Abstract
The present study shows the chemical profile and the in vitro properties (antioxidant and inhibition of nitric oxide production) of the Origanum heracleoticum L. (Lamiaceae). The ethanolic extract of the aerial parts is characterized by terpenes and fatty acids. The extract, with high total phenol and flavonoid content, showed a significant radical-scavenging activity (IC50 value of 12.8 μg/mL) using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test and an interesting antioxidant activity with the β-carotene bleaching test (IC50 values of 12.9 and 14.1 μg/mL at 30 and 60 min of incubation, respectively). The test for the inhibition of NO production, performed using the murine monocytic macrophage RAW 264.7 cell line, showed that the extract had significant activity with an IC50 value of 108.5 μg/mL. The cytotoxic effect of O. heracleoticum extract in presence of lipopolysaccharide (LPS) (1 μg/mL) was evaluated but found to be negligible.
Introduction
In traditional societies, nutrition and healthcare are strongly interconnected and many plants have been consumed both as food and for medicinal purposes.Citation1,Citation2 The consumption of noncultivated botanicals plays a central role in the diet, but very few ethnopharmacological and phytopharmacological studies have dealt exhaustively with the potential health benefits of such diets. The number of studies on the antioxidant properties of specific plant foods and their phenolic constituents has become very impressive.Citation3–5 Oxidative stress is intricately linked with aging-related diseases (ARD), and longevity has been related with variants of the Mediterranean diet and specifically with a high consumption of olive oil, fruits, and vegetables.Citation6
It is commonly accepted that in a situation of oxidative stress, reactive oxygen species (ROS) such as superoxide (O2−·, OOH·), hydroxyl (OH·), and peroxyl (ROO·) radicals are generated. ROS play an important role in the pathogenesis of various serious diseases, such as neurodegenerative disorders, cancer, cardiovascular diseases, atherosclerosis, cataracts, and inflammation.Citation7,Citation8 Several anti-inflammatory drugs have recently been shown to have an antioxidant and/or radical-scavenging mechanism as part of their activity.Citation9 Thus the neutralization of free radicals by antioxidants and radical scavengers can attenuate inflammation.Citation10
Nitric oxide (NO) is a diatomic free radical produced from l-arginine by constitutive and inducible nitric oxide synthase (cNOS and iNOS) in numerous mammalian cells and tissues. NO, superoxide (O2−), and their reaction product peroxynitrite (ONOO−) may be generated in excess during the host response against viral and antibacterial infections and contribute to some pathogenesis by promoting oxidative stress, tissue injury, and even cancer.Citation11
The vernacular name “oregano” is attributed to a vast number of species in over a dozen genera of different families. Today, it is generally accepted that oregano as a matter of fact is a characteristic flavour produced by a number of plant species that yield carvacrol-rich essential oils.Citation12
Oregano plays a primary role among temperate culinary herbs in world trade. Origanum vulgare L. is the most variable species of the genus and the only one commonly known as oregano in most countries. Origanum heracleoticum L. is endemic to the Mediterranean area.Citation13 Oregano is found in many areas and is a perennial shrub native to the dry, rocky calcareous soils in the mountainous area of southern Europe and Southwest Asia. It is also cultivated because of its uses as an herb and its therapeutic properties, which have been known since ancient times.Citation14
The aim of this work was to evaluate the chemical composition and the antioxidant activities of O. heracleoticum extract using 2,2-diphenyl-1-picrylhydrazyl (DPPH) test and β-carotene bleaching test. Moreover, the inhibition of ΝΟ production in the murine monocytic macrophage cell line RAW 264.7 of O. heracleoticum extract was performed to evaluate the anti-inflammatory activity.
Materials and methods
Chemicals
Ethanol, dimethyl sulphoxide (DMSO), sodium carbonate, sodium nitrite, and NaOH were obtained from VWR International s.r.l. (Milan, Italy). Ascorbic acid, β-carotene, propyl gallate, linoleic acid, Tween-20, DPPH, Griess reagent (1% sulphonamide and 0.1% N-(1-naphtyl)ethylenediamine dihydrochloride in 2.5% H3PO4), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco’s modified Eagle’s medium (DMEM), l-glutamine, fetal bovine serum (FBS), antibiotic/antimicotic solution (penicillin/streptomycin), lipopolysaccharide (LPS), indomethacin, Folin-Ciocalteau reagent, AlCl3, quercetin, and chlorogenic acid were obtained from Sigma-Aldrich S.p.A. (Milan, Italy). All other reagents, of analytical grade, were Carlo Erba products (Milan, Italy).
Plant material and sample preparation
The aerial parts of O. heracleoticum L. used in this study were collected in August 2007 in Calabria (Southern Italy) and authenticated by Dr. Dimitar Uzunov, Natural History Museum of Calabria and Botanic Garden, University of Calabria (CLU; Italy). A voucher specimen is deposited in the Botany Department Herbarium at the CLU.
The aerial parts (251 g) were exhaustively extracted with ethanol (3 × 2.50 L) through maceration (144 h × 3 times). The resultant total extract was filtered, dried under reduced pressure (37 g), and analyzed by gas chromatography-mass spectrometry (GC-MS).
GC-MS analyses
To determine the nonpolar composition of the O. heracleoticum ethanolic extract, analytical GC was carried out on a Hewlett-Packard 6890 gas chromatograph equipped with a SE30 capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness) and interfaced with a Hewlett-Packard 5973 Mass Selective. Ionization of the sample components was performed in Electron Impact (EI, 70 eV) mode, with helium as the carrier gas. Column temperature was initially kept at 50°C for 5 min, then gradually increased to 250°C at 5°C/min rate and finally held for 10 min at 250°C. Injector and detector were maintained at 250°C and 280°C, respectively. Constituents of O. heracleoticum extract were identified by comparison of their mass spectra with those stored in Wiley 138 and NIST 98 libraries or with mass spectra from literature and by comparison of their GC retention times with those of standards available in our laboratories.Citation15
Determination of total phenol content
The amount of total phenolics was estimated spectrophotometrically in the O. heracleoticum ethanolic extract using Folin-Ciocalteau reagent.Citation16 A total of 100 μL of the extract were diluted with 2 mL of distilled water, 0.2 mL of Folin-Ciocalteau phenol reagent was added, and the flasks were shaken vigorously. Subsequently, 1.0 mL of 15% sodium carbonate solution was added and the mixtures were mixed thoroughly again. The mixtures were allowed to stand for 2 h protected from light. The absorbance of the blue colour produced was measured with a spectrophotometer (UV–Vis Jenway 6003) at 765 nm. The levels of total phenolics content were calculated in triplicate on the basis of a standard curve obtained using chlorogenic acid as standard and were expressed in mg/g of extract.
Determination of total flavonoid content
The flavonoid content was determined spectrophotometrically using a method based on the formation of a flavonoid–aluminium complex.Citation17 One millilitre of the extract was added to a 10-mL volumetric flask. Distilled water was added to make a volume of 5 mL. At time 0, 0.3 mL of 5% (w/v) sodium nitrite was added to the flask. After 5 min, 0.6 mL of 10% (w/v) AlCl3 was added and then at 6 min 2 mL of 1 M NaOH were also added to the mixture, followed by the addition of 2.1 mL distilled water. Absorbance at 510 nm was measured immediately. Quercetin was chosen as a standard and the levels of total flavonoid content were determined in triplicate and expressed in mg/g of extract.
DPPH assay
Free radical-scavenging activity was determined using a method based on the reduction of a methanolic solution of the coloured free radical DPPH. This experimental procedure was adapted from Wang et al.Citation18 In an ethanol solution of DPPH radical (final concentration was 1.0 × 10−4 M), the extract at different concentrations (5–100 μg/mL) was added. The reaction mixtures were shaken vigorously and then kept in the dark for 30 min. The absorbance of the resulting solutions was measured in 1 cm cuvettes, using a PerkinElmer Lambda 40 UV/VIS spectrophotometer at 517 nm, against blank without DPPH. A decrease in the absorbance of the DPPH solution indicates an increase of DPPH radical-scavenging activity. This activity is given as % DPPH radical scavenging, which is calculated in the equation:
The DPPH solution without sample solution was used as control. All tests were run in triplicate and the mean values were calculated. Ascorbic acid was used as positive control.
β-Carotene bleaching−linoleic acid assay
Antioxidant activity was determined using the β-carotene bleaching testCitation19 with some modifications. In brief, 1 mL of β-carotene solution (0.2 mg/mL in chloroform) was added to 0.02 mL of linoleic acid and 0.2 mL of 100% Tween-20. Chloroform was evaporated and the mixture was diluted with 100 mL of water, 5 mL of the emulsion were transferred into different test tubes containing 0.2 mL of sample in 70% ethanol at different concentrations. Propyl gallate at the same concentration was used as positive control. The tubes were then gently shaken and placed at 45°C in a water bath for 60 min. The absorbances of the samples, standard and control, were measured at 470 nm using a PerkinElmer Lambda 40 UV/Vis spectrophotometer against a blank, consisting of an emulsion without β-carotene. The measurement was carried out at initial time (t = 0) and successively at 30 and 60 min. All samples were assayed in triplicate and the mean value calculated. The antioxidant activity was measured in terms of successful preventing of β-carotene bleaching by using the following equation:
where A0 and A0· are the absorbance values measured at the initial incubation time for samples/standard and control, respectively, whereas At and At· are the absorbance values measure in the samples/standard and control, respectively, at t = 30 min and t = 60 min.
Cell culture
The murine monocytic macrophage cell line RAW 264.7 (European Collection of Cell Cultures, London, UK) was grown in plastic culture flask in DMEM with l-glutamine supplemented with 10% FBS and 1% antibiotic/antimicotic solution (penicillin/streptomycin) under 5% CO2 at 37°C. After 4–5 days, cells were removed from culture flask by scraping and centrifugating for 10 min under 1500 rpm. The medium was then removed and the cells were resuspended with fresh DMEM. Cell counts and viability were performed using a standard trypan blue cell counting technique. The cell concentration was adjusted to 1 × 106 cells/mL in the same medium. One hundred microlitres of the above concentration were cultured in 96-well plate for 1 day to become nearly confluent. Concentrations ranging from 10 to 100 μg/mL of the samples were prepared from the stock solutions by serial dilution in DMEM to give a volume of 100 μL in each well of a microtiter plate (96-well). Then cells were cultured with vehicle, O. heracleoticum EtOH extract in the presence of 1 μg/mL LPS for 24 h.
Assay for cytotoxic activity
Cytotoxicity was determined using the MTT assay reported by Tubaro et al.Citation20 with some modifications. The assay for each sample analyzed was performed in triplicate and the culture plates were kept at 37°C with 5% (v/v) CO2 for 1 day. After 24 h of incubation, 100 μL of medium was removed from each well. Subsequently, 100 μL of 0.5% w/v MTT (Sigma, Italy), dissolved in phosphate-buffered saline, was added to each well and allowed to incubate for a further 4 h. After 4 h of incubation, 100 μL of DMSO was added to each well to dissolve the formazan crystals. Absorbance values at 550 nm were measured with a microplate reader (GDV DV 990 B/V, Roma, Italy). Cytotoxicity was expressed as IC50, which is the concentration to reduce the absorbance of treated cells by 50% with reference to the control (untreated cells).
Inhibition of NO production in LPS-stimulated RAW 264.7 cells
The presence of nitrite, a stable oxidized product of NO, was determined in cell culture media by Griess reagent (1% sulphonamide and 0.1% N-(1-naphtyl) ethylenediamine dihydrochloride in 2.5% H3PO4).Citation21 One hundred microlitres of cell culture supernatant were removed and combined with 100 μL of Griess reagent in a 96-well plate followed by spectrophotometric measurement at 550 nm using a microplate reader (GDV DV 990 B/V, Rome, Italy). Nitrite concentration in the supernatants was determined by comparison with a sodium nitrite standard curve.
Statistical analysis
All experiments were carried out in triplicate. Data were expressed as means ± SD. The concentration giving 50% inhibition (IC50) was calculated by nonlinear regression with the use of Prism Graphpad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). The dose–response curve was obtained by plotting the percentage inhibition versus concentration.
Results and discussion
After maceration with ethanol (99.8%), the total phenol and flavonoid content of O. heracleoticum aerial parts extract (yield of 15% relative to dry weight material) was evaluated. The O. heracleoticum extract showed a total phenol content, determined by Folin-Ciocolteau assay, of 38.7 mg chlorogenic acid equivalent/g of extract. The flavonoid content, determined using a method based on the formation of a flavonoid–aluminium complex, was 14.4 mg quercetin equivalent/g of extract.
Previously, Tsimogiannis et al.Citation22 examined O. heracleoticum as potential source of phenolic antioxidants. The components of the plant were extracted in a Soxhlet apparatus sequentially with solvents of increasing polarity, and in particular with petroleum ether, diethyl ether, and ethanol.Citation22 The O. heracleoticum ethanol extract was found to be characterized by rosmarinic acid, apigenin, apigenin glycoside, and carvacrol as major components. According to Vekiari et al.,Citation23 flavonoid constituents of oregano (O. vulgare) are apigenin, eriodictyol, taxifolin, and dihydrokaempferol. Apigenin and luteolin were detected in acetone extracts of Greek oregano (O. vulgare ssp. hirtum) by Exarchou et al.Citation24 JustesenCitation25 and Justesen and KnuthsenCitation26 identified rosmarinic acid, luteolin, luteolin glycoside, apigenin, apigeninacetyl-diglycoside, and diosmetin-acetyl-glucuronide.
In order to identify putative active nonpolar compounds present within the O. heracleoticum extract, the GC-MS analyses were realized. The GC-MS analysis revealed that the O. heracleoticum extract is characterized by the presence of four terpenes, namely p-cymene, thymol, carvacrol, and neophytadiene, and nine fatty acids and methyl esters, namely methyl palmitoleate, methyl palmitate, myristic acid, methyl heptadecanoate, methyl linoleate, methyl linolenate, methyl stearate, methyl arachidate, and methyl behenate, as major nonpolar constituents ().
Figure 1. Chromatograms of O. heracleoticum extract and structures of the major components: (A) terpenes and (B) fatty acids.
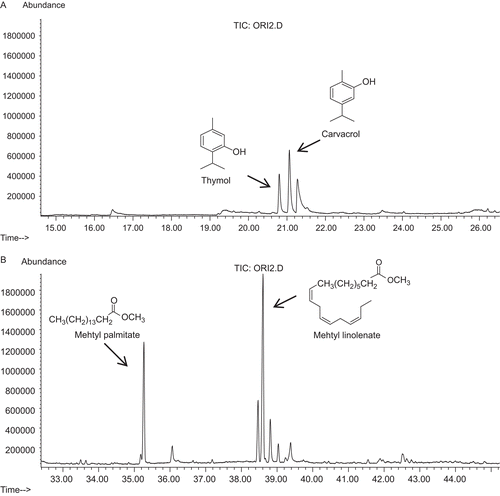
In addition to the above-referred studies on the polyphenols constituents, oregano was previously analyzed especially for its essential oil. The chemical analysis has shown its constituents to be principally carvacrol and thymol.Citation27–29 Vokou et al.,Citation30 examining essential oils of O. vulgare ssp. hirtum from 23 localities scattered all over Greece, found that in spite of the high variability of individual compounds in the essential oil, the sum of carvacrol, thymol, p-cymene, and γ-terpinene was stable amounting to >80%. Carvacrol and thymol appear to be the main components of all O. vulgare ssp.,Citation31–33 although their content may vary considerably among different populations.Citation30,Citation33
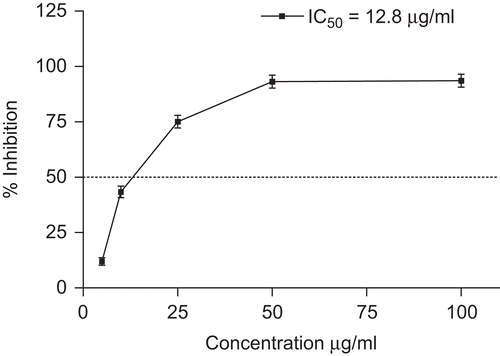
The model of scavenging stable DPPH free radicals can be used to evaluate antioxidant activity in a relatively short time. The absorbance decreases as a result of a colour change from purple to yellow as the radical is scavenged by antioxidants through donation of hydrogen to form the stable DPPH-H molecule,Citation34 although a recent work suggests that, on the basis of kinetic analysis of the reaction between phenols and DPPH, the reaction in fact behaves like a single electron-transfer reaction.Citation35 It was found that the rate-determining step for this reaction consists of a fast electron-transfer process from the phenoxide anions to DPPH. The hydrogen atom abstraction from the neutral ArOH by DPPH becomes a marginal reaction path, because it occurs very slowly in strong hydrogen-bond-accepting solvents, such as methanol and ethanol. The scavenging effects of extract on DPPH were examined at different concentrations (range between 5 and 1000 μg/mL). The extract was able to reduce the stable free radical DPPH to the yellow-coloured DPPH with an IC50 value of 12.8 μg/mL ( and ).
Table 1. IC50 values of antioxidant and NO production inhibition activities of EtOH extract from oregano.
Figure 2. Free radical-scavenging activity of O. heracleoticum extract. Data are mean ± SD (n = 3). Ascorbic acid (IC50 value of 2 μg/mL) was used as positive control.
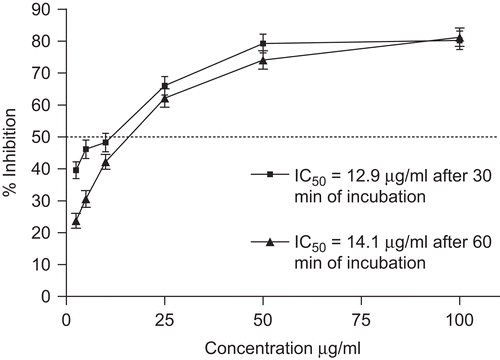
A second test, oriented towards lipophilic antioxidants, was selected. In the β-carotene/linoleic acid model system, β-carotene undergoes rapid discolouration in the absence of an antioxidant. Ethanol extract of O. heracleoticum was able to inhibit the discolouration of β-carotene with IC50 values of 12.9 and 14.1 at 30 min and 60 min of incubation, respectively ( and ). As reference, IC50 of propyl gallate was 1 µg/mL, both after 30 and 60 min incubation. Membrane lipids are rich in unsaturated fatty acids that are most susceptible to oxidative processes. Especially, linoleic acid and arachidonic acid are major targets of lipid peroxidation. Radicalar chain reactions are widely accepted as a common mechanism of lipid peroxidation and it is generally thought that the inhibition of lipid peroxidation by antioxidants may be due to their free radical-scavenging activities. Lipophilic radical scavengers may directly react and quench peroxide radicals to reduce the peroxidation chain reaction and improve the quality and stability of biological membranes and food products.
Figure 3. Lipid peroxidation inhibition using β-carotene–linoleic acid system after 30 and 60 min of incubation of ethanolic extract of O. heracleoticum. Data are mean ± SD (n = 3). Propyl gallate (IC50 = 1 μg/mL) was used as positive control.
Several studies evaluated the relationships between antioxidant activity of plant products and their phenolic content. Some authors found a correlation between the phenolic content and the antioxidant activity, whereas others found no relationship. Velioglu et al.Citation36 reported a high correspondence between total phenolic content and antioxidant activity (using β-carotene bleaching method) in selected fruits, vegetables, and grain products. On the other hand, no correlation between antioxidant activity (using methyl linoleate oxidation assay) and phenolic content was observed by Kähkönen et al.Citation37 on some plant extracts containing phenolic compounds.
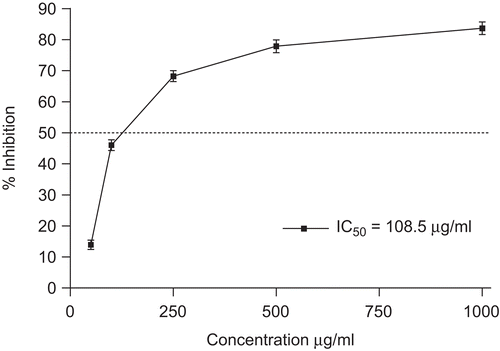
NO, which is derived from the oxidation of l-arginine through three isoforms of NOS, namely neuronal (nNOS), endothelial (eNOS), and iNOS, is recognized as a mediator and regulator in pathological reactions, especially in acute inflammatory responses.Citation38 High levels of NO cause a variety of pathophysiological processes including inflammationCitation39 and carcinogenesis.Citation40 iNOS mainly exists in macrophages, it is expressed by stimulation with endotoxins, tumor necrosis factors, or LPS. Proinflammatory agents, such as LPS, can significantly increase NO production in macrophages through activation of iNOS.Citation41,Citation42 In this study, the NO inhibitory activity of ethanolic extract of O. heracleoticum was evaluated by using a LPS-stimulated RAW 264.7 cell assay. The treatment of RAW 264.7 macrophages with LPS (1 μg/mL) for 24 h induces NO production, which can be quantified by utilizing the chromogenic Griess reaction, which measures the accumulation of nitrite, a stable metabolite of NO. The beneficial effect of O. heracleoticum extract on the inhibition of production of inflammatory mediators in macrophages can be mediated through oxidative degradation of products of phagocytes, such as O2− and HOCl. As shown in , incubation of RAW 264.7 cells with O. heracleoticum extract induced a significant inhibitory effect on the LPS-induced nitrite production. The EtOH extract of O. heracleoticum showed significant inhibition of LPS-induced NO production in RAW 264.7 cells in a dose-dependent manner, with an IC50 value of 108.5 μg/mL ( and ). Any cytotoxic effect of O. heracleoticum EtOH extract in presence of LPS (1 μg/mL) was also evaluated. O. heracleoticum extract did not show any cytotoxicity up to 250 μg/mL concentration.
Figure 4. Inhibition of NO production in the murine monocytic macrophage cell line RAW 264.7 of O. heracleoticum extract. Data are mean ± SD (n = 3). Indomethacin (IC50 value of 52.8 μg/mL) was used as positive control.
Previous studies showed that thymol and carvacrol inhibit the peroxidation of phospholipids liposomes and these compounds possess useful antioxidant properties.Citation43,Citation44 Carvacrol also showed inhibitory activity on NO production by human leucocytes, whereas thymol, an isomer of carvacrol, showed no activity.Citation45 Among polyphenol compounds, flavonoid such as apigenin was also markedly active inhibitors of free radicals and of transcriptional activation of iNOS.Citation46,Citation47
Conclusions
Oxidative damage may initiate and promote the progression of a number of chronic diseases, including inflammation. The present work showed for the first time the in vitro activity of O. heracleoticum ethanol extract. Further, in vivo investigations are needed for a possible usefulness of this extract in the treatment of inflammation. In this study, we have demonstrated that the EtOH extract of O. heracleoticum exhibited significant antioxidant activity and an inhibitory effect on NO production (an inflammatory mediator) in macrophages. The observed in vitro activities suggest that the investigated plant extract might also exert in vivo protective effects against oxidative and free radical injuries occurring in different pathological conditions. Therefore, we propose here the potential benefits of O. heracleoticum extract on the basis of the phytochemical characteristics and the observed bioactive properties. The antioxidative and anti-inflammatory properties of naturally occurring compounds appear to contribute to their chemopreventive or chemoprotective activity.
The anti-inflammatory activities of O. heracleoticum EtOH extract was evaluated to obtain an insight into the beneficial effects of this plant species in conditions related to inflammation, reduced risk for cardiovascular diseases, and cancer prevention by acting as anti-inflammatory agents. Further studies of the plant extracts and/or the identified compounds from O. heracleoticum on the pharmacokinetics or mode of action on mechanisms of chemopreventive properties are warranted. Also, the extraction technique should be investigated more widely, particularly in view of the application of supercritical fluids. Another point that should be strongly evaluated is the use of emulsions instead of solution in real applications, with the aim to prevent degradation of the extract activity due to oxygen exposure. In conclusion, this work reveals that O. heracleoticum can be an interesting source of anti-inflammatory and antioxidant principles with a potential use in different fields (food, cosmetics, pharmaceutical).
Declaration of interest
The authors report no conflict of interest.
References
- Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J Ethnopharmacol 2000;70:235–273.
- Etkin NL. Medicinal cuisines: diet and ethnopharmacology. Int J Pharmacogn 1996;34:313–326.
- Lionis C, Faresjö A, Skoula M, Kapsokefalou M, Faresjö T. Antioxidant effects of herbs in Crete. Lancet 1998;352:1987–1988.
- Cervato G, Carabelli M, Gervasi S, Cittera A, Cazzola R, Cestaro B. Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J Food Biochem 2000;24:453–465.
- Murcia MA, Jiménez AM, Martínez-Tomé M. Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. J Food Prot 2001;64:2037–2046.
- Trichopoulou A, Vasilopoulou E. Mediterranean diet and longevity. Br J Nutr 2000;84:S205–S209.
- Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr 2004;24:511–538.
- Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 1998;75:199–212.
- Perry EK, Pickering AT, Wang WW, Houghton PJ, Perru NS. Medicinal plants and Alzheimer’s disease: from ethnobotany to phytotherapy. J Pharm Pharmacol 1999;51:527–534.
- Delaporte RH, Sánchez GM, Cuellar AC, Giuliani A, Palazzo de Mello JC. Anti-inflammatory activity and lipid peroxidation inhibition of iridoid lamiide isolated from Bouchea fluminensis (Vell.) Mold. (Verbenaceae). J Ethnopharmacol 2002;82:127–130.
- Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry 1998;63:854–865.
- Lagouri V, Blekas G, Tsimidou M, Kokkini S, Boskou D. Composition and anti-oxidant activity of essential oils from oregano plants grown wild in Greece. Lebensmitt Z Unters Forsch 1993;197:20.
- Skoula M, Harborne JB. The taxonomy and chemistry of Origanum. In: Kintzios SE (Ed.), Oregano: The Genera Origanum and Lippia. Taylor & Francis, London, 2002, pp. 67–108.
- Bariceric D, Bartal T. The biological/pharmacological activity of the Origanum genus. In: Kintzios SE (Ed.), Oregano: The genera Origanum and Lippia. Taylor & Francis, London, 2002, pp. 177–214.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publishing, Carol Stream, IL, 2001.
- Menichini F, Tundis R, Bonesi M, Loizzo MR, Conforti F, Statti G, De Cindio B, Houghton PJ, Menichini F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem 2009;114:553–560.
- Yoo KM, Lee CH, Lee H, Moon BK, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem 2008;106:929–936.
- Wang M, Li J, Rangarajan M, Shao Y, La Voie EJ, Huang T-C Ho, C-T. Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem 1998;46:4869–4873.
- Amin I, Zamaliah M, Chin WF. Total antioxidant activity and phenolic content in selected vegetables. Food Chem 2004;87:581.
- Tubaro A, Florio C, Luxich E, Vertua R, Della Loggia R, Yasumoto T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 1996;34:965–974.
- Prakash H, Ali A, Bala M, Goel HC. Anti-inflammatory effects of Podophyllum hexandrum (RP-1) against lipopolysaccharides induced inflammation in mice. J Pharm Sci 2005;8:107–114.
- Tsimogiannis D, Stavrakaki M, Oreopoulou V. Isolation and characterisation of antioxidant components from oregano (Origanum heracleoticum). Inter J Food Sci Technol 2006;41:39–48.
- Vekiari SA, Oreopoulou V, Tzia C, Thomopoulos CD. Oregano flavonoids as lipid antioxidants. J Am Oil Chem Soc 1993;70:483–487.
- Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage and summer savory. J Agric Food Chem 2002;50:5294–5299.
- Justesen U. Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J Chromatogr A 2000;902:369–379.
- Justesen U, Knuthsen P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem 2001;73:245–250.
- Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJA. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J Food Prot 2005;68:919–926.
- Charai M, Mosaddak M. Chemical composition and antimicrobial activities of two aromatic plants: Origanum majorana L. and O. compactum Benth. J Essent Oil Res 1996;8:312–318.
- Dzamic A, Sokovic M, Ristic MS, Grujic-Jovanovic S, Vukojevic J, Marin PD. Chemical composition and antifungal activity of Origanum heracleoticum essential oil. Chem Nat Comp 2008;44:532–533.
- Vokou D, Kokkini S, Bessiere JM. Geographical variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem Syst Ecol 1993;21:287–295.
- D’Antuono LF, Galletti CG, Bocchini P. Variability of essential oil content and composition of Origanum vulgare L. populations from a North Mediterranean area (Liguria region, Northern Italy). Ann Bot 2000;8:471–478.
- Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004;85:633–640.
- Menaker A, Kravets M, Koel M, Orav A. Identification and characterization of supercritical fluid extracts from herbs. Comptes Rendus Chimie 2004;7:629–633.
- Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), a-tocopherol, BHT, and BHA. J Agric Food Chem 1997;45:632–638.
- Foti MC, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J Org Chem 2004;69:2309–2314.
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 1998;46:4113.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Phenolic contents and antioxidant activity of some food and medicinal plants. J Agric Food Chem 1999;47:3954.
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kB activation. Mutat Res 2001;480–481: 243–268.
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997;15:323–350.
- Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994;305:253–264.
- Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-α secretion, and NF-kB-dependent gene expression in RAW264.7 macrophages. FEBS Lett 2000;465:93–97.
- Kojima M, Morisaki T, Izuhara K, Uchiyama A, Matsunari Y, Katano M, Tanaka M. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-kB activation. Oncogene 2000;19:1225–1231.
- Vokou D, Kokkini S, Bessiere JM. Geographic variation of Greek oregano (Origanum vulgare subsp. hirtum) essential oils. Biochem Syst Ecol 1993;21:287.
- Aeschbach R, Löliger J, Scott BC, Murcia A, Butler J, Halliwell B, Aruoma OI. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 1994;32:31.
- Pérez-Rosés R, Risco E, Vila R, Peñalver P, Cañigueral S. Inhibitory activity of nine essential oils on nitric oxide production by human leukocytes. 57th International Congress of the Society for Medicinal Plant and Natural Product Research, Geneva (Switzerland), 16th–20th August 2009.
- Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 1999;20:1945–1952.
- Miyake Y, Mochizuki M, Okada M, Hiramitsu M, Morimitsu Y, Osawa T. Isolation of antioxidative phenolic glucosides from lemon juice and their suppressive effect on the expression of blood adhesion molecules. Biosci Biotechnol Biochem 2007;71:1911–1919.
