Abstract
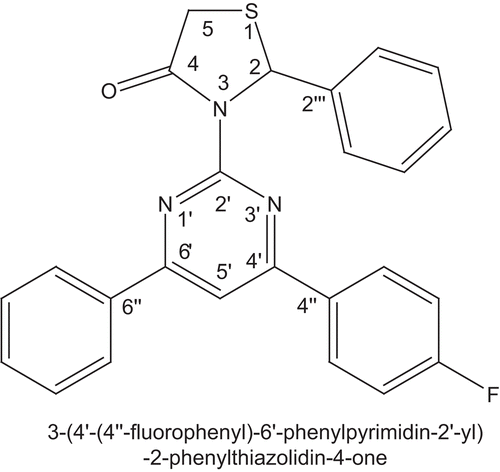
A new bis heterocycle comprising both bioactive 2-aminopyrimidine and thiazolidin-4-one nuclei namely 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one 3 was synthesized, characterized with the help of melting point, elemental analysis, FT-IR, MS, one-dimensional NMR (1H, 13C) spectra and we evaluated the chemopreventive potential of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one based on in vivo inhibitory effects on 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis. Administration of 3 effectively suppressed oral carcinogenesis initiated with DMBA as revealed by the reduced incidence of neoplasms. Lipid peroxidation, glutathione (GSH) content, and the activities of glutathione peroxidase (GPx), glutathione S-transferase (GST) were used to biomonitor the chemopreventive potential of 3. Lipid peroxidation was found to be significantly decreased, whereas GSH, GPx, GST, and GGT were elevated in the oral mucosa of tumor-bearing animals. Our data suggest that 3 may exert its chemopreventive effects in the oral mucosa by modulation of lipid peroxidation and enhancing the levels of GSH, GPx, and GST.
Introduction
In recent years, pyrimidine nucleus has attracted increase attention owing to their diverse biological activities [Citation1–5]. Substituted pyrimidines, particularly with an amino group at 2 or 4 positions, are known pharmacophores in several structure-based drug-design approaches in medicinal chemistry [Citation6–9]. For example, aryl-2-aminopyrimidines have been reported for the treatment of diseases modulated by the adenosine receptor. Drugs containing a pyrimidine with a hydroxy group such as tetrahydrofolate and 5-methyltetrahydrofolate possess high antioxidant activity [Citation10]. Biological importance of pyrimidines inspired us to report the synthesis, antibacterial and antifungal activity of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones and 4-(4-morpholinophenyl)-6-arylpyrimidin-2-amines [Citation11,Citation12]. Various 4-thiazolidinones have attracted considerable attention as they are endowed with wide range of pharmacological activities. A wide variety of biological properties such as hypolipidemic, antidegenerative, muscarinic receptor 1 agonist, antiproteolytic, anti-inflammatory, antiviral, antifungal, antibacterial, antitubercular, anticonvulsant, respiratory, and hypnotic activities have been reported for 4-thiazolidinones [Citation13–25].
Squamous cell carcinoma of the oral cavity is the sixth most common malignant neoplasm worldwide and constitutes 47% of all cancers in the Indian subcontinent [Citation26]. Despite advances in cancer detection and therapy, the mortality rate of oral cancer remains high and the 5-year survival rate is among the lowest of the major cancers [Citation27]. Patients with oral squamous cell carcinoma (OSCC) are susceptible to multiple primary and secondary tumors due to the phenomenon of “field cancerization” [Citation28,Citation29]. Furthermore, treatment of these tumors results in severe disfigurement and functional compromise.
Chemoprevention offers a novel approach to control the incidence of oral cancer. The buccal mucosa of the Syrian hamster is an excellent model for the investigation of oral cancer development and intervention by chemopreventive agents. Squamous cell carcinomas induced by the application of 7,12-dimethylbenz[a]anthracene (DMBA) to the cheek pouch of the Syrian hamsters are morphologically and histologically similar to human tumors [Citation30,Citation31]. In addition, hamster tumors express many metabolic and molecular markers that are expressed in human oral cancer [Citation32–34].
Several markers have been developed to biomonitor chemoprevention. These are based on the fact that chemopreventive agents can exert their anticarcinogenic effects by one or a combination of the following mechanisms: inhibiting formation of reactive carcinogenic metabolites, induction of enzymes that detoxify carcinogens, scavenging reactive oxygen species, influencing apoptosis, and inhibiting cell proliferation [Citation35,Citation36]. The tripeptide glutathione, a physiologically important nucleophile, and the enzymes glutathione peroxidase (GPx), glutathione S-transferase (GST), and gamma-glutamyltranspeptidase (GGT), which utilize reduced glutathione (GSH) as substrate, have assumed significance as biomarkers of chemoprevention owing to their antioxidant and detoxification properties [Citation37–39].
A large number of chemopreventive agents have been identified in epidemiological and experimental studies, preclinical systems, and clinical observations [Citation40,Citation41]. However, the toxic side effects produced by some of these agents have limited their extensive use [Citation42]. There is therefore a need to identify synthetic compounds that have significant chemopreventive potential. In view of our continued interest in the development of simpler and more convenient synthetic routes for achieving the biologically challenging hybrid heterocyclic systems [Citation43–51] and as part of our ongoing research program, we planned to design a system, which combines both bioactive 2-aminopyrimidine and thiazolidin-4-one nuclei together to give a compact structure like the title compound 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one 3, and we investigate the effects of 3 in a hamster buccal pouch (HBP) carcinogenesis model using lipid peroxidation, superoxide dismutase (SOD), catalase, GSH, GPx, and GST as biochemical endpoints of chemoprevention ().
Materials and methods
Chemicals
General remarks
We used thin-layer chromatography (TLC) to assess the reactions and the purity of the products. The reported melting point was taken in open capillaries and was uncorrected. IR spectra were recorded in KBr (pellet forms) on a Thermo Nicolet-Avatar-330 FT-IR spectrophotometer and noteworthy absorption values (per centimeter) alone were listed. One-dimensional 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, on Bruker AMX 400 NMR spectrometer using DMSO-d as solvent. The electron spray impact (ESI) positive (+ve) mass (MS) spectrum was recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer. All the chemicals were purchased from Fluka, Sigma-Aldrich Corp, St.Louis MO, USA, S.D.Fine Chemicals Ltd, 248, Worli Road, Mumbai, India and Spectrochem Pvt. Ltd, 221, Anand Bhuvan, Mumbai, India.&
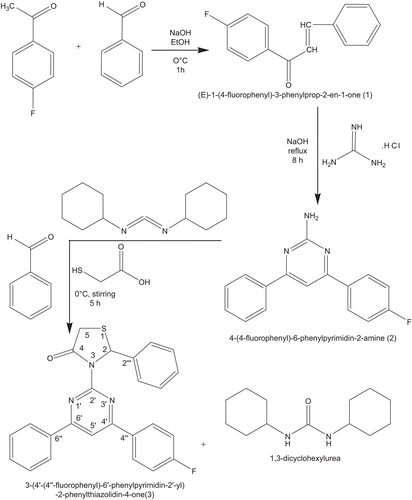
By adopting the literature precedent [Citation11] (E)-1-(4-fluorophenyl)-3-phenylprop-2-en-1-one 1 and 4-(4-fluorophenyl)-6-phenylpyrimidin-2-amine 2 were synthesized.
Typical method for the synthesis of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one 3
To an ice-cold stirred solution of 4-(4-fluorophenyl)-6-phenylpyrimidin-2-amine (0.01 mol) in dry dichloromethane was added benzaldehyde (0.012 mol) in drops followed by dicyclohexylcarbodiimide (DCC). After 5 min, thioglycolic acid (0.01 mol) was added and stirring was continued for additional 5 h at 0°C. Then the reaction mixture was filtered to remove dicyclohexyl urea followed by washing with 5% citric acid, 10% sodium bicarbonate, brine solution, finally with water and dried over anhydrous sodium sulfate. After evaporation of the solvent under reduced pressure, a gummy mass was obtained, which was solidified on treatment of petroleum ether (bp40–60). Final purification of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one was done by column chromatography using silica gel (100–200 mesh), with ethyl acetate–petroleum ether (bp40–60) in the ratio (2:8) as eluent (yield = 70%, melting point = 104°C).
Spectral data of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
IR (KBr) (per centimeter): 3071, 3027, 2928, 2852, 1712, 1626, 1575, 1352, 836, 769, 698; MS: m/z = 428, [M+1]+ Molecular formula C25H18FN3OS; elemental analysis: carbon 70.27cal (70.23found), hydrogen 4.21cal (4.18found), Nitrogen 9.79cal (9.83found); 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J = 15.24 Hz), 3.37 (d, 1H, CH2e at H5e, J = 15.28 Hz), 5.26 (s, 1H, CH at H2), 6.64–8.19 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 62.9 C-2, 108.9 C-5′, 127.3–143.1 Carom, 143.6 C-2′″, 145.1 C-4″, 146.1 C-6″, 166.8 C-4′, 167.0 C-6′, 163.9 C-2′, 171.4 C-4.
Animals
Male Syrian hamsters aged 6–10 weeks weighing between 90 and 110 g obtained from the Central Animal House, Annamalai University, India were used in this study. The animals were housed six in a polypropylene cage and provided with food and water ad libitum. The animals were maintained in a controlled environment under standard conditions of temperature and humidity with an alternating 12 h light:dark cycles. All animals were fed standard pellet diet (Mysore Snack Feed Ltd., Mysore, India).
Treatment schedule
The animals were randomized into experimental and control groups and divided into four groups of six animals each. Animals in group 1 were painted with a 0.5% solution of DMBA in liquid paraffin on the right buccal pouches using a number 4 brush three times per week for 14 weeks. Each application leaves ∼0.4 mg DMBA [Citation52]. Group 2 animals were painted with DMBA as in group 1. In addition, the animals were administered title compound, 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one, at a concentration of 1 mg/kg body weight (bw) orally three times per week on days alternate to DMBA application. Animals in group 3 received only 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one as in group 2. Group 4 (untreated control) animals received neither DMBA nor 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. The experiment was terminated at 14 weeks and all animals were sacrificed by cervical dislocation after an overnight fast. Fresh tissues were used for estimations.
Estimations
Thiobarbituric acid reactive substances (TBARS) released from endogenous lipid peroxides reflecting the lipid peroxidation process were assayed in tissues as described by Ohkawa et al. [Citation53]. The activity of SOD was assayed by the method of Oberley and Spitz [Citation54] based on the inhibition of the formation of NADPH phenazine methosulfate, nitroblue tetrazolium. Catalase was assayed colorimetrically by the method of Sinha [Citation55]. Reduced GSH was determined using the method of Beutler and Kelley [Citation56]. GPx activity was assayed by following the utilization of hydrogen peroxide according to the method of Rotruck et al. [Citation57]. The activity of GST was assayed using the method of Habig et al. [Citation58] using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. Tissue protein was estimated by the method of Lowry et al. [Citation59] using bovine serum albumin as the standard.
Statistical analysis
The data are expressed as mean ± standard deviation (SD). Statistical analysis on the data for bws and tumor burden was carried out using Student’s t-test. Tumor incidence was statistically compared using χ2-test. Statistical analysis on the data for biochemical assays was analyzed using analysis of variance (ANOVA) and the group means were compared by the least significant difference test (LSD). The results were considered statistically significant if the P < 0.05.
Results and discussion
Chemistry
Initial classical synthesis for the conversion of 4-(4-fluorophenyl)-6-phenylpyrimidin-2-amine 2 to 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one 3 is effected in the absence of DCC. No yield was achieved. Instead, if DCC was used as a dehydrating agent, the yield of the product has been improved significantly (i.e. about 70%) in stirring mode at about 0°C. The Claisen-Schmidt condensation of equimolar quantities of various p-fluoroacetophenone with benzaldehyde in the presence of sodium hydroxide base as a catalyst yields (E)-1-(4-fluorophenyl)-3-phenylprop-2-en-1-one. When (E)-1-(4-fluorophenyl)-3-phenylprop-2-en-1-one is treated with guanidine nitrate in the presence of potassium hydroxide alkali in refluxing ethanol for 8 h gives 4-(4-fluorophenyl)-6-phenylpyrimidin-2-amine. Novel title compound, 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one 3, is synthesized by the addition of benzaldehyde, followed by thioglycolic acid to 4-(4-fluorophenyl)-6-phenylpyrimidin-2-amine in dry dichloromethane at 0°C catalyzed by DCC. The schematic synthetic route representation of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one is given in The importance of the title compound is due to their diverse potential, broad-spectrum biological activity. The structure of the newly synthesized 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one is confirmed by melting point, elemental analysis, MS, FT-IR, one-dimensional NMR (1H and 13C) spectroscopic data.
Biology
The bw, tumor incidence, tumor multiplicity and tumor burden, and incidence of preneoplastic and neoplastic lesions in control and experimental animals are shown in . The mean final bws were significantly decreased in group 1 compared with control (group 4). No significant differences in the bws were observed in groups 2 and 3. In DMBA-painted animals (group 1), the incidence of SCC was 100% with a tumor multiplicity of 1.17 per hamster. These tumors were large and exophytic with a mean tumor burden of 208.00 mm3. In group 2, only one animal developed SCC, whereas others exhibited moderate to severe dysplasia without infiltration. Although no tumors were observed in group 3, histopathological examination of pouches revealed varying degrees of hyperplasia, hyperkeratosis, and dysplasia. While administration of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one (1 and 10 mg/kg bw) decreased tumor incidence as well as preneoplastic lesions, the inhibitory effect was more pronounced at 10 mg/kg bw of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. In group 4, the epithelium was normal, intact, and continuous.
Table 1. Body weight, tumor incidence, tumor multiplicity and tumor burden, and incidence of preneoplastic and neoplastic lesions in control and experimental animals (mean ± SD; n = 6).
The levels of TBARS and the activities of SOD, catalase, GPx, and GST in the buccal pouches of control and experimental animals are shown in . The changes in the levels of TBARS and GSH and the activities of GPx and GST were significantly increased with decrease in SOD and catalase activities in DMBA-painted animals (group 1) compared with control (group 4). Administration of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one significantly enhanced the lipid peroxidation and the activities of antioxidants in the buccal pouches of groups 2 and 3 animals compared with group 1. However, the antioxidant-enhancing effects were more significant in group 3 (10 mg/kg bw) compared with other groups. DMBA, an aryl hydrocarbon, is a potent carcinogen that induces significant histological and biochemical changes during HBP carcinogenesis. In the present study, topical application of DMBA to the cheek pouch for 14 weeks resulted in well-differentiated SCCs with very high tumor burden.
Table 2. The cellular redox status in the buccal pouches of experimental and control animals (mean ± SD; n = 10).
In DMBA-induced HBP tumors, low lipid was associated with enhanced activities of GSH redox cycle antioxidants. Lipid peroxidation, which prolongs the G1 phase of the cell cycle, has been suggested to control cell division. Das have demonstrated that tumor cells are more resistant to lipid peroxidation than normal cells [Citation60]. Several studies have documented decreased lipid peroxidation in rapidly proliferating tumors compared with their normal counterparts [Citation60,Citation61]. The thiol antioxidant GSH, which provides cellular protection against ROS in conjunction with GPx, γ-glutamyl tranferase (GGT), and glutathione reductase (GR), is recognized to play a key role in regulating cell proliferation [Citation62]. Overexpression of GSH- and GSH-dependent enzymes has been reported in a wide range of tumors including OSCC [Citation63]. The elevated GSH redox cycle antioxidants may serve to maintain a reduced environment providing a selective growth advantage to HBP tumors. Our results are in agreement with the hypothesis of Slater et al. [Citation64] that an increase in antioxidant capacity is associated with a decrease in lipid peroxidation. In contrast to GSH-dependent antioxidants, the activities of the antioxidant enzymes SOD and CAT were decreased in the HBP tumors. An increase in the activities of GPx with decreased SOD and CAT has been reported in various tumors, including the hamster cheek pouch carcinoma cell line HCPC-1 [Citation65–67]. Reduced activities of SOD and CAT reported in fast-growing tumor tissues can cause accumulation of superoxide anion (02•−) and H2O2 with deleterious consequences including oxidation of critical sulfhydryl groups and conformational changes in functional proteins as well as DNA strand breaks leading to oxidative stress. Oxidative stress has been implicated in cancer progression [Citation68].
Administration of 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one at two different doses (1 and 10 mg/kg bw) significantly suppressed tumor incidence, multiplicity, and tumor burden in the HBP by modulating oxidant–antioxidant status. The results of the present study substantiate the anticarcinogenic effects of bioactive 2-aminopyrimidines and thiazolidinones derivatives reported in literature [Citation4,Citation25,Citation69]. Administration of synthetic compounds reversed the susceptibility to lipid peroxidation while simultaneously increasing the antioxidant status in the buccal pouch. These findings support reports by us and other workers that chemopreventive agents exert an “electrophilic counterattack response” characterized by the elevation of antioxidant enzymes [Citation70]. Among these two different doses used in the present study, the maximum dose 10 mg/kg bw was more effective in chemoprevention. We have demonstrated that 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one suppresses DMBA-induced oral neoplasms as revealed by the reduced incidence of carcinomas. The present preliminary study suggests that 3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one has potential anticarcinogenic properties in experimental animals and are promising candidates for chemoprevention. Further development of this group of pyrimidino thiazolidine hybrid heterocycles, which may lead to compounds with better pharmacological profiles, is under progress.
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. One of the authors namely V. Kanagarajan is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry.
Declaration of interest
The authors report no conflicts of Interest.
References
- Rindhe, S.S., Mandhare, P.N., Patil, L.R., Mane, R.A. Synthesis and antifungal activity of 2-amino-6-substituted thiazolyl-pyrimidines. Indian J. Heterocycl. Chem. 2005, 15, 133–136.
- Siddiqui, A.A., Rajesh, R., Mojahid-Ul-Islam, Alagarsamy, V., Meyyanathan, S.N., Kumar, B.P., Suresh, B. Synthesis, antiviral, antituberculostic and antibacterial activities of some novel, 4-(4′-substituted phenyl)-6-(4″-hydroxyphenyl)-2-(substituted imino) pyrimidines. Acta Pol. Pharm. 2007, 64, 17–26.
- Patil, L.R., Mandhare, P.N., Bondge, S.P., Munde, S.B., Mane, R.A. Synthesis and antimicrobial activity of new pyrimidine incorporated 1,3,4-thiadiazoles. Indian J. Heterocycl. Chem. 2003, 12, 245–248.
- Moragues, J., Prieto, J., Spickett, R.G., Vega, A., Salazar, W., Roberts, D.J. Dopaminergic activity in a series of N-substituted 2-aminopyrimidines. Farmaco Sci. 1980, 35, 951–964.
- Hughes, T.V., Emanuel, S.L., Beck, A.K., Wetter, S.K., Connolly, P.J., Karnachi, P., Reuman, M., Seraj, J., Fuentes-Pesquera, A.R., Gruninger, R.H., Middleton, S.A., Lin, R., Davis, J.M., Moffat, D.F. 4-Aryl-5-cyano-2-aminopyrimidines as VEGF-R2 inhibitors: synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2007, 17, 3266–3270.
- Boudet, N., Knochel, P. Chemo- and regioselective functionalization of uracil derivatives. Applications to the synthesis of oxypurinol and emivirine. Org. Lett. 2006, 8, 3737–3740.
- Baraldi, P.G., Bovero, A., Fruttarolo, F., Romagnoli, R., Tabrizi, M.A., Preti, D., Varani, K., Borea, P.A., Moorman, A.R. New strategies for the synthesis of A3 adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169.
- Ferrero, M., Gotor, V. Biocatalytic selective modifications of conventional nucleosides, carbocyclic nucleosides, and C-nucleosides. Chem. Rev. 2000, 100, 4319–4348.
- Balzarini, J., Naesens, L., De Clercq, E. New antivirals—mechanism of action and resistance development. Curr. Opin. Microbiol. 1998, 1, 535–546.
- Rezk, B.M., Haenen, G.R., van der Vijgh, W.J., Bast, A. Tetrahydrofolate and 5-methyltetrahydrofolate are folates with high antioxidant activity. Identification of the antioxidant pharmacophore. FEBS Lett. 2003, 555, 601–605.
- Gopalakrishnan, M., Thanusu, J., Kanagarajan, V. Design, synthesis, spectral analysis and in vitro microbiological evaluation of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones. J. Enz. Inhib. Med. Chem. 2009, 24, 1088–1094.
- Gopalakrishnan, M., Thanusu, J., Kanagarajan, V. 4-(4-morpholinophenyl)-6-arylpyrimidin-2-amines—Synthesis, spectral analysis and in vitro microbiological evaluation. J. Enz. Inhib. Med. Chem. 2010, 25, 347–353.
- Andres, C.J., Bronson, J.J., D’Andrea, S.V., Deshpande, M.S., Falk, P.J., Grant-Young, K.A., Harte, W.E., Ho, H.T., Misco, P.F., Robertson, J.G., Stock, D., Sun, Y., Walsh, A.W. 4-Thiazolidinones: novel inhibitors of the bacterial enzyme MurB. Bioorg. Med. Chem. Lett. 2000, 10, 715–717.
- Nampurath, G.K., Mathew, S.P., Khanna, V., Zachariah, R.T., Kanji, S., Chamallamudi, M.R. Assessment of hypolipidaemic activity of three thiazolidin-4-ones in mice given high-fat diet and fructose. Chem. Biol. Interact. 2008, 171, 363–368.
- Ottanà, R., Maccari, R., Ciurleo, R., Vigorita, M.G., Panico, A.M., Cardile, V., Garufi, F., Ronsisvalle, S. Synthesis and in vitro evaluation of 5-arylidene-3-hydroxyalkyl-2-phenylimino-4-thiazolidinones with antidegenerative activity on human chondrocyte cultures. Bioorg. Med. Chem. 2007, 15, 7618–7625.
- Chandra, J.N., Malviya, M., Sadashiva, C.T., Subhash, M.N., Rangappa, K.S. Effect of novel arecoline thiazolidinones as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Neurochem. Int. 2008, 52, 376–383.
- Kishore, V., Narain, N.K., Kumar, S., Parmar, S.S. Relationship between antiinflammatory and antiproteolytic properties of substituted oxothiazolylacetic acids. Pharmacol. Res. Commun. 1976, 8, 43–51.
- Newbould, B.B. Suppression of adjuvant-induced arthritis in rats with 2-butoxycarbonylmethylene-4-oxothiazolidine. Br. J. Pharmacol. Chemother. 1965, 24, 632–640.
- Schauer, P., Likar, M., Tisler, M., Krbavcic, A., Pollak, A. Studies of some substances with antiviral activity. II. 2,4-Dioxo-5-thiazolidine acetic acid derivatives (DFT) as an inhibitor of growth of herpes virus and poliovirus type i in cell cultures of human embryonic kidneys. Pathol. Microbiol. (Basel) 1965, 28, 382–387.
- Choubey, V.N., Singh, H. Synthesis of some new fungicides. Bull. Chem. Soc. Jpn. 1970, 43, 2233–2236.
- Akerblom, E.B. Synthesis and structure–activity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2-furylpropenylidene) thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J. Med. Chem. 1974, 17, 609–615.
- Litvinchuk, M.D. Antitubercular activity with low toxicity associated with a few derivatives of 2-imino-4-thiazolidinones. Farmakol. Toksikol. 1963, 26, 725–728.
- Dimri, A.K., Parmar, S.S. Synthesis of 3-aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anticonvulsants. J. Heterocycl. Chem. 1978, 15, 335–336.
- Parmar, S.S., Dwivedi, C., Chaudhari, A., Gupta, T.K. Substituted thiazolidones and their selective inhibition of nicotinamide-adenine dinucleotide dependent oxidations. J. Med. Chem. 1972, 15, 99–101.
- Singh, S.P., Parmar, S.S., Krishna Raman, Stenberg, V.I. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981, 81, 175–203.
- Johnson, N.W. Epidemiology of oral cancer in risk markers of oral disease. In: Johnson, N.W. (Ed.), Oral Cancer, Vol. 2. Cambridge University Press, Cambridge, 1991, pp. 3–26.
- Boring, C.C., Squires, T.S., Tong, T. Cancer statistics, 1992. CA. Cancer J. Clin. 1992, 42, 19–38.
- Slaughter, D.P., Southwick, H.W., Smejkal, W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 1953, 6, 963–968.
- Licciardello, J.T., Spitz, M.R., Hong, W.K. Multiple primary cancer in patients with cancer of the head and neck: second cancer of the head and neck, esophagus, and lung. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 467–476.
- Gimenez-Conti, I.B., Slaga, T.J. The hamster cheek pouch model of carcinogenesis and chemoprevention. Adv. Exp. Med. Biol. 1992, 320, 63–67.
- Gimenez-Conti, I.B., Slaga, T.J. The hamster cheek pouch carcinogenesis model. J. Cell. Biochem. Suppl. 1993, 17F, 83–90.
- Solt, D.B., Shklar, G. Rapid induction of gamma-glutamyl transpeptidase-rich intraepithelial clones in 7,12-dimethylbenz(a)anthracene-treated hamster buccal pouch. Cancer Res. 1982, 42, 285–291.
- Kwong, Y.Y., Husain, Z., Biswas, D.K. c-Ha-ras gene mutation and activation precede pathological changes in DMBA-induced in vivo carcinogenesis. Oncogene 1992, 7, 1481–1489.
- Chang, K.W., Lin, S.C., Koos, S., Pather, K., Solt, D. p53 and Ha-ras mutations in chemically induced hamster buccal pouch carcinomas. Carcinogenesis 1996, 17, 595–600.
- Wattenberg, L.W. Chemoprevention of cancer. Cancer Res. 1985, 45, 1–8.
- Sharma, S., Stutzman, J.D., Kelloff, G.J., Steele, V.E. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. 1994, 54, 5848–5855.
- Anderson, M.E., Meister, A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc. Natl. Acad. Sci. U.S.A. 1983, 80, 707–711.
- Ketterer, B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat. Res. 1988, 202, 343–361.
- Hayes, J.D., Pulford, D.J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600.
- Boone, C.W., Kelloff, G.J., Malone, W.E. Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: a review. Cancer Res. 1990, 50, 2–9.
- Lippman, S.M., Benner, S.E., Hong, W.K. Cancer chemoprevention. J. Clin. Oncol. 1994, 12, 851–873.
- Ito, N., Fukushima, S., Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150.
- Gopalakrishnan, M., Kanagarajan, V., Thanusu, J. Novel one-pot synthesis of 1,2,4-triazolidin-3-thiones comprising piperidine moiety. Green. Chem. Lett. Rev. 2008, 1, 241–246.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J., Govindaraju, R. A simplified green chemistry approaches to organic synthesis in solid media activated fly ash, an industrial waste (pollutant) as an efficient and novel catalyst for some selected organic reactions in solvent-free conditions under microwave irradiation. ARKIVOC 2006, 13, 130–141.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J., Govindaraju, R., Ezhilarasi, M.R. Microwave-promoted facile and rapid solvent-free procedure for the efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones using ZrO2/SO42− as a reusable heterogeneous catalyst. Lett. Org. Chem. 2006, 3, 484–488.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J. Aluminium metal powder (atomized) catalyzed Friedel–Crafts acylation in solvent-free conditions: a facile and rapid synthesis of aryl ketones under microwave irradiation. Catalysis Commun. 2005, 6, 753–756.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J., Govindaraju, R. Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. J. Chem. Res. 2005, 5, 299–303.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J. Organic synthesis in solid media alumina supported sodium hydrogen sulfate as an effective and reusable catalyst for ‘one-pot’ synthesis of amides from ketones in dry media under microwave irradiation. Lett. Org. Chem. 2005, 2, 444–446.
- Gopalakrishnan, M., Sureshkumar, P., Kanagarajan, V., Thanusu, J. Design, ‘one-pot’ synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J. Sulfur Chem. 2007, 8, 383–392.
- Gopalakrishnan, M., Sureshkumar, P., Thanusu, J., Kanagarajan, V., Govindaraju, R., Jayasri, G. A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J. Enz. Inhib. Med. Chem. 2007, 22, 709–715.
- Gopalakrishnan, M., Thanusu, J., Kanagarajan, V. Heterogeneous NaHSO4.SiO2 catalyzed ‘one-pot’ synthesis and in vitro antibacterial and antifungal activities of pyridino-1,2,3-thiadiazoles. J. Sulfur Chem. 2008, 29, 179–185.
- Shklar, G. Experimental oral pathology in the Syrian hamster. Prog. Exp. Tumor Res. 1972, 16, 518–538.
- Ohkawa, H., Ohishi, N., Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358.
- Oberley, L.W., Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Method Enzymol. 1984, 105, 457–464.
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394.
- Beutler, E., Kelly, B.M. The effect of sodium nitrite on red cell GSH. Experientia 1963, 19, 96–97.
- Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G., Hoekstra, W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590.
- Habig, W.H., Pabst, M.J., Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275.
- Das, U.N. A radical approach to cancer. Med. Sci. Monit. 2002, 8, 79.
- Subapriya, R., Bhuvaneswari, V., Ramesh, V., Nagini, S. Ethanolic leaf extract of neem (Azadirachta indica) inhibits buccal pouch carcinogenesis in hamsters. Cell Biochem. Funct. 2005, 23, 229–238.
- Kohno, Y., Patel, V., Kim, Y., Tsuji, T., Chin, B.R., Sun, M., Bruce Donoff, R., Kent, R., Wong, D., Todd, R. Oncogene—P12CDK2-AP1 mediates DNA damage responses induced by DMBA. Oral Oncol. 2002, 38, 274–280.
- Abou Ghalia, A.H., Fouad, I.M. Glutathione and its metabolizing enzymes in patients with different benign and malignant diseases. Clin. Biochem. 2000, 33, 657–662.
- Slater, T.F., Benedetto, C., Burton, G.W., Cheeseman, K.H., Ingold, K.U., Nodes, J.T. Lipid peroxidation in animal tumours: a disturbance in the control of cell division. In: Thaler-Dao, H., Paoletti, R., Crastes de Paulet, A. (Eds.), Icosanoids and Cancer. Raven Press, New York, 1984, pp. 21–29.
- Chandra Mohan, K.V.P., Nagini, S. Combination chemoprevention by tomato and garlic in the hamster buccal pouch carcinogenesis model. Nutr. Res. 2003, 23, 1403–1406.
- Moghadasian, M.H., Freeman, H.J., Godin, D.V. Endogenous antioxidant status in neoplastic and adjacent tissues in 1,2-dimethylhydrazine-induced colon cancer in rats: effects of olsalazine. Carcinogenesis 1996, 17, 983–987.
- Lam, E.W., Zwacka, R., Seftor, E.A., Nieva, D.R., Davidson, B.L., Engelhardt, J.F., Hendrix, M.J., Oberley, L.W. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic. Biol. Med. 1999, 27, 572–579.
- Cheng, S.C., Prakash, A.S., Pigott, M.A., Hilton, B.D., Lee, H., Harvey, R.G., Dipple, A. A metabolite of the carcinogen 7,12-dimethylbenz[a]anthracene that reacts predominantly with adenine residues in DNA. Carcinogenesis 1988, 9, 1721–1723.
- Sinhababu, A.K., Thakker, D.R. Prodrugs of anticancer agents. Adv. Drug Delivery. Rev. 1996, 19, 241–273.
- Prestera, T., Zhang, Y., Spencer, S.R., Wilczak, C.A., Talalay, P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv. Enzyme Regul. 1993, 33, 281–296.