, J = 9 Hz, m, Ar-H), 8.14 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 122.7, 124.6, 125.8, 129.7, 129.8, 132.3, 151.3, 155.2, 158.7, 169.3, 174.5; Mass (m/z): 344 (M+).Analysis for C15H9ClN4O2S (344.78): C; 52.25, H; 2.63, N; 16.25, S; 9.30. Found: C; 52.22, H; 2.61, N; 16.23, S; 9.27.
(7g). IR (KBr) νmax (cm−1): 637 (C-S-C benzothiazole), 828 (C-Cl), 1378 (NO2), 1468 (C-N benzothiazole), 1496 (C-O-C oxadiazole), 1521 (C = C aromatic), 3336 (N-H). 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.21–7.24 (7H, J = 9 Hz, m, Ar-H), 8.16 (1H, s, NH); 13C NMR δ (ppm): 118.3, 121.2, 122.8, 123.9, 125.8, 127.0, 129.8, 130.1, 132.3, 133.6, 148.4, 151.3, 164.5, 169.3, 174.5; Mass (m/z): 373 (M+).Analysis for C15H8ClN5O3S (373.77); C; 48.20, H; 2.16, N; 18.74, S; 8.58. Found: C; 48.23, H; 2.18, N; 18.76, S; 8.60.
General procedure for the synthesis of 5-(6-Chlorobenzo[d]thiazol-2-ylamino)-1,3,4-oxadiazole-2-thiol (8)
A mixture of 3 (0.005 mol), KOH (0.005 mol) and carbon disulphide (5 mL) in ethanol (50 mL) was refluxed on a steam bath for 12 h. The solution was then concentrated, cooled and acidified with dilute HCl. The solid mass that separated out was filtered, washed with ethanol, dried and recrystallized from ethanol.
(8). IR (KBr) νmax (cm−1): 614 (C-S-C benzothiazole), 826 (C-Cl), 1421 (C-N), 1505 (C-O-C oxadiazole), 1514 (C = C), 1614 (C = O), 2516 (SH), 3316 (N-H); 1H NMR (DMSO-d6) (300 MHz) δ (ppm) 7.17–7.19 (3H, J = 6 Hz, m, Ar-H), 8.05 (1H, s, NH), 10.41(1H, s, SH); 13C NMR δ (ppm): 118.3, 121.2, 125.8, 129.8, 132.3, 151.3, 169.3, 174.5; Mass (m/z): 284 (M+). Analysis for C9H5ClN4OS2 (284.75): C; 37.98, H; 1.80, N; 19.71, S; 22.56. Found: C; 37.96, H; 1.77, N; 19.68, S; 22.52.
Antimicrobial activity
Antibacterial activity of the synthesized compounds were determined in vitro by using serial plate dilution methodCitation25,Citation26 against E. coli (ATCC-25922), S. aureus (ATCC-25923), P. aeruginosa (ATCC-27853) and K. pneumoniae (ATCC-700603) at 100 µg/mL, 50 µg/mL, 25 µg/mL and 12.5 µg/mL concentrations, respectively, in the nutrient agar media. Standard antibiotic ofloxacin was used as reference drug at 25 µg/mL, 12.5 µg/mL and 6.25 µg/mL concentrations.
Newly synthesized compounds were screened in vitro against pathogenic fungal strains C. albicans (ATCC 2091), A. niger (MTCC 281), Aspergillus flavus (MTCC 277), M. purpureus (MTCC 369) and P. citrinum (NCIM 768) by serial plate dilution methodCitation27,Citation28 at 100 µg/mL, 50 µg/mL, 25 µg/mL and 12.5 µg/mL concentrations in sabouraud dextrose medium. Ketoconazole was used as standard drug at 25 µg/mL, 12.5 µg/mL and 6.25 µg/mL concentrations. Solutions of required concentrations of test compounds were prepared by dissolving the compounds in DMSO. The minimum inhibitory concentration (MIC) obtained for the test compounds and reference drugs are reported in and . The minimum inhibitory concentration (MIC) was defined as the lowest concentration of the compounds that inhibited visible growth of microorganisms on the plate.
Results and discussion
Chemistry
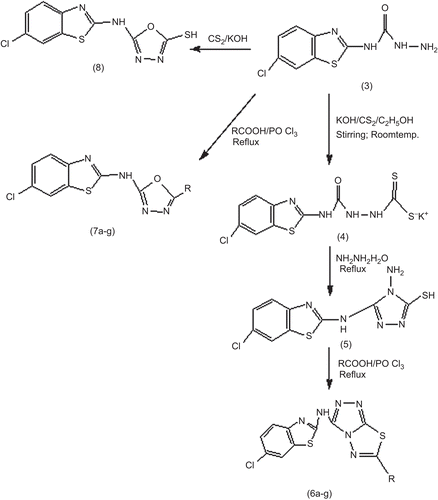
6-substituted-1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole (6a–g) and 1,3,4-oxadiazole (7a–g, 8) were prepared according to the procedure outlined in and . The required dithiocarbazinate (4) was synthesized by reacting N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide with carbon disulfide and potassium hydroxide in ethanol. This salt underwent ring closure with an excess of 99% hydrazine hydrate to give the 4-amino-5-(6-chlorobenzo[d]thiazol-2-ylamino)-4H-1,2,4-triazole-3-thiol (5). Hence resulted triazole (5) was then further converted to the 6-chloro-N-(6-substituted-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-3-yl)benzo[d] thiazol-2-amine (6a–g) in a one-pot reaction, by condensation with aromatic acids in the presence of POCl3. Phosphorus oxychloride was necessary for this condensation, which activate the carbonyl group of aromatic acids and increases its electrophilicity to enhance the addition of triazole to it. The synthesis of compounds (7a–g) was accomplished in a single step by reacting the benzothiazole carboxamide with aromatic acids in the presence of POCl3 and compound (8) by reacting with carbon disulfide and potassium hydroxide in ethanol, respectively. The structure of synthesized compounds was confirmed by elemental analysis and spectral data (IR, 1H NMR, Mass).
Physicochemical data for the N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide derivatives (6a–g, 7a–g, 8) is given in . The synthetic route for the title compounds is shown in and .
Table 1. Physicochemical data of N-(6-chlorobenzo[d]thiazol-2-yl)hydrazine carboxamide derivatives (6a–g, 7a–g, 8).
Antimicrobial activity
The newly synthesized compounds were screened for their antimicrobial activity. The results of antimicrobial effect of all the tested compounds were reported as minimal inhibitory concentrations (MICs, µg/mL).The investigation of antibacterial and antifungal screening data revealed that all the tested compounds 6a–g, 7a–g and 8 showed moderate to good inhibition at 12.5–100 µg/mL in DMSO.
The compounds 6c, 6d, 6g, 7a, 7c, 7f and 8 showed comparatively good activity against all the bacterial strains (). The good activity is attributed to the presence of pharmacologically active 2,4-dichloro (6c), methyl (6d), 4-nitro (6g) substituent attached to phenyl group at position 6 of the triazolo-thiadiazole ring (MIC 12.5 µg/mL & 25 µg/mL), whereas phenyl (7a), 2,4-dichlorophenyl (7c), phenoxy groups (7f) and mercapto (8), attached at second position of 1,3,4-oxadiazole moiety (MIC 12.5 µg/mL & 25 µg/mL). When this group was replaced by 2-chlorophenyl (6b and 7b) and acetyl groups (6e and 7e) it caused sharp decrease in activity against most of the strains (MIC 100 µg/mL). The compounds 6a, 6f, 7d and 7g, exhibited moderate activity compared to that of standard against all the bacterial strains (MIC 25 µg/mL & 50 µg/mL). Further, the result also shows that gram-negative (E. coli, P. aeruginosa, K. pneumoniae) exhibited better activity than gram positive (S. aureus) organisms.
Table 2. Antibacterial activity of the title compounds (6a–g, 7a–g and 8).
The compounds 6c, 6d, 6f, 7e, 7g and 8 showed comparatively good activity against all the fungal strains (). The structure of these compounds contains biologically active 2,4-dichloro phenyl, methyl phenyl and phenoxy group attached at position 6 of the triazolo-thiadiazole ring (MIC 12.5 µg/mL & 25 µg/mL) and mercapto, acetyl and 4-nitro phenyl group attached at second position of oxadiazole ring (MIC 12.5 µg/mL & 25 µg/mL), respectively. The compounds 6a, 6g, 7a, 7c and 7d showed moderate activity compared to that of standard (6.25 µg/mL) against all the fungal strains (MIC 25 µg/mL & 50 µg/mL). It has been observed that triazolo-thiadiazole derivatives are found to be more active than 1,3,4-oxadiazole derivatives against all pathogenic bacterial and fungal strains.
Table 3. Antifungal activity of the title compounds (6a–g, 7a–g and 8).
The antimicrobial activity study revealed that all the compounds tested showed good to moderate antibacterial and antifungal activities against all pathogenic strains (MIC 12.5 µg/mL, 25 µg/mL and 50 µg/mL). Structure and biological activity relationship of title compounds showed that the presence of 2,4-dichloro phenyl, methyl phenyl,4-nitro phenyl group at sixth position of triazolo-thiadiazole (MIC 12.5 µg/mL and 25 µg/mL) and 2-mercapto, phenyl, 2,4-dichlorophenyl and phenoxy group at second position of oxadiazole nucleus (MIC 12.5 µg/ mL and 25 µg/mL) are responsible for good antibacterial activity.
Similarly, presence of 2,4,-dichloro phenyl, methyl phenyl and phenoxy group attached at sixth position of triazolo-thiadiazole (MIC 12.5 µg/mL and 25 µg/mL), whereas mercapto (–SH), acetyl and 4-nitro phenyl attached at second position of oxadiazole nucleus (MIC 12.5 µg/mL and 25 µg/mL) are responsible for good antifungal activity.
Thus, various triazolo-thiadiazole (6a–g) and oxadiazole (7a–g, 8) derivatives of benzothiazole hydrazine carboxamide were prepared with the objective of developing better antimicrobial agents. The triazolo-thiadiazole and oxadiazole derivatives were found to have a promising class of compounds with an interesting pharmacological profile. Further, it is clear from structure activity relationship (SAR), that the triazolo-thiadiazole derivatives were found to be more active than oxadiazole derivatives.
Acknowledgements
The authors are thankful to the Head of the Department, Pharmaceutical Chemistry for providing laboratory facilities, Central Drug Research Institute (CDRI) for spectral analysis of the compounds. Authors are also thankful to Dr. Qadir, Head, IGIB, New Delhi for providing bacterial and fungal strains.
Declaration of interest
The authors report no conflict of interest.
References
- Swamy NS, Basappa Priya, SB, Prabhuswamy B, Doreswamy BH, Prasad S.J., Rangappa S. Synthesis of pharmaceutically important condensed heterocyclic 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivatives as antimicrobials. Eur J Med Chem 2006; 41: 531–538.
- Nogrady T., Weaver FD. Medicinal Chemistry: A Molecular & Biochemical Approach. Oxford University Press, 2005, pp. 559–582.
- Thomasco LS, Gadwood CR, Weaver EA, Ochoada JM, Ford CW, Zurenko GE, Hamel JC, Stapert D, Moerman JK, Schaadt RD, Yagi BH. The synthesis and antibacterial activity of 1,3,4-Thiadiazole phenyl oxazolidinone analogues. Bioorg Med Chem Lett 2003;13: 4193–4196.
- Onkol T, Dogruer DS, Uzun L, Adak S, Ozkan S, Sahin MF. Synthesis and antimicrobial activity of new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. J Enzyme Inhib Med Chem 2008; 23:277–284.
- Rana A, Siddiqui N, Khan SA, Haque SE, Bhat MA. N-{[(6-Substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: Synthesis and pharmacological evaluation. Eur J Med Chem 2007; 1–9.
- Shi DF, Bradshaw TD, Chua MS, Westwell AD, Stevens MF. Antitumour benzothiazoles. Part 15: The synthesis and physico-chemical properties of 2-(4-aminophenyl)benzothiazole sulfamate salt derivatives. Bioorg Med Chem Lett 2001; 11:1093–1095.
- Rana A, Siddiqui N, Khan SA. Benzothiazoles: A new profile of biological activities. Indian J Pharmaceut Sciences 2007; 69:10–17.
- Khan MSY, Chawla G, Mueed MA. Synthesis and biological activity of some isoniazid based 1,3,4-oxadiazole derivatives. Indian J Chem 2004; 43B:1302–1305.
- Jimonet P, Audiau F, Barreau M, Blanchard JC, Boireau A, Bour Y, Coléno MA, Doble A, Doerflinger G, Huu CD, Donat MH, Duchesne JM, Ganil P, Guérémy C, Honor E, Just B, Kerphirique R, Gontier S, Hubert P, Laduron PM, Le Blevec J, Meunier M, Miquet JM, Nemecek C, Mignani S. Riluzole series. Synthesis and in vivo “antiglutamate” activity of 6-substituted-2-benzothiazolamines and 3-substituted-2-imino-benzothiazolines. J Med Chem 1999; 42:2828–2843.
- Paramashivappa R, Phani Kumar P, Subba Rao PV, Srinivasa Rao A. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett 2003; 13:657–660.
- Mathew V, Keshavayya J, Vaidya VP, Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur J Med Chem 2007; 42:823–840.
- Karegoudar P, Prasad DJ, Ashok M, Mahalinga M, Poojary B, Holla BS. Synthesis, antimicrobial and anti-inflammatory activities of some 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines bearing trichlorophenyl moiety. Eur J Med Chem 2008; 43:808–815.
- Ibrahim DA. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur J Med Chem 2009; 44:2776–2781.
- Mathew V, Keshavayya J, Vaidya VP. Heterocyclic system containing bridgehead nitrogen atom: synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur J Med Chem 2006; 41:1048–1058.
- Amir M, Kumar H, Javed SA. Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives of naproxen. Bioorg Med Chem Lett 2007; 17:4504–4508.
- Padmavathi V, Nagendra Mohan AV, Thriveni P, Shazia A. Synthesis and bioassay of a new class of heterocycles pyrrolyl oxadiazoles/thiadiazoles/triazoles. Eur J Med Chem 2009; 44:2313–2321.
- Amir M, Shikha K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur J Med Chem 2004; 39:535–545.
- Kumar H, Javed SA, Khan SA. Amir M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: synthesis and preliminary evaluation of biological properties. Eur J Med Chem 2008; 43:2688–2698.
- Joshi CK, Giri SJ. Synthesis of some new fluoro ketones and their thiosemicarbazones. Indian Chem Soc 1963; 40: 42.
- Keshavan B, Seetharamappa J. Synthesis and structural investigation of biologically active complexes of N-alkylphenothiazines with dioxouranium (VI). Inorganica Chimica Acta 1987; 138:135–138.
- Mathew V, Keshavayya J, Vaidya PV. Synthesis, characterization and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo [3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. J Chem 2007; 4:320–342.
- Nussbaumer P, Leitner I, Stütz A. Synthesis and structure-activity relationships of the novel homopropargylamine antimycotics. J Med Chem 1994; 37:610–615.
- Nussbaumer P, Leitner I, Mraz K, Stütz A. Synthesis and structure-activity relationships of side-chain-substituted analogs of the allylamine antimycotic terbinafine lacking the central amino function. J Med Chem 1995; 38:1831–1836.
- Chongxi YU. Transdermal Delivery Systems of Beta-Lactam Antibiotics. International Application No.: PCT.IB2006/054724 [Online] WO/2008/072032. Available at: http://www.wipo.int/pctdb/en/wo.jsp. Accessed on 27 July 2010.
- Barry AL. Procedures and theoretical considerations for testing antimicrobial agents in agar media. In: Lorian V (ed.), Antibiotics in Laboratory Medicine, 3rd edn. Baltimore: Williams & Wilkins Co., 1991, pp. 1–16.
- James D, Lowry M, Jaua MJ, Selepak ST. Appl Microbiol 1970; 20: 46.
- Arthington-Skaggs BA, Motley M, Warnock DW, Morrison CJ. Comparative evaluation of PASCO and national committee for clinical laboratory standards M27-A broth microdilution methods for antifungal drug susceptibility testing of yeasts. J Clin Microbiol 2000; 38:2254–2260.
- Verma SR, Khan ZK, Singh AP. Antifungal Agents: Past, Present and Future Prospects. National Academy of Chemistry and Biology: India, 1998, p. 55.

![Scheme 1. Synthetic pathways to N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide.](/cms/asset/fd23c3f4-53ec-4a6f-870a-962b64e69834/ienz_a_508441_f0001_b.gif)