Abstract
Bioactivity-guided investigation for the rhizomes of Anemarrhena asphodeloides using the Fms-like tyrosine kinase 3 (FLT3) inhibition assay led to the identification of an active xanthone, mangiferin. Mangiferin was found to inhibit activity of the FLT3 wild type and a mutated form of FLT3 with IC50 values of 0.7 and 1.2 μM, respectively. Furthermore, this compound was assessed with a small panel of select kinases anaplastic lymphoma kinase (ALK), insulin receptor, and epidermal growth factor receptor) and was also found to be active in ALK assay.
| Abbreviations | ||
| NMR | = | Nuclear magnetic resonance |
| HPLC | = | High performance liquid chromatography |
| HR-ESI-MS | = | High resolution electrospray ionization mass spectrometry |
| HSCCC | = | High-speed counter-current chromatography |
| FLT3 | = | Fms-like tyrosine kinase 3 |
| AML | = | Acute myeloid leukaemia |
| ALK | = | Anaplastic lymphoma kinase |
| EGFR | = | Epidermal growth factor receptor |
Introduction
The Fms-like tyrosine kinase 3 (FLT3), a receptor tyrosine kinase, is involved in the proliferation, differentiation, and apoptosis of haematopoietic cells. FLT3 is expressed in acute myeloid leukaemia (AML) in the majority of patients, and activating mutations of FLT3, the FLT3 internal tandem duplication mutation and point mutations (tyrosine kinase domain) which are located in the juxtamembrane domain and the intracellular tyrosine kinase domain, respectively, are frequently found in many AML casesCitation1. Therefore, inhibition of FLT3 kinase activity has been proposed as a novel therapy for AML patients. Several small-molecule FLT3 inhibitors, such as lestaurtinib (CEP-701), midostaurin (PKC-412), tandutinib (MNL-518), sorafenib, KW-2449, and AC-220 are currently under clinical trialsCitation2.
As a part of discovering small-molecule inhibitors of plant origin, the rhizomes of Anemarrhena asphodeloides Bunge (Anthericaceae) were selected for further investigation due to their inhibitory effect against FLT3 activity in the initial screening. The rhizomes of A. asphodeloides have been used as an anodyne, an antidiabetic, an antiphlogistic, an antipyretic, a diuretic, and a sedative in mainland China, Japan, and KoreaCitation3. Previous investigations on this species have led to the identification of norlignans, phenolics, steroidal saponins, and xanthenes, exhibiting various biological activities including antiadipogenic, antidepressant, antidiabetic, antifungal, antioxidant, and antiproliferative effectsCitation4. The present study describes the isolation of mangiferin from A. asphodeloides rhizomes and the evaluation of its inhibitory activity against the FLT3 wild type and an FLT3 mutant in an in vitro assay as well as in a small panel of select kinases.
Material and methods
General experimental procedures
UV spectra were obtained with a Shimadzu UV-1601 spectrophotometer. Nuclear magnetic resonance (NMR) spectroscopic data were recorded at room temperature on a Varian Unity 400 FT-NMR spectrometer with tetramethylsilane as the internal standard. Electrospray ionization (ESI) mass spectrometric analysis was performed with a Waters Q-Tof Premier mass spectrometer. A Perfectsil ODS column [150 × 4.6 mm internal diameter (i.d.), 3 μm] was used for high performance liquid chromatography (HPLC) separation on a Finnigan ChromQuest model instrument (Thermo Electron). Column chromatography was performed using RP-18 resin (Cosmosil 75C18-PREP, Kyoto, Japan). Thin-layer chromatography was carried out on pre-coated Silica gel 60 F254 (0.25 mm, Merck, Darmstadt, Germany) and RP-18 plates (0.25 mm, Merck, Darmstadt, Germany). Preparative high-speed counter-current chromatography (HSCCC) was carried out with a model TBE-1000A (Shanghai Tauto Biotech Co. Ltd, Shanghai, China) with three serially connected multilayer coil separation columns (i.d. of the tubing = 1.6 mm; total volume = 1000 mL) and an 80 mL sample loop. This system was equipped with TBP5002 LPLC pumps (Shanghai Tauto Biotech Co. Ltd, Shanghai, China), a Thermo Finnigan SSI 500 UV Detector (Thermo Electron Co., San Jose, CA), and Autochro-WIN software (version 2.0, Youngin-Tech, Korea). The upper (stationary) and lower phase (mobile) were simultaneously pumped into the multilayer-coiled column in a 3:1 volumetric ratio. When the column was completely filled with the two phases, the apparatus was rotated at 500 rpm; simultaneously, the mobile phase was pumped through the column at a flow-rate of 5.0 mL/min. The temperature was set at 25°C. The sample solution (15 mL) containing the crude BuOH extract was loaded onto the HSCCC and the effluent monitored at 254 nm.
Plant material
A dried powder of the rhizomes of A. asphodeloides (Anthericaceae), purchased in a Korean oriental market, was used for the present study and identified by Dr. Joongku Lee, International Biological Material Research Center, Korea Research Institute of Bioscience & Biotechnology. A representative sample (KRIBBCYW-0002) has been deposited as a powder in the Korean Research Institute of Bioscience & Technology, ChungBuk, Korea.
Extraction and isolation
The powder of the rhizomes of A. asphodeloides (600 g) was extracted by maceration with MeOH three times (1000 mL each) at room temperature, for up to 1 day each repetition, with the extractives pooled and then evaporated in vacuo. The dried MeOH extract (80 g) was suspended with H2O (500 mL) and partitioned sequentially with hexanes, CHCl3 (3 × 500 mL), EtOAc (3 × 500 mL) and n-BuOH (3 × 500 mL). Of these, the BuOH-soluble partition (23 g) exhibited inhibitory activity against FLT3 (62% inhibition at 40 μg/mL), using the protocol indicated below. For preliminary screening of the active partition, a portion of the BuOH-soluble fraction (250 mg) was subjected to HSCCC, eluting the lower phase of the two-phase solvent system composed of ethyl acetate/isopropanol/water (3:2:5, v/v), and pooled into six sub-fractions (ABF1-ABF6). Of these sub-fractions, ABF4 (tR 212–260 min, 18 mg) was found to be active (95% at 40 μg /mL) in FLT3 kinase inhibition assay. This sub-fraction was further purified by HPLC separation using a Perfectsil ODS column (25% MeOH, 1 mL/min) and yielded mangiferin (tR 11.5 min, 14 mg).
Mangiferin
Yellow powder. HR-ESI-MS m/z 423.0938 [M + H]+ (calcd for C19H19O11, 423.0927). The spectroscopic data (1H and 13C NMR) of this compound were consistent with published valuesCitation5.
In vitro kinase assay
Inhibition of kinase activity against a variety of recombinant kinases [FLT3, FLT3 D835Y, anaplastic lymphoma kinase (ALK), insulin receptor, and epidermal growth factor receptor (EGFR)] was measured using homogeneous time-resolved fluorescence (HTRF) assaysCitation6. In brief, the assays are based on the phosphorylation of peptide substrates in the presence of adenosine triphosphate (ATP). Resulting phosphorylated substrates are detected by a time resolved-fluorescence resonance energy transfer (TR-FRET) signal. Recombinant proteins containing a kinase domain were purchased from Millipore (Billerica, MA). Optimal enzyme, ATP, and substrate concentrations were established for each enzyme using an HTRF KinEASE kit (Cisbio, France) according to the manufacturer’s instructions. Assays consist of enzymes mixed with serially diluted compounds and peptide substrates in a kinase reaction buffer (250 mM HEPES (pH 7.0), 0.5 mM orthovanadate, 0.05% bovine serum albumin, 0.1% NaN3). Following the addition of reagents for detection, the TR-FRET signal was measured using an EnVision multi-label reader (Perkin Elmer, Waltham, MA). IC50 was calculated by a non-linear regression using Prism version 5.01 (GraphPad, La Jolla, CA). Each partition and chromatographic fraction was tested against FLT3 and the isolated compound was tested in FLT3, FLT3 D835Y, ALK, insulin receptor and EGFR.
Results and discussion
The preliminary screening results of the rhizomes of A. asphodeloides demonstrated that a BuOH-soluble partition exhibited the inhibitory activity (62% inhibition at 40 μg/mL) against FLT3 in an in vitro kinase assay. Fractionation of the BuOH-soluble extract using HSCCC and evaluation of the sub-fractions thereof afforded an active sub-fraction (IC50 4.2 μg/mL) containing mangiferin as a major constituent. The structure of mangiferin, xanthone C-glycoside (1,3,6,7-tetrahydroxyxanthone-2-C-glucopyranoside), was confirmed by comparison of the measured 1H NMR, 13C NMR, and high resolution electrospray ionization mass spectrometry with published values (). To assess direct inhibition of kinase activity by mangiferin, purified recombinant FLT3 protein was pre-treated with the aforementioned compound and subjected to kinase reaction by adding substrates and ATP. As shown in , mangiferin inhibited FLT3 activity with an IC50 value of 0.7 μM, as compared with the positive control, lestaurtinib (IC50 1.2 nM), used in this assay system. Moreover, mangiferin exhibited equivalent inhibitory activity (IC50 1.2 μM) against FLT3 D835Y, an activating mutation of FLT3 found in AMLCitation7. In addition, in order to give more information on FLT3-inhibitory activity of xanthones, five prenylated xanthones [α-mangostin (1), γ-mangostin (2), gartanin (3), 1-isomangostanin (4), and garcinone E (5)] previously isolated from Garcinia mangostana () were tested in the same assay system and it was found that none of them inhibited FLT3 activity (IC50> 10 μM). Even though γ-mangostin (2) and garcinone E (5) have the same 1,3,6,7-tetrahydroxyxanthone-type skeleton as mangiferin, these compounds were found to be inactive in this assay system, which may be due to the prenyl substituents in these compounds. Furthermore, mangiferin was tested against ALK, insulin receptor, and EGFR, for kinase selectivity and found to inhibit ALK activity with an IC50 value of 0.8 μM, whereas it was found inactive against insulin receptor (> 10 μM), and EGFR (> 10 μM; ). As described elsewhereCitation6,Citation8,Citation9, small-molecule FLT3 inhibitors in clinical development, midostaurin, lestaurtinib, tandutinib and linifanib, are known to target multi-tyrosine kinase. This xanthone used in the current study was also found to display a non-selective inhibitory effect against FLT3 and ALK. Given that FLT3 is highly activated in AML and acute lymphocytic leukaemia, and given that small-molecule FLT3 kinase inhibitors are under clinical trials, inhibition against FLT3 and mutant FLT3 activity is recognized as an important process for cancer therapy. Thus, FLT3-inhibitory activity of mangiferin in the present study, which has not been reported in this xanthone-type structure, was deemed to demonstrate this structure to be worthy of exploiting as a potential FLT3 kinase inhibitor. To date, mangiferin isolated from several plant sources has been known to possess biological activities relevant to cancer intervention, such as apoptosisCitation10, modulation of nuclear factor-κB signal transductionCitation11 and cancer chemopreventionCitation12. There has been no previous report of xanthone with inhibitory activity against FLT3 tyrosine kinase. Current finding in the present study seems to disclose a new mode of action of this compound germane to cancer intervention. In this regard, this xanthone may merit further work including chemical modifications and mechanistic study.
Figure 2. Effects of mangiferin on the kinase activity of FLT3 and FLT 3 D835Y. Positive control, lestaurtinib, was used.
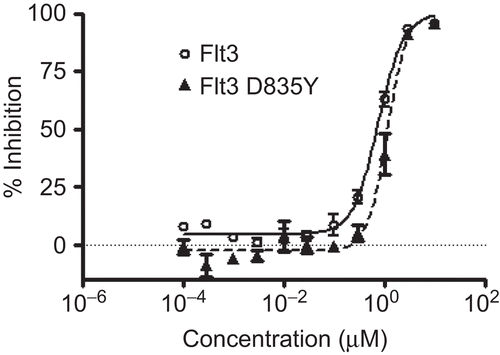
Figure 3. Structures of compounds 1–5 from Garcinia mangostana fruits α-mangostin (1), γ-mangostin (2), gartanin (3), 1-isomangostin (4) and garcinone E (5).
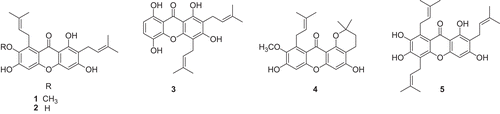
Table 1. In vitro activity of mangiferin against select kinases (μM).
Declaration of interest
This work was supported by a grant from the KRIBB Research Initiative program (Y.-W. Chin) and a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100096, S-Y Han).
References
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003;3:650–665.
- Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leuk Lymphoma 2008;49:852–863.
- Bae G, Yu JR, Lee J, Chang J, Seo EK. Identification of nyasol and structurally related compounds as the active principles from Anemarrhena asphodeloides against respiratory syncytial virus (RSV). Chem Biodivers 2007;4:2231–2235.
- Youn UJ, Lee YS, Jeong H, Lee J, Nam JW, Lee YJ, Hwang ES, Lee JH, Lee D, Kang SS, Seo EK. Identification of antiadipogenic constituents of the rhizomes of Anemarrhena asphodeloides. J Nat Prod 2009;72:1895–1898.
- Kim CY, Ahn M-J, Kim J. Preparative isolation of mangiferin from Anemarrhena asphodeloides rhizomes by centrifugal partition chromatography. J Liq Chromatogr Relat Technol 2006;29:869–875.
- Choi SJ, Moon MJ, Lee SD, Choi SU, Han SY, Kim YC. Indirubin derivatives as potent FLT3 inhibitors with anti-proliferative activity of acute myeloid leukemic cells. Bioorg Med Chem Lett 2010;20:2033–2037.
- Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol 2001;113:983–988.
- Pandey A, Volkots DL, Seroogy JM, Rose JW, Yu JC, Lambing JL, Hutchaleelaha A, Hollenbach SJ, Abe K, Giese NA, Scarborough RM. Identification of orally active, potent, and selective 4-piperazinylquinazolines as antagonists of the platelet-derived growth factor receptor tyrosine kinase family. J Med Chem 2002;45:3772–3793.
- Dai Y, Hartandi K, Ji Z, Ahmed AA, Albert DH, Bauch JL, Bouska JJ, Bousquet PF, Cunha GA, Glaser KB, Harris CM, Hickman D, Guo J, Li J, Marcotte PA, Marsh KC, Moskey MD, Martin RL, Olson AM, Osterling DJ, Pease LJ, Soni NB, Stewart KD, Stoll VS, Tapang P, Reuter DR, Davidsen SK, Michaelides MR. Discovery of N-(4-(3-amino-1H-indazol-4-yl)phenyl)-N’-(2-fluoro-5-methylphenyl)urea (ABT-869), a 3-aminoindazole-based orally active multitargeted receptor tyrosine kinase inhibitor. J Med Chem 2007;50:1584–1597.
- Sarkar A, Sreenivasan Y, Ramesh GT, Manna SK. beta-d-Glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor kappaB but potentiates apoptosis. J Biol Chem 2004;279:33768–33781.
- Leiro J, Arranz JA, Yanez M, Ubeira FM, Sanmartin ML, Orallo F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by modulated by mangiferin. International Immunopharmcol 2004;4:763–778.
- Rajendran P, Ekambaram G, Magesh V, Sakthisekaran D. Chemopreventive efficacy of mangiferin against benzo(α)pyrene induced lung carcinogenesis in experimental animals. Environ Toxicol Pharmacol 2008;26:278–282.