Abstract
A series of polyhydroxy benzophenone were synthesized and evaluated as mushroom tyrosinase inhibitors. The results demonstrated that most of the target compounds had remarkable inhibitory activities on mushroom tyrosinase. Among all these compounds, 2,3,4,3′,4′,5′-hexahydroxy-diphenylketone 10 was found to be the most potent tyrosinase inhibitor with IC50 value of 1.4 μM. In addition, the inhibition kinetics analyzed by Lineweaver–Burk plots revealed that such compounds were competitive inhibitors. These results suggested that such compounds might be utilized for the development of new candidate for treatment of dermatological disorders.
Keywords::
Tyrosinase (EC 1.14.18.1; polyphenol oxidase, PPO), a multifunctional copper-containing enzyme, catalyzed two distinct reaction of melanin synthesis: the hydroxylation of monophenols and the oxidation of the o-phenols.Citation1,Citation2 Thereby, the enzyme converts tyrosine to 3,4-dihydroxyphenylalanine (l-dopa) and oxidizes l-dopa to form dopaquinone, which plays important roles in the process of melanin biosynthesis.Citation3 Tyrosinase is widespread in plants and animals. Therefore, tyrosinase inhibitors have become increasingly important in agriculture,Citation2,Citation4 cosmetic industry,Citation5 and medicationCitation6 due to decreasing the excessive accumulation of pigmentation resulting from the enzyme action.Citation7–12
Presently, tyrosinase inhibitors have been established as important constituents of cosmetic materials, food preservative, and depigmenting agents for hyperpigmentation. Many tyrosinase inhibitors have been reported, for example, hydroquinone,Citation13–16 kojic acid,Citation17 azelaic acid,Citation18,Citation19 electron-rich phenols,Citation20 corticosteroids,Citation21,Citation22 resinoids,Citation23,Citation24 resveratrol,Citation25 oxyresveratrol,Citation26 and arbutin have been utilized as cosmetic agents. Most of these compounds have the structures of benzene rings and hydroxyl radicals.
In the last decades, polyhydroxy benzophenones have been widely utilized for the production and development of plastic, resin, coating materials, synthetic rubber, light-sensitive materials, and cosmetic materials. However, its other properties such as antioxidant activity and tyrosinase inhibitory activity have not been reported yet.
Since polyhydroxy benzophenones have the similar structures to the tyrosinase inhibitors mentioned earlier, we speculated that polyhydroxy benzophenones might exhibit potent tyrosinase inhibitory activity. Therefore, our team synthesized a series of polyhydroxy benzophenones and evaluated their inhibitory effects on the diphenolase activity of mushroom tyrosinase. To the best of our knowledge, this is the first time to report the inhibitory effects on the diphenolase activity of mushroom tyrosinase of polyhydroxy benzophenones.
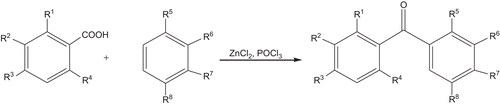
The synthetic routes of compounds 1–18Footnote1 were outlined in , and the chemical structures of polyhydroxy benzophenones 1–18 were given in .
Table 1. Chemical structure of polyhydroxy benzophenones 1–18 and inhibitory effects on mushroom tyrosinase of polyhydroxy benzophenones and kojic acid as reference inhibitor.
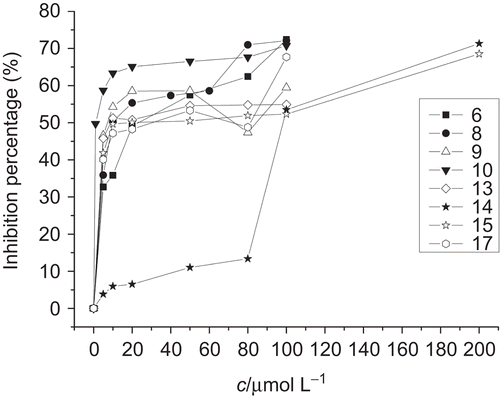
Tyrosinase inhibition assays were performed according to the developed method described earlier by HearingCitation27 with some modification.Footnote2 The IC50 values of all compounds investigated were summarized in and . As the results reported in , compounds 6, 8, 9, 10, 13, 15, and 17 exhibited more potent tyrosinase inhibitory activities than kojic acid, especially compound 10 (IC50 = 1.4 μmol/L), which demonstrated excellent in vivo activity.
Of all polyhydroxy benzophenones 1–18, compounds 1, 18, and 3, which are dihydroxy-substituted diphenylketones, display none or very weak inhibitory effect on tyrosinase, whereas trihydroxy-, tetrahydroxy-, pentahydroxy-, and hexahydroxy-substituted diphenylketones exhibited a significant increase inhibitory activity on tyrosinase along the number of hydroxyl group grows. It seems to be a tendency that the inhibitory activity of polyhydroxy benzophenones on tyrosinase increase along the number of hydroxyl group grows.
Interestingly, most of the compounds that display potent tyrosinase inhibitory activities are substituted by hydroxyl group on positions R1, R2, and R3. The result suggests that the introduction of hydroxyl group on positions R1, R2, and R3 strengthens the tyrosinase inhibitory activity. Compared with compounds 2 and 12, compound 13 (IC50 = 7.3 μmol/L) bearing a hydroxyl substituent at position R5 displays potent inhibitory activity. When position R7 is replaced by hydroxyl group, such as compounds 4 (IC50 ≥ 200 μmol/L) and 7 (IC50 ≥ 100 μmol/L), a significant decrease in inhibition potency was observed, whereas the IC50 values of compounds 13 and 17 are 7.3 μmol/L and 10.5 μmol/L, respectively.
In addition, compounds substituted by methoxyl group exhibited more potent tyrosinase inhibition potencies than unsubstituted compounds with similar structure, such as compounds 9 and 7 or compounds 2 and 5.
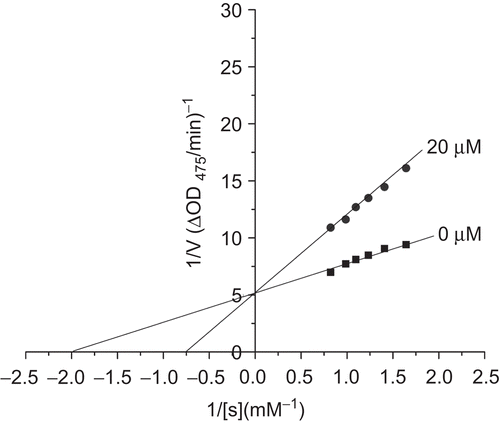
As the compounds that showed potent inhibitory activities on tyrosinase have similar structures to each other, we can get the conclusion that they have the same inhibition type on mushroom tyrosinase. Therefore, 2,3′,4,4′,5′-pentahydroxy-diphenylketone (compound 6) had been chosen to investigate the kinetic behavior of mushroom tyrosinase during the oxidation of l-dopa ()Footnote3. 29 The result shows that 2,3′,4,4′,5′-pentahydroxy-diphenylketone is a competitive inhibitor because increasing its concentration resulted in a family of lines with a common intercept on the 1/V axis but with different slopes.
Figure 2. Lineweaver–Burk plots for inhibition of 2,3′,4,4′,5′-pentahydroxydiphenylketone on mushroom tyrosinase for the catalysis of dopa at 25°C, pH 6.8. Concentrations of 2,3′,4,4′,5′-pentahydroxydiphenylketone (compound 6) for curves were 0 and 20 μmol/L, respectively.
In conclusion, in the present investigation, a series of polyhydroxy benzophenone were synthesized and evaluated as mushroom tyrosinase inhibitors. The results demonstrated that most of target compounds had remarkable inhibitory activities on mushroom tyrosinase. Particularly, compounds 6, 8, 9, 10, 13, 15, and 17 exhibited more potent tyrosinase inhibitory activities than kojic acid. This research indicates that both the number and position of hydroxyl groups in diphenylketone seem to play a critical role in exerting the inhibitory effect on dopa oxidase activity of tyrosinase. In addition, the inhibition kinetics analyzed by Lineweaver–Burk plots revealed that such compounds were competitive inhibitors. These results suggested that such compounds might be utilized for the development of new candidate for treatment of dermatological disorders.
Declaration of interest
This work was supported by the Undergraduate Research Programmes of Sun Yat-sen University, China (2009-73).
Notes
1The synthesis of compounds 1–18: To the mixture of appropriate phenol (50 mmol) and polyhydroxybenzoic acid (55.6 mmol), anhydrous zinc chloride (27.2 g) and phosphorus oxychloride (25 mL) were added. The reaction mixture was refluxed for 2.5 h in the water bath of 70°C with stirring. Then add the mixture to appropriate ice and cool to 4°C for 24 h. The precipitate solid was filtered, washed with 3% sodium bicarbonate twice, and purified by recrystallization from boiling water to afford compounds 1–18 (). The synthetic route of compounds 1–18 is shown in . Compound 10: 2,3,4,3′,4′,5′-hexahydroxydiphenylketone (10). Yield: 90%; yellow powders; mp 272–273°C; 1H NMR (dimethyl sulfoxide-d6, 300 MHz) δ (ppm): 6.4 (1H, d, J = 6.9 Hz, ArH), 6.6 (2H, s, ArH), 7.0 (1H, d, J = 4.2 Hz, ArH), 8.6 (1H, s, OH-R3), 8.9 (1H, s, OH-R2), 9.3 (2H, s, OH-R6, OH-R8), 10.0 (1H, s, OH-R7), 12.1 (1H, s, OH-R1). 13C NMR(dimethyl sulfoxide-d6, 300 MHz) δ: 13C NMR(dimethyl sulfoxide-d6, 300 MHz) δ: 198.60(Ccarbonyl), 175.94 (Cphenyl-4), 152.43 (Cphenyl-3′), 145.96 (Cphenyl-3,5), 138.27 (Cphenyl-4′), 133.45 (Cphenyl-2′), 128.61 (Cphenyl-5′), 125.31 (Cphenyl-6′), 113.76 (Cphenyl-1′), 109.64 (Cphenyl-2,6), 107.83 (Cphenyl-1).
2Tyrosinase Inhibition Assay: In brief, a 10 μL sample was added to an assay mixture containing with 10 μL tyrosinase solution (0.5 mg/mL) and 900 μL phosphate buffer (pH 6.8) to a total volume assay mixture of 920 μL, for 20 min at 25°C. Then 80 μL of l-dopa (1.50 mg/mL) was added to the reaction mixture and the enzyme reaction was monitored by measuring the change in absorbance at 475 nm of the l-dopa for 1 min. IC50 value, a concentration giving 50% inhibition of tyrosinase activity, was determined by interpolation of the dose–response curves. Here, kojic acid (IC50 = 19.0 μmol/L) was used as the reference inhibitors.
3Kinetic Assay of Tyrosinase Inhibition: Various concentrations of l-dopa (0.1–1.2 mM) as the substrate, 40 μL of mushroom tyrosinase (1 units/mL), and 50 mM potassium phosphate buffer (pH 6.8) were added to test tube in a total volume assay mixture of 1000 μL. The initial rate of dopa chrome formation in the reaction mixture was determined by the increase of absorbance at 475 nm/min (ΔOD475/min). Michaelis constant (Km) and maximal velocity (Vmax) of the tyrosinase activity were determined by the Lineweaver–Burk plot.
References
- Song K. K., Huang H., Han P., Zhang C. L., Shi Y., Chen Q. X., Inhibitory effects of cis- and trans-isomers of 3,5-dihydroxystilbene on the activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 2006, 342, 1147–1151.
- Yokochi N., Morita T., Yagi T., Inhibition of diphenolase activity of tyrosinase by vitamin B6 compounds. J. Agric. Food Chem. 2003, 51, 2733–2736.
- Hearing V. J., Tsukamoto K. Enzymic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909.
- Nerya O., Musa R., Khatib S., Tamir S., Vaya J., Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395.
- Maeda K., Fukuda M., In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–363.
- Maeda K., Fukuda M., Arbutin: mechanism of its depigmenting action in human melanocyte culture. J. Pharm. Exp. Ther. 1996, 276, 765–769.
- Chen Q. X., Kubo I., Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112.
- ShiinoM., Watanabe Y., Umezawa K., Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of mushroom tyrosinase. Bioorg. Med. Chem. 2001, 9, 1233–1240.
- Seo S. Y., Sharma V. K., Sharma N., Mushroom tyrosinase: recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853.
- Kubo I., Kinst-Hori I., 2-Hydroxy-4-methoxybenzaldehyde. A potent tyrosinase inhibitor from African medicinal plants. Planta Med. 1999, 65, 19–22.
- Okombi S., Rival D., Bonnet S., Mariotte A. M., Perrier E., Boumendjel A., Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J. Med. Chem. 2006, 49, 329–333.
- Khatib S., Nerya O., Musa R., Tamir S., Pete T., Vaya J., Enhanced substituted resorcinol hydrophobicity augments tyrosinase inhibition potency. J. Med. Chem. 2007, 50, 2676–2681.
- Arandt K. A., Fitzpatrick T. B., Topical use of hydroquinine as a depigmenting agent. J. Am. Med. Assoc. 1965, 194, 117–119.
- Fitztrick T. B., Arandt K. A., El-Mofty A. M. M. A., Hydroquinone and psoralens in the therapy of hypermelanosis and vitiligo. Arch. Dermatol. 1966, 93, 589–600.
- Kligman A. M., Willis I., A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48.
- Bleehen S. S., Skin bleaching preparations. J. Soc. Cosmet. Chem. 1977, 28, 407–412.
- Mishima Y., Hatta S., Ohyama Y., Inazu M., Induction of melanogenesis suppression: cellular pharmacology and mode of differential action. Pigment Cell Res. 1998, 1, 367–374.
- Breathnach A. C., Nazzaro-Porro M., Passi S., Azelaic acid therapy in disorders of pigmentation. Clin. Dermatol. 1989, 7, 106–119.
- Verallo-Rowell V. M., Verallo V., Graupe K., Lopez-Villafuerte L., Garcia-Lopez M., Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Dermatol. Venereol. 1989, 143, 58–61.
- Jimbow K., N-Acetyl-4-S-cysteaminylphenol as a new type of depigmenting agent for the melanoderma of melasma patients. Arch. Dermatol. 1991, 127, 1528–1534.
- Neering H., Treatment of melasma (chloasma) by local application of steroid cream. Dermatologica 1975, 151, 349–353.
- Kanwar A. J., Dhar S., Kaur S., Treatment of melasma with potent topical corticosteroids. Dermatology 1994, 188, 170.
- Griffiths C. E. M., Finkel L. J., Ditre C. M., Hamilton T. A., Ellis C. N., Voorhees J. J., Topical tretinonin (retinoic acid) improves melasma: a vehicle-controlled clinical trial. Br. J. Dermatol. 1993, 129, 415–421.
- Kimbrough-Green C. K., Griffiths C. E. M., Finkel L. J., Hamilton T. A., Burengo-Ransby S. M., Ellis C. N., Voorhees J. J., Topical retinoic acid (tretinoin) for melasma in black patients: a vehicle-controlled clinical trial. Arch. Dermatol. 1994, 130, 727–733.
- Kenji O., Toshiyuki T., Tet suro I., Inhibitory effects of resveratrol derivatives from Dipterocarpaceae plants on tyrosinase activity. J. Biosci. Biotechnol. Biochem. 2003, 67, 1587–1589.
- Shin N H., Ryu S. Y., Choi E J., Oxyresveratrol as the potent inhibitor on DOPA oxidase activity of mushroom tyrosinase. J. Biochem. Biophys. Res. Commun. 1998, 243, 801–803.
- Hearing V. J., Jr., Methods in enzymology. Academic, 1987, 142, 155.
- Chen Q. X., Song K. K., Qiu L., Liu X. D., Huang H., Guo H. Y., Inhibitory effects on mushroom tyrosinase by p-alkoxybenzoic acids. Food Chem. 2005, 91, 269–274.