Abstract
Transition metal complexes containing bidentate N, S donor ligands i.e., carvone thiosemicarbazone [(RS)-5-isopropenyl-2-methylcyclohex-2-en-1-one thiosemicarbazone (IPMCHTSC)] and carvone N1-phenylthiosemicarbazone [(RS)-5-isopropenyl-2-methylcyclohex-2-en-1-one phenylthiosemicarbazone (IPMCHPhTSC)] have been synthesized. All the metal complexes (1–8) have been characterized by elemental analysis, molar conductance measurement and various spectral studies [infrared (IR), electronic, fast-atom bombardment (FAB) mass and NMR (for ligands)] and thermogravimetric analysis. FAB mass spectroscopic studies of (1), (3), (4), (5), (6) (7), and (8) suggest their monomeric nature. Metal complexes are [M(LH)Cl2] and [M(LH)2Cl2] type, where M = Fe(III), Co(II), and Cu(II) and LH = IPMCHTSC and IPMCHPhTSC. The proposed geometries of the complexes were octahedral for 1:2 complexes, square planar for 1:1 complexes and distorted octahedral for Cu(II) complexes (1:2). The free radical scavenging activity of ligands (IPMCHTSC and IPMCHPhTSC) and their metal complexes have been determined at the concentration range of 10–400 μg/mL by means of their interaction with the stable free radical 2,2′-diphenyl-1-picrylhydrazyl and 5–200 μg/mL by 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulphonic acid. All the compounds have shown encouraging antioxidant activities.
Introduction
During the recent years transition metal complexes with N, S donor ligands have attracted considerable interest because of their encouraging antibacterial and antifungalCitation1–4 activities than those of the parent ligands. Thiosemicarbazones are well established as an important class of sulfur donor ligands particularly for transition metal ions.Citation5–7 The activity of thiosemicarbazones and its substituted derivatives is usually increased by complexation therefore to understand the properties of both ligands and metal can lead to the synthesis of highly active compounds.Citation8–15 Previously, we have reported structural and spectral studies of transition metal complexes with semicarbazone and thiosemicarbazones of some terpenoids.Citation16–18 Our ongoing research work on transition metal complexes with thiosemicarbazones involving such systems led to describe the synthesis, characterization and antioxidant activity of some transition metal complexes with new thiosemicarbazones derived from carvone [(RS)-5-isopropenyl-2-methyl cyclohex-2-en-1-one]. Carvone is a monocyclic monoterpenone and important constituent of several essential oils, e.g. oil of caraway seeds (carum carvi).Citation19
Antioxidants are the compounds that terminate the attack of reactive species like free radicals and prevent it from ageing and different disease associate with oxidative damage inside the body system.Citation20 Antioxidant activity of a synthetic compound can be measured using the scavenging potential of that compound for the trapping of free radicals. These free radicals can oxidize biomolecules viz. nucleic acids, proteins, lipids, DNA, tissue damage, and can initiate degenerative diseases, oxidative damage plays a significantly pathological role in human diseases such cancer, emphysema, cirrhosis, and arthritis etc.Citation21,Citation22 Almost all organisms are protected upto some extent by free radical (peroxide, hydroperoxide, or lipid peroxyl) damage by enzymes such as superoxide dismutase and catalase or compounds such as ascorbic acid (AA), tocopherols, phenolic acids, polyphenols, flavonoids, and glutathione.Citation23 Some compounds like rutin, quercetin having nitrogen, and glutathione having sulfur are also most effective antioxidants.Citation24–27
However, antioxidant supplements or dietary antioxidants may be sources of protection that the body needs to protect against the damaging effects of free radicals.Citation28 Presently, synthetic antioxidants are widely used because they are effective and cheaper than natural antioxidants.
Material and methods
All the chemicals and reagents used were of AR grade. Solvents were dried by conventional methods and distilled prior to use. Metal contents were measured by complexometric titrations. Sulfur was estimated gravimetrically as BaSO4 and chloride content was determined by Volhard’s method.Citation29 Elemental analyses were carried out on thermoquest analyzer. The infrared (IR) spectra were recorded with KBr pellets in the 4000–225 cm−1 range on Nicolet Megna 550 FT-IR spectrometer. The 1H and 13C{1H} NMR spectra of ligands were collected in CDCl3 solvent using Tetramethylsilane (TMS) as internal standard on JEOL FX 300 FT NMR spectrometer at 300.4 and 75.45 MHz frequencies for 1H and 13C{1H} NMR, respectively, electronic spectra were recorded on Agilent UV/visible spectrometer. Molar conductivity of 10−3 dimethylsulphoxide (DMSO) solutions were measured on a microprocessor based conductivity meter model 1601/E. Thermogravimetric analysis (TGA) was performed by PerkinElmer Thermal analyzer with the heating rate 35-900/20°C under nitrogen atmosphere. Mass spectra were recorded on Schimadzu mass spectrophotometer. Antioxidant activity was measured in spectro UV-Vis double beam PC scanning spectrophotometer (Labomed Inc., Culver City, CA) vortex (Spinix).
Synthesis of ligands
Carvone thiosemicarbazone (IPMCHTSC) and carvone N1-phenylthiosemicarbazone (IPMCHPhTSC) were prepared according to known method.Citation15
IPMCHTSC
Yield: 91% (2.01 gm); m.pt. 109°C; IR (cm−1) 3441 s, 3240 s, br v(NH2); 3155 s, v(NH); 1592, v(C=N); 840 m, v(C=S); 1H NMR (CDCl3, δ ppm): 9.05 (s, 1H, NH); 7.32, 7.05 (2 s, 2H, NH2); 6.20–6.77 (m, 1H, =CH–CH2); 4.83, 4.79 (2 s, 2H, =CH2); 2.79 (dd, 1H, J = 3.0Hz, –CH2–CH–CH2–); 2.22–2.69 (m, 2H, CH2); 2.09–2.18 (m, 2H, CH2); 1.86 (s, 3H, CH3); 1.75 (s, 3H, CH3); 13C NMR (CDCl3, δppm): 178.7 (C=S); 150.2 (C=N); 146.8 (C-7); 135.3 (C-2); 132.0 (C-3); 110.7 (C-8); 40.6 (C-5); 30.1 (C-4); 29.2 (C-6); 20.6 (C-9); 17.7 (C-10). Anal. Found for C11H17N3S (223.34): C, 59.40; H, 7.91; N, 18.01; S, 14.40%. Calcd. C, 59.15; H, 7.67; N, 18.81; S, 14.36%.
IPMCHPhTSC
Yield: 71% (2.19 gm); m.pt. 99°C; IR (cm−1) 3280 s, 3150 s, br v(NH); 3030w, v(Ar-H); 1585 s, v(C=N); 832 m, v(C=S); 1H NMR (CDCl3, δ ppm): 9.36 (br, s, 1H, NHCS); 8.77 (br, s, 1H, NHPh); 7.20–7.69 (m, 5H, NHPh); 1.78, 1.92 (2 s, 6H, CH3); 6.26 (t, 1H, =CH–CH2); 4.85, 4.79 (2 s, 2H, =CH2); 2.72–2.79 (dd, 1H, J=3.9Hz, –CH2–CH–CH2); 2.32-2.51 (m, 2H, CH2); 2.10–2.20 (m, 2H, CH2); 13C NMR (CDCl3, δppm): 175.8 (C=S); 149.0 (C=N); 146.7 (C-7); 137.9 (C-2); 135.3 (C-3); 131.8–123.8 (–C6H5); 110.8 (C-8); 40.5 (C-5); 30.4 (C-4); 29.1 (C-6); 20.5 (C-9); 17.6 (C-10). Anal. Found for C17H21N3S (299.44): C, 68.13; H, 7.10; N, 14.10; S, 10.78%. Calcd. C, 68.19; H, 7.06; N, 14.03; S, 10.70%.
Synthesis of complexes
Synthesis of [MCl2(IPMCHTSC)n]
To an ethanolic solution (∼20 mL) of CuCl2.2H2O (0.4310 g, 2.5 mmol), a hot ethanolic solution (∼25 mL) of ligand (IPMCHTSC) (1.1167 g, 5 mmol) was added dropwise with constant stirring. After complete addition the reaction mixture was refluxed for 6 h and cooled to room temperature. The resulting greenish-yellow precipitate was filtered and washed with ethanol and diethyl ether and dried in vaccum to give greenish-yellow colored solid.
Similar route have been employed for the preparation of other IPMCHTSC complexes.
Synthesis of [MCl2(IPMCHPhTSC)2]
To an ethanolic solution (∼20 mL) of CuCl2.2H2O (0.5966 g, 2.5 mmol), a hot ethanolic solution (∼25 mL) of ligand (IPMCHPhTSC) (1.3514 g, 5 mmol) was added dropwise with constant stirring for 6 h. After complete addition the reaction mixture was filtered and washed with ethanol and diethyl ether and dried in vaccum to give grayish-black colored solid.
Similar route have been employed for the preparation of other IPMCHPhTSC complexes.
Antioxidant activity
Antioxidant activity of the compounds was estimated by 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical scavenging effect. The method for estimating free radical scavenging activity of the methanolic solutions of bioactive compounds were undertaken as suggested by Hatano et al. 1989.Citation30 The DPPH reagent evidently offers a convenient and accurate method for titrating the oxidizable groups of natural or synthetic antioxidant.Citation31 To different concentration (10–400 μg/mL) of methanolic solution of test compound, 5 mL methanolic solution of DPPH (0.01 mm) was added and mixed thoroughly. The absorbance of the mixture was measured after 40 min at 517 nm against methanol as blank. AA and catachin (CAT) were used as reference standard. The radical scavenging activities (%) of tested samples were evaluated by comparing with a control (5.0 mL DPPH and 0.5 mL of methanol). Each sample was then measured in duplicate and averaged.
The method of Re et al.Citation32,Citation33 was adopted for ABTS radical scavenging assay. A solution containing a mixture of 7.0 mM ABTS and 2.4 mM potassium persulphate in equal measures was allowed to react for 12 h at room temperature in the dark. This working solution was diluted by mixing 1.0 mL of ABTS radical solution and 60.0 mL of methanol. AA and butylated hydroxytoluene (BHT) were used as standard antioxidants and the testing samples were compared with them. For this purpose solution of different concentrations range (5–200.0 μg/mL) were prepared for AA, BHT, and testing samples. A quantity of 1.0 mL of each sample was allowed to react with 1.0 mL of ABTS radical solution for 7.0 min and the absorbance was recorded at 734 nm. Percentage inhibition was calculated as ABTS radical scavenging activity.
The % scavenging activity was calculated by using the formula:
% Scavenging activity = [(Ac−As/Ac)] × 100
Where, Ac = Absorbance of control (DPPH or ABTS radical + methanol)
As = Absorbance of sample (DPPH or ABTS radical + sample/standard)
The scavenging activity was expressed as IC50, which is defined as the concentration (μg/mL) of compound required for the 50% inhibition of the DPPH and ABTS radical. IC50 values were determined by linear regression analysis using at least five different concentrations in duplicate.Citation34
Results and discussion
A systematic study of the reaction of metal chlorides with ligand IPMCHTSC in 1:1 and 1:2 and IPMCHPhTSC in 1:2 molar ratio in EtOH have been carried out. The reaction can be represented by following equation:
[Where M = Fe(III), Co(II), Cu(II); X = 2 for Co(II), Cu(II) and 3 for Fe(III); m = 0 for Fe(III), 2 for Cu(II) and 6 for Co(II); y = 1 or 2 for LH = IPMCHTSC, y = 2 for LH = IPMCHPhTSC; n = 0 for Co(II), Cu(II) and 1 for Fe(III)]
Several analytical techniques were used to characterize the complexes including microanalysis (CHN), spectral studies (IR, fast-atom bombardment (FAB) mass, and UV), TGA and conductometric measurements. Analytical data for the newly synthesized complexes are given in . All the metal complexes are non-hygroscopic in nature, stable at room temperature, insoluble in water partially soluble in ethanol and methanol but completely soluble in DMSO.
Table 1. Analytical and physical data for complexes.
The molar conductance values of the complexes are well presented in . The molar conductivity shows that all the complexes are non-electrolyte with λ = 17.4–31.8 Ω−1 cm2·mol−1 in DMSO (10−3 M) solution at room temperature.
Electronic spectra
The electronic spectra of Fe(III) complexes show 810 nm and 570 nm may be assigned to Citation6A1g→ 4T1g and 6A1g→ 4T2g transitions, respectively, which suggest the octahedral geometry around Fe(III).Citation35 The electronic spectra of the Co(II) complexes exhibit four bands at 890, 685, 602, and 256 nm, which are assigned to 4T1g→ 4T2g (F), 4T1g→ 4T1g (P), 4T1g→ 4A2g and charge transfer transitions of the d7 system. Therefore, octahedral geometry was proposed for Co(II) complex.Citation35–37
The Cu(II) complexes show bands at 930, 620, and 405 nm which are assigned to 2B1g→ 2A1g (v1), 2B1g→ 2B2g (v2), and 2Eg (v3) transitions. The positions of these bands and their assignments suggest distorted octahedral geometry.Citation38 The absorption bands appearing in the UV domain are considered to the characteristics of ligand. The assignments of n-π* and π-π* transitions as being due to the (C=S) bond.
The electronic spectrum of [Co(IPMCHTSC)Cl2] exhibits three bands at 1090, 985, and 650 nm. The first two bands are assigned to 2B2g→ 2Eg and 2B2g→ 2A1g transitions, respectively, in a square-planner environment of Co(II).Citation39 The spectrum of [Cu(IPMCHTSC)Cl2] shows a band 485 nm indicates square-planar geometry for the Cu(II) complex.Citation39,Citation40
IR spectra
The main IR spectral bands of complexes and their assignments are presented in . The ligands IPMCHTSC and IPMCHPhTSC exhibit bands at 840 and 832 cm−1, which shifted to the downward region in complexes suggested the coordination of metal of through the C=S group. The spectra of both ligands exhibit a band in the 1580–1595 cm−1 region due to C=N mode of azomethine linkage. In the metal complexes this band shifted to lower frequency suggesting that the unsaturated nitrogen of azomethine linkage is coordination to metal. In IPMCHTSC the highest frequency bands observed in 3441 and 3240 cm−1 are assigned to asymmetric and symmetric stretching of terminal NH2 group vibration. The second highest bands observed at 3280, 3150, and 3155 cm−1 due to NH group stretching vibrations in IPMCHPhTSC and IPMCHTSC, respectively. In IPMCHPhTSC a band observed at 3030 cm−1 due to Ar-H stretching vibration. In the complexes the above bands are not affected indicating non-participation of NH2, NH and Ar-H in coordination. The non-ligand bands occuring the 420–472, 325–395, and 310–370 cm−1 regions are tentatively assigned to v(M–N), v(M–S) and v(M–Cl) modes, respectively.
Table 2. Main IR spectral bands for complexes.
Thermal studies
The thermogram for complex (3) and (6) revealed three step decomposition behavior []. These TG steps are connected with exothermic events caused due to the pyrolysis of organic by products. The thermogram, also exhibits completion of the decomposition at 900°C. The residual for complex (3) was 29.85% corresponding to the formation of Co2S3 (Calcd. 30.29%). The residual for complex (6) was 21.60%, corresponding to the formation of Cu2S (Calcd. 22.23%).
Mass spectra
The FAB mass spectra studies of seven of the representative compounds [Fe(C11H17N3S)2Cl2] (1), [Co(C11H17N3S)Cl2] (3), [Co(C11H17N3S)2Cl2] (4), [Co(C17H21N3S)2 Cl2] (5), [Cu(C11H17N3S)Cl2] (6), [Cu(C11H17N3S)2Cl2] (7), and [Cu(C17H21N3S)2Cl2] (8) indicate their monomeric nature. The splitting patterns of mass spectra of compounds are shown in . The molecular ion peak of (2), (5), and (6) appears at m/z 608.0, 576.0, and 581.0, respectively, thus confirming the formation a metal ion complex in 1:2 ratio. Appearance of some molecular ion peaks at higher m/z than molecular ion in the FAB mass spectra may be due to re-association of fragments.
Table 3. Fragmented molecular ions vs m/z values of following metal complexes.
On the basis of above analysis, the following structural formula () may be suggested for the complexes.
Free radical scavenging activity of methanolic solutions using DPPH and ABTS assay
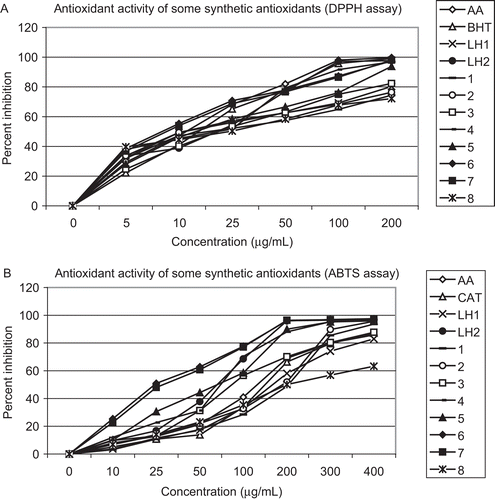
A variety of thiosemicarbazone complexes show antioxidant activity.Citation41–43 Various researchers have used scavenging effect of a chemical on DPPH and ABTS radicals as a quick and reliable parameter to assess the in vitro antioxidant activity. The results of free radical scavenging activity of methanolic solutions of compounds at different concentrations are shown in . It is evident from results that free radical scavenging activity of these compounds was concentration dependent. Among the examined compound the complex [Co(C11H17N3S)2Cl2] (4) and [Cu(C11H17N3S)2Cl2] (7) showed a strong interactive ability with DPPH and ABTS, respectively, compound (4) shows maximum free radical scavenging activity (96.97%) for DPPH radicals and compound (7) shows maximum free radical scavenging activity (98.03%) for ABTS radicals, followed by (97.56%), (97.70%) in IPMCHPhTSC for DPPH and ABTS radicals, respectively, while least activity (82.99%) and (80.33%) were observed from IPMCHTSC for DPPH and ABTS radicals, respectively. The complex (4) and (7) expressed an IC50 value of 73.94 μg/mL and 28.90 μg/mL for DPPH radical, lower than that of the standards [i.e. AA (131.9 μg/mL) and CAT (149.8 μg/mL)]. The compound IPMCHTSC, (2), (6), and (7) expressed an IC50 values of 11.32, 11.42, 8.42, and 8.85 μg/mL, respectively, for ABTS radical which is lower than that of the standards [i.e. AA (12.0 μg/mL) and BHT (15.58 μg/mL)].
Table 4. Antioxidant activity of metal complexes and ligands (DPPH assay).
Table 5. Antioxidant activity of metal complexes and ligands (ABTS assay).
Table 6. IC50 values of test compounds (µg/mL).
The comparative antioxidant activity of compounds against AA, CAT, and BHT as a standard is shown by graphs ().
All DPPH and ABTS scavenging activities were carried out only to evaluate the relative antioxidant activities. In the experiment standard antioxidants and other synthetic antioxidants were mixed with DPPH and ABTS in same fraction ratio which was not 1:1 ratio but actually it was 1:6 ratio that’s why 50% scavenging was observed with high concentration of antioxidants and large IC50 values were observed although it was only to compare synthetic antioxidants with standard antioxidants.
Conclusion
The metal complexes isolated during the present study demonstrated that the interaction of metal chloride with thiosemicarbazones of carvone leads to complexes with 1:1 and 1:2 stoichiometry and are found to be mononuclear. The bidentate nature of both type of ligands have been suggested on the basis of spectral evidences. The antioxidants activity of considered compounds are very large which indicate that among the test compound ligand IPMCHTSC, IPMCHPhTSC and their complexes showed potent antioxidant activity. The free redical scavenging activity at different concentrations (10–400, 5–200 μg/mL) of metal complexes in DPPH and ABTS assay are clearly mentioned in and , respectively. The conditions for carrying out the antioxidant activities in the laboratory shows the results that indicate the good potentiality of the synthesized compounds as synthetic antioxidants and they can serve as dietary antioxidants accordingly but clinical trials are necessary to find out the Lethal Dose LD50 for the induction of any material in dietary supplements.
Acknowledgements
The authors are thankful to Defence Institute of Bio-Energy Research, Pithoragarh (Uttrakhand), for carrying out antioxidant activity and also grateful to Zydus Research Center, Ahmedabad for recording FAB Mass, TGA, elemental analysis and electronic spectra. One of the authors (R.S.) is grateful to CSIR, New Delhi for the award of Research Associateship to her.
Declaration of interest
The authors report no conflicts of interest.
References
- Chandra S, Gupta LK, Sangeetika. Spectroscopic, cyclic voltammetric and biological studies of transition metal complexes with mixed nitrogen-sulphur (NS) donor macrocyclic ligand derived from thiosemicarbazide. Spectrochim Acta A Mol Biomol Spectrosc 2005;62:453–460.
- Mishra AP, Soni M. Synthesis, structural, and biological studies of some schiff bases and their metal complexes. Met Based Drugs 2008;2008:875410.
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enzyme Inhib Med Chem 2006;21:749–755.
- Chandra S, Sharma AK. Antifungal and Spectral Studies of Cr(III) and Mn(II) Complexes Derived from 3,3′-Thiodipropionic Acid Derivative. Research Letters in Inorganic Chemistry 2009;2009:1–4.
- Singh NK, Agrawal S, Aggarwal RC. Synthetic, Structural and Antifungal Studies of Some 3d-Metal Complexes of Salicylaldehyde-2-furanthiocarboxyhydrazone. Synth React Inorg Met Org Chem 1985;15:75.
- Padhye SB, Kauffman GB. Transition metal complexes of semicarbazones and thiosemicarbazones. Coord Chem Rev 1985;63:127–160.
- West DX, Padhye SB, Sonawane PB, Chikte RC. Structural and physical correlation in the biological properties of transition metal N-heterocyclic thiosemicarbazones and S-alkyldithiocarbazate complexes. Structure and Bonding 1991;76:1.
- West DX, Padhye SB, Sonawane PB, Chikte RC. Copper(II) complexes of tridentate (O, N, S) thiosemicarbazones. Asian J Chem Rev 1990;4:125.
- Ali MA, Livingstone SE. Metal complexes of sulphur-nitrogen chelating agents. Coord Chem Rev 1974;13:101.
- Campbell MJM. Transition metal complexes of thiosemicarbazide and thiosemicarbazones. Coord Chem Rev 1975;15:279.
- Padhyé S, Kauffman GB. Transition metal complexes of semicarbazones and thiosemicarbazones. Coord Chem Rev 1985;63:127.
- Hughes MN. The Inorganic Chemistry of Biological Processes, (Wiley, London) 1972.
- Byrnes RW, Mohan M, Antholine WE, Xu RX, Petering DH. Oxidative stress induced by a copper-thiosemicarbazone complexes. Biochemistry 1990;29:7046–7053.
- West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yerande RG. Thiosemicarbazone complexes of copper (II): structural and biological studies. Coord Chem Rev 1993;123:49–71.
- Ferrari MB, Fava GG, Leporati E, Pelosi G, Rossi R, Tarasconi P, Albertini R, Bonati A, Lunghi P, Pinelli S. Synthesis, characterisation and biological activity of three copper (II) complexes with a modified nitrogenous base: 5-formyluracil thiosemicarbazone. J Inorg Biochem 1998;70:145–154.
- Sharma R, Bansal AK, Nagar M. Transition metal complexes of cis-3,7-dimethyl-2,6-octadienthiosemicarbazone:Synthesis, characterization and biological studies. Indian J Chem 2005;44A: 2255–2258; Sharma R, Agarwal SK, Rawat S, Nagar M. Synthesis, characterization and antibacterial activity of some transition metal cis-3,7-dimethyl-2,6-octadiensemicarbazone complexes. Transition Met Chem 2006;31:201–206.
- Sharma R, Nagar M. Synthesis, structural and antibacterial studies of some mixed ligand complexes of Zn (II), Cd (II) and Hg (II) derived from citral thiosemicarbazone and N-phthaloyl amino acids. Phosphorus, Sulfur and Silicon 2006;181:2863–2875.
- Sharma R, Nagar M, Agarwal M, Sharma H. Synthesis, Characterization and Antimicrobial Activities of Some Mixed Ligand Complexes of Co(II) with Thiosemicarbazones and N-Protected Amino Acids. J Enz Inhib Med Chem 2009;24:197–204.
- De Carvalho CCCR, Da Fonseca MMR. “Carvone: why and how should one bother to produce this terpene.” Food Chem 2006;95:413–422.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996;20:933–956.
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984;219:1–14.
- Maxwell SR. Prospects for the use of antioxidant therapies. Drugs 1995;49:345–361.
- Niki E, Shimaski H, Mino M. Antioxidantism-free and biological defense, Gakkai Syuppn Center, Tokyo, 1994:3–16.
- Reiter M, Rupp K, Baumeister P, Zieger S, Harréus U. Antioxidant effects of quercetin and coenzyme Q10 in mini organ cultures of human nasal mucosa cells. Anticancer Res 2009;29:33–39.
- Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr 2006;45:19–28.
- Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull 2003;26:1398–1402.
- Vidhya A, Indira M. Protective effect of Quercetin in the Regression of Ethanol-Induced Hepatotoxicity. Indian J Pharm Sci 2009;71:527–532.
- Prior RL, Cao G. Variability in Dietary Antioxidant Related Natural Product Supplements: The Need for Methods of Standardization. J Am Nutraceutical Assoc 1999;2:46–56.
- Vogel AI. Text Book of Practical Quantitative Chemical Analysis, 5th Edn (ELBS, London) 1989.
- Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effect of tannins and related polyphenols on super-oxide anion radical and on DPPH radical. Chem Pharm Bull 1989;37:2016–2021.
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 1997;22:749–760.
- Re R, Pellegrini N, Proteggente A, Pannala M, Yang C, EVANS R. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 1999;26:1231–1237.
- Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis africana. Rec Nat Prod 2009;3:23–31.
- Panteleon V, Kostakis IK, Marakos P, Pouli N, Andreadou I. Synthesis and free radical scavenging activity of some new spiropyranocoumarins. Bioorg Med Chem Lett 2008;18:5781–5784.
- Lever ABP. “Inorganic Elecrtronic Spectroscopy”, 2nd Ed, Elsevier, 1984.
- Lewis J, Wilkins RG. Modern Coordination Chemistry (Interscience, New York), 1967.
- Casella L, Gullotti M. Coordination modes of histidine. 2. Stereochemistry of the reaction between histidine derivatives and pyridoxal analogs conformational properties of zinc(II) complexes of histidine Schiff bases. J Am Chem Soc 1981;103:6338.
- Patel KC, Goldberg DE. Aralkylpolyamine complexes-III: Some thiocyanate and selenocyanate complexes of copper(II), nickel(II) and cobalt(II) with n-benzylethylenediamine and N,N′-dibenzylethylenediamine. J Inorg Nucl Chem 1972;34:637.
- Nishida Y, Kida S. Splitting of d-orbitals in square planar complexes of copper(II), nickel(II) and cobalt(II). Coord Chem Rev 1979;27:275–298.
- Proctor IM, Hathaway BJ, Nicholls P. The electronic properties and stereochemistry of the copper(II) ion. Part I. Bis(ethylenediamine)copper(II) complexes. J Chem Soc A 1968:1678.
- Karatepe M, Karatas F. Antioxidant, pro-oxidant effect of the thiosemicarbazone derivative Schiff base (4-(1- phenylmethylcyclobutane -3-yl)-2-(2-hydroxybenzylidenehydrazino) thiazole) and its metal complexes on rats. Cell Biochem Funct 2006;24:547–554.
- Zhong Z, Zhong Z, Xing R, Li P, Mo G. The preparation and antioxidant activity of 2-[phenylhydrazine (or hydrazine)-thiosemicarbazone]-chitosan. Int J Biol Macromol 2010;47:93–97.
- Ghosh S, Misra AK, Bhatia G, Khan MM, Khanna AK. Syntheses and evaluation of glucosyl aryl thiosemicarbazide and glucosyl thiosemicarbazone derivatives as antioxidant and anti-dyslipidemic agents. Bioorg Med Chem Lett 2009;19:386–389.

![Figure 1. TGA curve of {weight (%) vs temperature (°C)} (A) [Co(C11H17N3S)Cl2] and (B) [Cu (C11H17N3S)Cl2].](/cms/asset/97720107-1d9a-44dc-bcfc-b8130c24a073/ienz_a_518966_f0001_b.gif)
![Figure 2. Structural formula for the complexes (A) [M(LH)Cl2] and (B) [M(LH)2Cl2].](/cms/asset/bb4af39f-e118-40d7-9f8b-3fbdd47c239d/ienz_a_518966_f0002_b.gif)