Abstract
Medicinal plants are becoming an important research area for novel and bioactive molecules for drug discovery. Novel therapeutic strategies and agents are urgently needed to treat different incurable diseases. Many plant derived active compounds are in human clinical trials. Currently ursolic acid is in human clinical trial for treating cancer, tumor, and skin wrinkles. This review includes the clinical use of ursolic acid in various diseases including anticancer, antitumor, and antiwrinkle chemotherapies, and the isolation and purification of this tritepernoid from various plants to update current knowledge on the rapid analysis of ursolic acid by using analytical methods. In addition, the chemical modifications of ursolic acid to make more effective and water soluble derivatives, previous and current information regarding, its natural and semisynthetic analogs, focusing on its anticancer, cytotoxic, antitumor, antioxidant, anti-inflammatory, anti-HIV, acetyl cholinesterase, α-glucosidase, antimicrobial, and hepatoprotective activities, briefly discussion is attempted here for its research perspectives. This review article contains fourteen medicinally important ursolic acid derivatives and 351 references.
1.Introduction
In the context of our previous communication on bioactivities of oleanolic acid and related derivatives,Citation1 now we report the bioactivities of ursolic acid (1) and related derivatives of ursolic acid along with other triterpenoidal compounds like, ursolic acid glycosides and few sugars were isolated from Eriobotrya japonica.Citation2 Ursolic acid is of interest to scientists in the area of oncology because of its cytotoxicity, induction of differentiation, antimutagenic, antiviral, and anti-invasive activities. It may occur in its free acid form, as shown in 1, or as aglycones for triterpenoid saponins which are comprised of a triterpenoid aglycone linked to one or more sugar moieties.
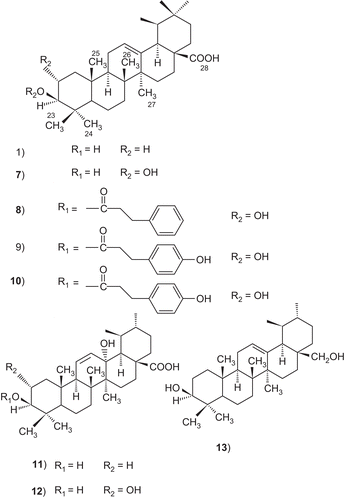
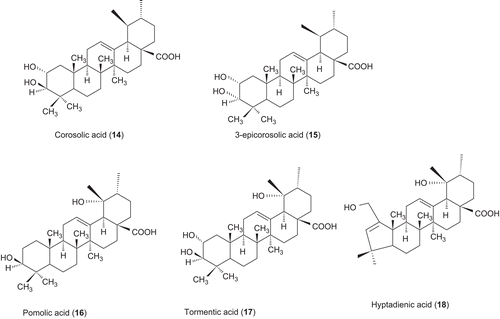
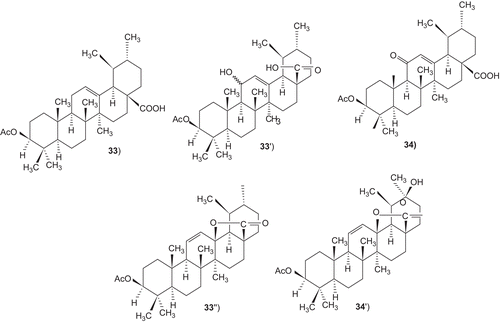
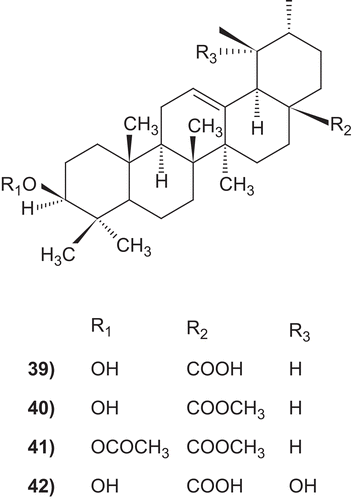
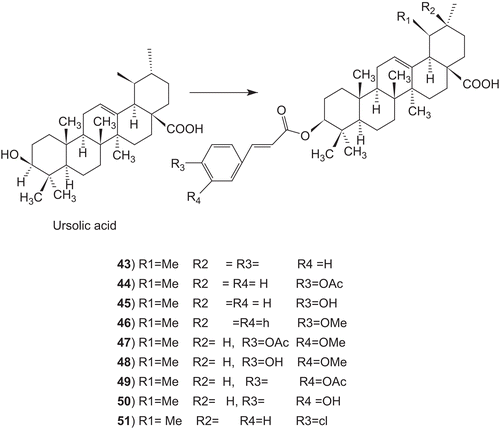
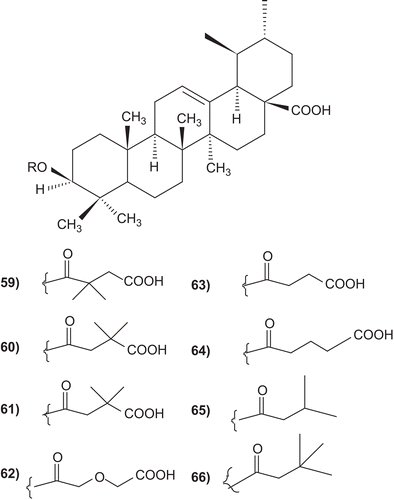
A number of ursolic acid derivatives showed high potential anticancer activities against three human cancer cell lines. 2α-hydroxyursolic acid (7–13), showed higher antiproliferative activity toward HepG2 cancer cells. Four ursolic type triterpene (14–17), showed a marked anti-inflammatory effect on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. Ursolic acid and their lipophilic 3-O-fatty acid ester chains (C12–C18), were evaluated for their antimicrobial activity against a series of Gram-positive and Gram-negative bacteria. Significantly these compounds (29–31) were found to exhibit potent activity against Gram-negative bacteria Pseudomonas syringae. Ursolic acid acetate (33) and 11,12-dehydroursolic lactone, 3-O-acetyl-9,11-dehydro-12α–hydroxyoleanolic lactone (33″), were found to exhibit potent activity against α-glucosidase. The presence of free hydroxy or carboxy groups is necessary for the trypanocidal activity of ursolic acid as could be deduced from the effect of the acetates (39), methyl ester (40), and aldehyde derivatives. Ursolic acid has been modified at the C-3 position to cinnamate-based esters (43–51). The results indicated that modification of the parent structures of ursolic acid to the p-coumarate and the ferulate ester analogues resulted in high antimycobacterial activity.
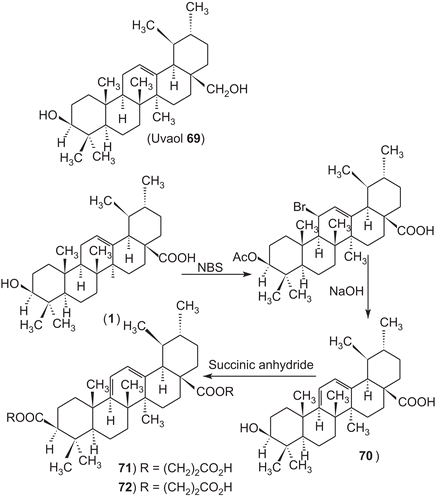
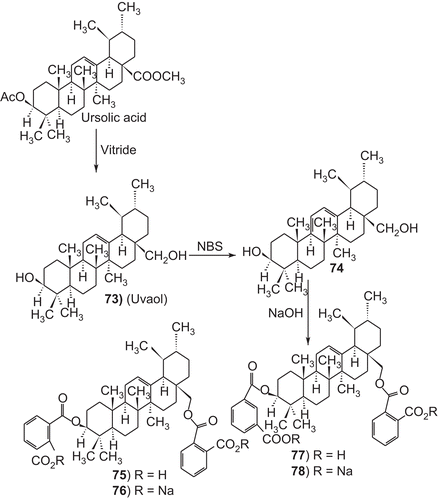
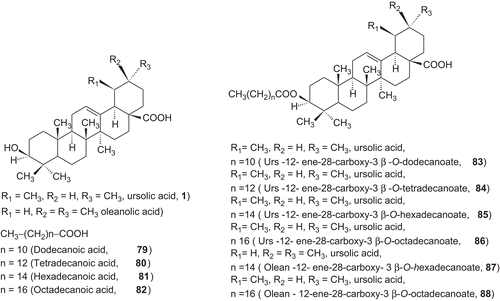
Several 3-O-acyl-ursolic acids were prepared and evaluated for anti-HIV activity. 3-O-Diglycoryl-ursolic acid (62, ) demonstrated relatively potent anti-HIV activity. 3-O-(3′, 3′-dimethylsuccinyl) ursolic acid (60), displayed only weak anti-HIV activity. The ursolic acid derivatives, dihemisuccinate sodium salt 72 demonstrated a good separation between anti-ulcer and mineralocorticoid activities. In particular, 75 and 76 (, ), showed a potent anti-ulcer activity, 3- to 25-fold higher than carbenoxolone. The 3-O-fatty acid ester derivatives of ursolic acid (79–84 ), Urs-12-ene-28-carboxy-3β-octadecanoate and olean-12-ene-28-carboxy-3β-hexadecanoate were found to exhibit exceptionally potent antifeedant activities. These derivatives have attracted considerable interest due to there biological activity. Only few publications in the compiled form have appeared on the activities of ursolic acid and its derivatives.Citation3–11
2.Isolation of ursolic acid from different plant sources
Isolation of ursolic acid from various plants by different workers has been known. Its isolation from Catharanthus trichophyllus roots,Citation12 Chamobates pusillus,Citation13 has been reported. Keller auf dem et al., isolated ursolic acid as content of Rhododendron ponticum.Citation14 Presence of ursolic acid has been reported in Retanilla ephedra,Citation15 Cestrum diurnum Linn,Citation16 in the blossoms of Viburnum opulus l. var. sterile dc (Caprifoliaceae),Citation17 and Viburnum latana.Citation18 The presence of ursolic acid in the leaves of Funtumia latifolia Stapf, has been reported by Takahashi.Citation19 Incidence of ursolic acid in the leaves of Pyrus communis (L.),Citation20 has been reported. The leaves of little periwinkle and Vinca minor, revealed the presence of ursolic acid.Citation21 Isolation of ursolic acid from leaves of Ilex aquifolium L. I. has been reported by Schindler et al.Citation22 Chemical investigation of bark, peel, and leaf of Punica granatum (L.) also revealed the presence of ursolic acid. The other plants contained ursolic acid were Coussarea paniculata,Citation23 Salvia sochifolia,Citation24 Gentiana tizuensis,Citation25 Alysicarpus monolifer,Citation26 Ilex psammophila,Citation27 Debregeasia salicifolia,Citation28 Uncaria macrophylla,Citation29 Rabdosia excise,Citation30 Schizonepeta tenuifolia Briq,Citation31 Eriobotrya japonica,Citation32 Synurus deltoids,Citation33 Origanum majorana (L.),Citation34 Viscum album (L.),Citation35 Punica granatum (L.),Citation36 Echites hirsuta (Apocynaceae),Citation37 Rabdosia japonica var. glaucocalyx,Citation38 Arbutus unedo,Citation39 Paederia scandense,Citation40 Potentilla chinensis,Citation41 Elaeagnus bockii,Citation42 Schizonepeta tenuifolia,Citation43 Mentha haplocalyx,Citation44 Chirita longgangensis var. hongyao,Citation45 Launaea nudicaulis,Citation46 Rhaponticum uniflorum,Citation47 Rabdosia japonica var. galaucocalyx,Citation48 Ludwigia octovalvis,Citation49 Mallotus apelta Muell,Citation50 Salacia hainanensis,Citation51 Rubus chingi I,Citation52 Incarvillea arguta,Citation53 Tadehagi triquetrum,Citation54 Forsythia viridissima flowers,Citation55 Calophyllum polyanthum,Citation56 Madagascar rain forest,Citation57 Wendlandia formosana,Citation58 Ixora coccinea,Citation59 Lasianthus gardneri,Citation60 Couepia ulei,Citation61 Ilex kudingcha,Citation62 Dalbergia hainanensis,Citation63 Schisandra sphaerandra,Citation64 Psidium guajava,Citation65 Potentilla multifida (L.),Citation66 Ludwigia octovalvis,Citation67 Prunus serrulata var.,Citation68 Salvia przewalskii Maxim,Citation69 Hyssopus officinalis,Citation70 Eucalyptus globulusCitation71 Gaultheria leucocarpa var.,Citation72 Callicarpa bodinieri Levl.,Citation73 Alyxia sinensis,Citation74 Eucommia ulmoides,Citation75 Liquidambaris-lulutong,Citation76 Rabdosia japonica (Burm.f). Hara var. glaucocalyx (Maxin) Hara,Citation77 Acanthopanax sessiliflorus,Citation78 Ilex hainanensis,Citation79 Acanthopanax sessiliflorus,Citation80 Pyrrosia gralla,Citation81 Syzygium buxifolium Hook,Citation82 Eclipta alba,Citation83 Campylotropis hirtella (Franch. Schindl.),Citation84 Isodon oresbius,Citation85 Ilex latifolia,Citation86 Eucalyptus camaldulensis var. obtuse,Citation87 Lonicera angustifolia,Citation88 Isodon phyllostachys,Citation89 Syzygium formosanum,Citation90 Baccharis gaudichaudiana,Citation91 Fraxinus ornus (L.),Citation92 Leucoseptrum stellipillum,Citation93 Osbeckia chinensis (L.),Citation94 Hedyotis chrysotricha (Palib.) Merr.,Citation95 Actinidia arguta (Sieb. et Zucc.) Planch. ex Miquel,Citation96 Lichen Ramalina hierrensis.,Citation97 Isodon albopilosus (C.Y. Wu et H.W. Li) Hara,Citation98 Patrinia scabiosaefolia Fischer.,Citation99 Salvia wagneriana,Citation100 Cynomorium songaricum,Citation101 Lantana camara,Citation102 Punica granatum,Citation103 Ilex paraguariensis,Citation104 Aralia decaisneana,Citation105 Fagonia arabica,Citation106 almond hulls,Citation107 Mentha haplocalyx,Citation108 Fagonia glutinosa,Citation109 Potentilla chinesis,Citation110 Origanum majorana (L.),Citation111 radix Ranunculus ternati,Citation112 Liriope platyphylla,Citation113 Pyrola calliatha,Citation114 Actinidia macrosperma,Citation115 Urtica dioica (L.),Citation116 Sambucus adnata,Citation117 Patrinia villosa,Citation118 Eriophyton wallichii,Citation119 Calligonum polygonoides,Citation120 Psidium guajava,Citation121 Paulownia fortunei (seem.) Hemsl,Citation122 Vaccinium iteophyllum,Citation123 Homonoia riparia,Citation124 Uncaria macrophylla,Citation125 Palicourea coriacea (Rubiaceae),Citation126 Carapa guianensis Aubl. (Meliaceae),Citation109 Potentilla chinesis,Citation127 Isodon oresbius,Citation128 Crataegus pinnatifida Bge. var. major N. E. Br,Citation129 Satureja khuzistanica.,Citation130 Perovskia abrotanoides,Citation131 Sambucus chinensis Lindl,Citation131 Leucoseptrum stellipillum,Citation92 Ajuga postii,Citation132 Morus nigra,Citation133 Valeriana officinalis,Citation134 Chondrilla piptocoma,Citation135 Rhodobryum roseum,Citation136 Swertia speciosaCitation137,Citation138 and Mitragyna speciosa.Citation139
From the stem bark extract of Amanoa oblongifolia a number of non-cytotoxic isolates were found, that comprised the lignans, (+)-sesamin, and paulownin, friedelin, canophyllol, betulinic acid; and the sterols, β-sitosterol, daucosterol, and ursolic acid.Citation140
3.Clinical applications of ursolic acid
3.1.Ursolic acid-induced changes in tumor growth, O2 consumption, and tumor interstitial fluid pressure
The antitumor effect of ursolic acid and ursolic acid-induced changes in tumor physiology in tumor-bearing mice were examined. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) colorimetric assay, clonogenic assay, and growth-delay assay for the determination of tumoricidal effects of ursolic acid were evaluated.Citation141 See supplementary material 3.1.
3.2.Clinical applications of ursolic acid for treating tumor patients
Ursolic acid is a promising antitumor agent. It is capable of inducing apoptosis in tumor cells on one side and to prevent malignant transformation of normal cells on the other side. It also interferes with numerous enzymes, including the ones serving directly to DNA synthesis. It can inhibit proliferation and induce apoptosis of many tumor cell lines. This compound have been shown to act at various stages of tumor development. They effectively inhibit angiogenesis, invasion of tumor cells and metastasis. It is relatively non-toxic and could be use as chemopreventive/chemoprotective agent in clinical praxis.Citation142
Ursolic acid had been isolated from Glechoma hederacea as inhibitors of Epstein–Barr virus activation induced by TPA, were tested against inhibitory effect on tumor promotion by TPA in vivo.Citation143
3.3.The treatment of wrinkles with ursolic acid in clinical trials (liposomal ursolic acid (Merotaine) increases ceramides and collagen in human skin)
Ursolic acid liposomes increased the ceramide content of the skin of human subjects, with increases in hydroxy ceramides occurring after only 3 days of treatment. Both ursolic acid liposomes and retinoic acid decreased markers of keratinocyte differentiation (keratin 1, keratin 10, and involucrin) in cultured normal human epidermal keratinocytes.Citation144
Skin wrinkling and xerosis associated with aging result from decreases of dermal collagen and stratum corneum ceramide content. Ursolic acid incorporated into liposomes increases both the ceramide content of cultured normal human epidermal keratinocytes and the collagen content of cultured normal human dermal fibroblasts. In clinical tests, Merotaine increased the ceramide content in human skin over an 11-day period. Merotaine has effects on keratinocyte differentiation and dermal fibroblast collagen synthesis similar to retinoids. However, unlike retinoids, Merotaine increases ceramide content of human keratinocytes. Ursolic acid may bind to members of the glucocorticoid receptor family to initiate changes in keratinocyte gene transcription. As ursolic acid represents a promising chemical entity for the protection of human skin, in agreement with tests done by the cosmetic industry.Citation145,Citation146
4.Structure similarity of ursolic acid
4.1.Induction of apoptosis in HeLa cells by 3β-hydroxyurs-12-en-27-oic acid
3-β-Hydroxy urs-12-en-27-oic acid (4), was structurally very similar to ursolic acid, with the only difference being the interchange of the COOH and Me group at C-14 and C-17.
3β-Hydroxyurs-12-en-27-oic acid was found to exhibit more distinctive cytotoxicity toward human cervical squamous carcinoma cells than ursolic acid, suggesting that the position of the COOH group significantly affects the cytotoxicity of ursane-type pentacyclic triterpenes with a COOH group.Citation142 See supplementary material 4.1.
4.2.Antiproliferative and antiviral mechanisms of ursolic acid and dexamethasone in cervical carcinoma cell lines
The structure of ursolic acid is very similar to that of dexamethasone (5). Antiproliferative and antiviral effects of ursolic acid and dexamethasone in human papillomavirus associated cervical cancer cells was determined. MTTs assay was performed to measure antiproliferative activity and also characterized apoptosis by DNA fragmentation, 4′-6-diamidino-2-phenylindole staining, and flow cytometry analysis.Citation147
5.Rapid analysis of ursolic acid by using different methods
5.1.Study on the extracting of ursolic acid by uniform design
Uniform design method was adopted to research the extraction of ursolic acid. The ethanol concentration, the liquid to solid ratio, and the extraction times were researched. The optimal conditions were achieved by optimization treatment. The ethanol concentration being 75%, liquid to solid ratio being 7, extraction time 60 min, were extracted two times.Citation148
5.2.Quantitative determination of ursolic acid by means of the Liebermann–Burchard reactionCitation149
5.3.Determination of ursolic acid by reversed phase-high-performance liquid chromatography
The new sesquiterpenoid, together with ursolic acid have been isolated from the roots of Euphorbia chrysocoma Lévl. et Vant.Citation150
Simple high-performance liquid chromatography (HPLC) methods were developed for the determination of ursolic acid in loquat flower.Citation151 To evaluate the quality of Perilla frutescens, a simple HPLC method was developed for the assessment of ursolic acid.Citation152 See supplementary material 5.3.
5.4.Determination of free isomeric ursolic acid in Pterocephalus hookeri by capillary zone electrophoresis.Citation153 See supplementary material 5.4
5.5.Quantitative determination of ursolic acid by spectrophotometry
Ursolic acid was isolated from Herba cynomorii and was quantitatively determined by ultraviolet spectrophotometry. The results indicate that the content of ursolic acid is 0.78%. The average recovery rate of ursolic acid is 97.4%, relative standard deviation (RSD) = 0.45% (n = 4).Citation154
5.6.Determination of ursolic acid of Liuwei Dihuangwan simulation samples by near infrared
Determine the content of ursolic acid of Liuwei Dihuangwan by using near infrared with partial least squares, principal component analysis-back-propagation artificial neural network and wavelet transform-back-propagation artificial neural network was carried out. The predication recoveries were 100.7, 100.6, and 100.1% and the RSD were 5.42, 6.49, and 6.52%, respectively.Citation155
5.7.Quantitative determination of ursolic acid in Folium ilicis cornutae by thin-layer chromatography method
The content of ursolic acid in Folium ilicis cornutae gathered in different periods from Yongfeng country of Jiangxi province was determined by thin-layer chromatography (TLC) method. The result shows that the content appears to be low in samples gathered in April, but about the same in samples of February, June, August, October, and December.Citation156
5.8.Comparative analysis of ursolic acid in Crataegus pinnatifida Bge. var. major N.E. Br.
The contents of ursolic acid in Crataegus pinnatifida var. major before and after processing determined by CS-920 TLC scanner: 0.274 and 0.265%, coefficients of variation 1.180 and 1.150%, respectively.Citation157
The MeOH extract of the leaves of rosemary completely inhibited the motility of cultured epimastigotes of Trypanosoma cruzi at the concentration of 2 mg/mL after 2 h of incubation.Citation158
5.9.Development of liquid chromatography-mass spectrometry method for determination of ursolic acid
A reproducible liquid chromatography-mass spectrometric assay was developed for the determination of ursolic acid in laboratory-made mixtures and in leaves and twigs extracts of Staphylea holocarpa Hemsl.Citation159
Another rapid liquid chromatography-mass spectrometry (LC-MS) method for the determination of ursolic acid in rat plasma was developed and validated.Citation160 See supplementary material 5.9.
5.10.In situ analysis by microspectroscopy reveals triterpenoid compositional patterns.Citation161 See supplementary material 5.10
5.11.Use of liquid chromatography-atmospheric pressure chemical ionization-ion trap mass spectrometry for identification of ursolic acid
A quantitative method, consisting of supercritical fluid extraction followed by liquid chromatography-atmospheric pressure chemical ionization-ion trap mass spectrometry (LC-APCI-IT-MS) analysis, was developed for identification of ursolic acid in Anoectochilus roxburghii.Citation162
5.12.Dereplication of pentacyclic triterpenoids in plants by gas chromatography/electron impact/MS
Ursolic acid has been found to exhibit moderate antitubercular activity in a microplate alamar blue assay. To facilitate the discovery of novel antitubercular leads with diverse chemical structures, a new gas chromatography (GC)/electron impact/MS method was developed to dereplicate it as its methyl ester with limits of detection of 25.6, 26.9, and 26.8 ng.Citation163
Isolation and GC/MS quantitative determination of ursolic acid in the herb of Nepeta cataria var. citriodora have been performed. The content of this compound was in the range 0.95–1.30%.Citation164 A high performance GC procedure has been developed for the quantitation of ursolic acid in different parts of Xiakucao collected in different periods. The study provides a scientific foundation for increased availability and rational collecting periods of Xiakucao.Citation165
To develop a GC method to determine ursolic acid in Spica Prunellae, the sample was derivatized with CH2N2 solution. The GC conditions were as follows: comumn-10% SE-30 (2 m × 3 mm) and column temperature −270°C. Ursolic acid well separated and had good linearity in the range of 0.0025–0.4000 mg/mL. The method is good for determining ursolic acid in Chinese medicines.Citation166
6.Biological and pharmacological activity of plants extract containing ursolic acid
Some plant extracts containing ursolic acid as one of the constituents have been tested for various biological and microbial activities by different scientists. It has been reported to possess a wide range of diverse pharmacological properties, including strong anti-inflammatory activity, anticancer, antioxidant and is one of the most promising chemopreventive agents for cancer and showed that ursolic acid can inhibit proliferation and induce apoptosis of many tumor cell lines.
6.1.Prevention of dental caries by oriental folk medicines. Active principles of Zizyphi fructus for inhibition of insoluble glucan formation by cariogenic bacterium Streptococcus mutans
The Z. fructus extract had inhibitory activity against insoluble glucan formation by glucosyltransferase from the cariogenic bacterium S. mutans. The active substances were isolated from the plant and the chemical structures were elucidated to be oleanolic acid and ursolic acid.Citation167
6.2.Tumor inhibitory agents from Vauquelinia corymbosa
V. corymbosa Correa plant has shown activity against the P-388 lymphocytic leukemia test system. The constituents responsible for this activity were identified as uvaol, betulinic acid, and ursolic acid.Citation168
6.3.Antimicrobial activity of the extract and compounds from Baccharis dracunculifolia D. C.Citation169,Citation170 See suplementary material 6.3
6.4.Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham.Citation171 See supplementary material 6.4
6.5.Central nervous system activity of the extract of Mallotus peltatus (Geist) Muell Arg. Leaf.Citation172 See supplementary material 6.5
6.6.A potent sperm motility-inhibiting activity of ursolic acid from an ethnomedicine of Onge, Alstonia macrophylla Wall ex A. DC.Citation173 See suplementary material 6.6
6.7.Antibacterial constituents from fruit bodies of Ascomyce Bulgaria inquinans
Two ergosterins betuinic acid, cerevisterol, (24R) ergosta-7, and triterpenoids 22E-diene-3β, 5α, 6β-triol-3-O-palmitate and ursolic acid were isolated from the dried fruit bodies of Ascomyce Bulgaria inquinans.Citation174
6.8.Antipyretic activity of ursolic acid: an ethnomedicine of Andaman Islands.Citation175 See supplementary material 6.8
6.9.α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes.Citation176 See suplementary material 6.9
6.10.A new ursane triterpene inhibits DNA polymerase β−lyase
Bioassay-directed fractionation of a butanone extract of Monochaetum vulcanicum resulted in the isolation of a new triterpene, 3β-acetoxy-2α-hydroxyurs-12-en-28-oic acid and four known compounds, ursolic acid, 2α-hydroxyursolic acid, 3-(p-coumaroyl) ursolic acid, and β-sitosteryl-β-d-galactoside. Compounds 3β-acetoxy-2α,-hydroxyurs-12-en-28-oic acid, 2α-hydroxy ursolic acid, and β sitosteryl-β-d-galactoside exhibited polymerase β-lyase activity.Citation177
6.11.Antiviral activities of extracts and ursolic acid constituents of Ocimum basilicum
O. basilicum extracts were used to identify possible antiviral activities against DNA viruses (herpes viruses, adenoviruses and hepatitis B virus) and RNA viruses (coxsackievirus B1 and enterovirus.Citation178
6.12.In vitro transforming growth factor-β1 antagonistic activity of ursolic acid.Citation4 See suplementary material 6.12
6.13.Dual activity of triterpenoids: apoptotic versus antidifferentiation effects.Citation179 See suplementary material 6.13
6.14.Apoptosis inducing activity of ursolic acid, from Viscum album (L.).Citation180 See supplementary materials 6.14
6.15.Antioxidant, anti-inflammatory, and anticancer activities of extracts from Ledum groenlandicum Retzius.Citation181,Citation182 See suplementary material 6.15
7.Biological and pharmacological significance of isolated ursolic acid
7.1.Ursolic acid: an anti- and pro-inflammatory triterpenoid
Some studies have recently revealed that the effects of ursolic acid on normal cells and tissues are occasionally pro-inflammatory. Thus, ursolic acid may be designated as a double-edged sword with both positive and negative effects, and further evaluations of the effects of ursolic acid on the biological status of target cells or tissues are necessary.Citation183
7.2.Effect of ursolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-α.Citation184 See suplementary material 7.2
7.3.Non-enzymatic antioxidative and antiglycative effects of ursolic acid.Citation185 See supplementary material 7.3
7.4.Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats.Citation186 See suplementary material 7.4
7.5.Evaluation of hepatoprotective activity of ursolic acid.Citation187–192 See suplementary material 7.5
7.6.Triterpenoids (ursolic acid) from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. See suplementary material 7.6
Antiproliferative activities of the thirteen triterpenoids from apple peels against human HepG2 liver cancer cells, MCF-7 breast cancer cells, and Caco-2 colon cancer cells were evaluated. Most of the triterpenoids showed high potential anticancer activities against the three human cancer cell lines. Among the compounds isolated, 2α-hydroxyursolic acid (7, ), 2α-hydroxy-3β-[(2E)-3-phenyl-1-oxo-2-propenyl] oxy-olean-12-en-28-oic acid (8) and 3β-trans-p-coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid (9) showed higher antiproliferative activity toward HepG2 cancer cells. Ursolic acid, 2α-hydroxyursolic acid, and 3β-trans-p-coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid exhibited higher antiproliferative activity against MCF-7 cancer cells. All triterpenoids showed antiproliferative activity against Caco-2 cancer cells, especially 2α-hydroxyursolic acid, maslinic acid, 2α-hydroxy-3β-{[(2E)-3-phenyl-1-oxo-2-propenyl]oxy}olean-12-en-28-oic acid, and 3β-trans-p-coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid, which displayed much higher antiproliferative activities.Citation186
7.7.Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells.Citation193 See supplementary material 7.7
7.8.Ursolic acid demonstrates anticancer activity on human prostate cancer cells.Citation194 See supplementary material 7.8
7.9.MCF-7 cell-cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts.Citation195,Citation196 See supplementary material 7.9
7.10.Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart.Citation197 See supplementary material 7.10
7.11.Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice
Ursolic acid markedly reversed the d-gal induced learning and memory impairment by behavioral tests.Citation198
7.12.Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by ursolic acid in Cornelian cherry.Citation199 See supplementary material 7.12
7.13.Effects of ursolic acid on mucin release from airway goblet cells.Citation200 See supplementary materials 7.13
7.14.Inhibition of cytochrome P450 activities by ursolic acid in human liver microsomes.Citation201 See supplementary material 7.14
7.15.Ursolic acid induces apoptosis through caspase-3 activation and cell-cycle arrest in HaCaT cells
The pro-apoptotic effect of ursolic acid on HaCaT derived keratinocyte cell line was determined.Citation202 See supplemntary material 7.15.
7.16.Ursolic acid triggers calcium-dependent apoptosis in human Daudi cells
Ursolic acid was found to decrease cell viability in human lymphoma Daudi cells in a dose-dependent manner.Citation203 See supplemntary material 7.16.
7.17.Cytotoxic ursolic acid from Erica andevalensis
The cytotoxic activity of ursolic acid and α-amyrine has been assessed against three human cancer cell lines, TK-10 (renal adenocarcinoma), MCF-7 (breast adenocarcinoma), and UACC-62 (melanoma) and scientists also evaluated the antimitotic effect in root meristematic cells of Allium cepa. Ursolic acid was found to possess the highest cytotoxic activity.Citation204
7.18.Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A 549 cells.Citation205 See supplementary materials 7.18
7.19.Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells.Citation206 See supplementary materials 7.19
7.20.Proteomic analysis of ursolic acid-induced apoptosis in cervical carcinoma cells.Citation207 See supplementary materials 7.20
7.21.Molecular mechanism of ursolic acid-induced apoptosis in poorly differentiated endometrial cancer HEC108 cells.Citation208 See supplementary material 7.21
7.22.Inhibition by ursolic acid of calcium-induced mitochondrial permeability transition and release of two pro-apoptotic proteins
The possible inhibition by ursolic acid of mitochondrial permeability transition in mouse liver was investigated to identify the mechanisms underlying the hepatoprotective effect of ursolic acid.Citation209
7.23.Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells
Ursolic acid effects were investigated on the M4Beu human melanoma cell line. Ursolic acid had a significant antiproliferative effect on M4Beu, associated with the induction of an apoptotic process, characterized by caspase-3 activation, the downstream central effector of apoptosis.Citation210 See supplementary material 7.23.
7.24.Induction of apoptosis by ursolic acid through activation of caspases and downregulation of c-IAPs in human prostate epithelial cells
The effects of ursolic acid on the growth of human prostate epithelial cells were investigated.Citation211 See supplementary material 7.24.
7.25.Apoptotic activity of ursolic acid may correlate with the inhibition of DNA replication
The pro-apoptotic effect of ursolic acid on HepG2 human hepatoblastoma cells was investigated.Citation212 See supplementary material 7.25.
7.26.Mechanisms of inhibiting proliferation and inducing apoptosis of human gastric cancer cell line SGC7901 by ursolic acid
This study was carried out to investigate the effect of ursolic acid on cyclooxygenase-2 (COX-2), Bcl-2 and Bax expression in human gastric cancer cell line SGC7901, and explore its potential mechanisms of inhibiting proliferation and inducing apoptosis.Citation213
7.27.Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells
Agents that can suppress STAT3 activation have potential for prevention and treatment of cancer. Ursolic acid tested, for its ability to suppress STAT3 activation. Ursolic acid, inhibited both constitutive and interleukin-6-inducible STAT3 activation in a dose- and time-dependent manner in multiple myeloma cells.Citation214
7.28.Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes
The possible protective effects of ursolic acid against CCl4-induced alterations of antioxidant defense enzymes in vivo as well as its effects against CCl4-intoxication in vitro studied.Citation215
7.29.The effect of ursolic acid on experimental liver injury in mice.Citation216 See supplementary material 7.29
7.30.Antiallergic and anti-inflammatory ursolic acid derevative from the herb of Prunella vulgaris.Citation217 See supplementary material 7.30
7.31.Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease
The methanol extract of Crataegus pinnatifida showed potent inhibitory activities against HIV-1 protease at a concentration of 100 µg/mL. The isolation of the extract gave two compounds, uvaol, and ursolic acid and inhibit HIV-1 protease with IC50 values of 5.5 and 8.0 µM.Citation218
7.32.Treatment with ursolic acid induces apoptosis in human leukemia K562 cells and downregulates protein levels of bcl-xL. Treatment also increases phospho-Jun N-terminal kinase (JNK) in a dose- and time-dependent manner but does not alter phospho-Erk1/2 and phospho-P38. These results suggest that JNK may participate in ursolic acid-induced apoptosis in K562 cells.Citation219
7.33.Reduction of DNA-damaging effects of anti-HIV drug 3′-azido-3′-dideoxythymidine on human cells by ursolic acid.Citation220 See supplementary materials 7.33
7.34.In vitro and in vivo evaluation of ursolic acid as an antimalarial
The ursolic acid isolated from the root bark of the Tanzanian tree Uapaca nitida Müll-Arg. It exhibited IC50 values of 36.5 µg/mL and 28 µg/Ml.Citation221
7.35.Induction of differentiation of the cultured rat mammary epithelial cells by ursolic acid
The effects of ursolic acid and oleanolic acid, on the induction of proliferation and differentiation of normal rat mammary epithelial cells or organoids cultured in Matrigel or primary culture system was investigated.Citation222
7.36.Natural-product inhibitors of human DNA ligase I.Citation223 See supplementary material 7.36
7.37.Anti-angiogenic activity of ursolic acid.Citation224 See supplementary material 7.37
7.38.Effects of ursolic acid on different steps of the angiogenic process.Citation225 See supplementary material 7.38
7.39.Heme oxygenase-1 inducing ursane derevatives of Prunella vulgaris in HepG2 cells.Citation226 See supplementary material 7.39
7.40.Endogenous reverse transcriptase as a mediator of ursolic acid’s antiproliferative and differentiating effects in human cancer cell linesCitation227
The effects of ursolic acid on the proliferation and differentiation of human tumor cell lines from melanoma (A375), glioblastoma (U87) and thyroid anaplastic carcinoma, and on the proliferation of a non-transformed human fibroblast cell line (WI-38) was examined.Citation227
7.41.Antigenotoxic effects of ursolic acid, evaluation by the comet assay
Quercetin and ursolic acid prevented DNA damage and had antiproliferative properties in HepG2 cells suggesting an anticarcinogenic potential for these compounds.Citation228
7.42.Antimicrobial activity of Ursolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococciCitation229. See supplementary materials 7.42
7.43.Ursolic acid from the Chinese herb danshen [Salvia miltiorrhiza (L.)] upregulates endothelial nitric oxide synthase and downregulates Nox4 expression in human endothelial cells
Danshen, the dried root of Salvia miltiorrhiza Bunge, is the most commonly used traditional Chinese medicines for cardiovascular indications. Ursolic acid is responsible for this effect of the plant.Citation230
7.44.Experimental study on apoptosis induced by ursolic acid isolated from asparagus in HL-60 cellsCitation231
7.45.Ursolic acid inhibits proliferation and stimulates apoptosis in HT-29 cells following activation of alkaline sphingomyelinase
The anticancer effects of ursolic acid on human colon cancer cells was studied. HT-29 cells were treated with ursolic acid. Cell proliferation was determined by cleavage of WST-1.Citation232
7.46.Group IIA secretory PLA2 inhibition by ursolic acid
Ursolic acid inhibited secretory PLA2 enzymes purified from Vipera russelli, Naja naja venom and human pleural fluid and synovial fluid. IC50 values determined for these enzymes ranged from 12 to 18 µM.Citation233
7.47.Aggregated ursolic acid, a natural triterpenoid, induces IL-1β release from murine peritoneal macrophages: role of CD36.Citation234 See supplementary material 7.47
7.48.Upregulation of matrix metalloproteinase family gene involvement in ursolic acid-induced human lung non-small carcinoma cell apoptosis.Citation235 See supplementary materials 7.48
7.49.Ursolic acid: a potent inhibitor of superoxides produced in the cellular system.Citation236 See supplementary material 7.49
7.50.Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis.Citation237 See supplementary material 7.50
7.51.Carbenoxolone and ursolic acid inhibited mucin secretion from airway epithelial cells.Citation238 See supplementary material 7.51
7.52.Ursolic acid enhances COXs and tumor necrosis factor-α expression in mouse skin.Citation239 See supplementary materials 7.52
7.53.Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via nitric oxide release in isolated rat thoracic aorta.Citation240 See supplementary material 7.53
7.54.Protection of peroxynitrite-induced DNA damage by dietary antioxidants
Dietary antioxidants such as epigallocatechin gallate, quercerin, rutin, resveratrol, and ursolic acid inhibit single strand breaks in supercoiled plasmid DNA induced by 3-morpholinosydnomine N-ethylcarbamide (SIN-1), a generator of peroxynitrite through the reaction between nitric oxide (NO) and superoxide anion.Citation241 See supplementary material S-7.54.
7.55.Anti-complementary activity of ursane-type triterpenoids from Weigela subsessilis.Citation242 See supplementary material 7.55
7.56.Effect of ursolic acid on caspase-3 and PARP expression of human MCF-7 cells.Citation243 See supplementary material 7.56
7.57.Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata.Citation244 See supplementary material 7.57
7.58.Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production.Citation245 See supplementary material 7.58
The activity of ursolic acid on pro-inflammatory and anti-inflammatory cytokines in the peripheral blood of arthritic balb/c mice was investigated.Citation245 See supplementary material 7.58.
7.59.The effect of ursolic acid on high glucose-induced apoptosis in U937 cells was investigated.Citation246 See supplementary material S-7.59
7.60.Ursolic acid promotes the release of macrophage migration inhibitory factor via ERK2 activation in resting mouse macrophages.Citation247 See supplementary material 7.60
7.61.Antiviral activities of extracts and selected pure constituents of O. basilicum.Citation248 See supplementary material 7.61
7.62.Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats.Citation249 See supplementary material 7.62
7.63.Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid.Citation250 See supplementary material 7.6
7.64.Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi
From the whole plant of Dracocephalum kotschyi BOISS. ursolic acid (6.2 µM), was effective against epimastigotes of T. cruzi.Citation251
7.65.In vitro anti-inflammatory activity of 23-hydroxyursolic acid isolated from Cussonia bancoensis in murine macrophage RAW 264.7 cells.Citation146 See supplementary material 7.65
7.66.Antifungal constituents of Clytostoma ramentaceum and Mansoa hirsuta
The activity-guided fractionation of C. ramentaceum extract afforded ursolic acid and 2-(3′, 4′-dihydroxyphenyl) ethanol, both active against the test fungi (100 µg).Citation252
7.67.Antitubercular constituents of Valeriana laxiflora
In a microplate alamar blue assay against Mycobacterium tuberculosis, compounds (+)-1-hydroxy-2, 6-bis-epi-pinoresinol, along with eleven known compounds, betulin, betulinic acid, 5,7-dihydroxy-3, 6,4′-trimethoxyflavone, 23-hydroxyursolic acid, oleanolic acid, tricin, ursolic acid exhibited MIC of 15.5–127 µg/Ml.Citation253
7.68.Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats.Citation254 See supplementary material 7.68
7.69.Anti-platelet ursolic acid from leaves of Campsis grandiflora.Citation255 See supplementary material 7.69
7.70.Ursolic acid, an antagonist for transforming growth factor-β1.Citation256 See supplementary material 7.70
7.71.Triterpene acids from the leaves of P. frutescens and their anti-inflammatory and antitumor-promoting effects
Ursolic acid, corosolic acid (14), 3-epicorosolic acid (15), pomolic acid (16), tormentic acid (17) and hyptadienic acid (18), were isolated from the leaves of P. frutescens (L.). Except 3-epicorosolic acid all compounds, were evaluated for its inhibitory effects on TPA-induced inflammation in mice. It showed a marked anti-inflammatory effect, with a 50% inhibitory dose of 0.09–0.3 mg per ear. In addition, an evaluation against the Epstein–Barr virus early antigen (Epstein–Barr virus-EA) activation induced by TPA-showed five compounds, ursolic acid, corosolic acid, 3-epicorosolic acid, tormentic acid, and 3-epimaslinic acid, with a potent inhibitory effect on Epstein–Barr virus-EA induction (91–93% inhibition at 1 × 10(3) mol ratio/TPA).Citation5
7.72.Inhibition of ultraviolet-A-modulated signaling pathways by ursolic acid in HaCaT human keratinocytes
Scientists investigated the effects of ursolic acid and asiatic acid on the ultraviolet A-modulated signaling pathways using HaCaT human keratinocytes as a model cellular system.Citation257
7.73.Ursolic acid inhibits nuclear factor-κβ activation induced by carcinogenic agents through suppression of Iκβα kinase and p65 phosphorylation: correlation with downregulation of COX-2, matrix metalloproteinase-9, and cyclin D1
Ursolic acid suppressed nuclear factor-κβ activation induced by various carcinogens including tumor necrosis factor, phorbol ester, okadaic acid, H2O2, and cigarette smoke.Citation258 See supplementary material 7.73.
7.74.Sterol and triterpenoid constituents of Verbena littoralis with NGF-potentiating activity
Ursolic acid and oleanolic acid were isolated from Paraguayan medicinal plant showed an enhancing activity of nerve growth factor-mediated neurite outgrowth in PC12D cells.Citation259
7.75.Biological activity of some resins, gums and pigments against in vitro LDL oxidation
Amyrin, oleanolic acid, ursolic acid, lupeol, 18-α-glycyrrhetinic acid, and hydroxyl naphthoquinones (naphthazarin, shikonin, and alkannin) showed 53.5–78.8% and 27.0–64.1% low-density lipoprotein (LDL) protective activity. The combination effects (68.7–76.2% LDL protection) of ursolic, oleanolic, and ursodeoxycholic acids were almost equal to the effect (75.3%) of the Chios mastic gum extract in comparable doses.Citation260,Citation261
7.76.Cardiovascular, antihyperlipidemic, and antioxidant effects of ursolic acids in hypertension.Citation262 See supplementary material 7.76
7.77.Antileukemic activity of selected natural products in Taiwan
Ursolic acid possessed strong activity against human leukemia and lymphoma cell lines. Ursolic acid was effective against P3HR1 cells (IC50: 2.5 µg/mL, SI: 262.6) and chronic myelogenous leukemia cells K562 (IC50: 17.79 µg/mL, SI: 36.91).Citation263
7.78.Suppressive effects of edible Thai plants and ursolic acid on superoxide and NO generationCitation264
7.79.In vivo anti-nociceptive and anti-inflammatory effect of ursolic acid and 23-hydroxyursolic acid, from Cussonia bancoensis
Ursolic acid and 23-hydroxyursolic acid significantly inhibited 1%-carrageenan-induced edema in the rat. These results suggest that ursolic acid and 23-hydroxyursolic acid, are responsible for the anti-nociceptive and anti-inflammatory effect of C. bancoesnsis.Citation265
Ursolic acid and 2α-hydroxyursolic exhibited strong antiallergic and anti-inflammatory inhibitory activities (IC50 values, 17 and 27 µM, respectively).Citation217
7.80.Isolation and biological activity of ursolic acid from Vitex negundo (L.)
Ursolic acid and betulinic acid’s antifeedant activity against the larvae of an agricultural pest, the castor semilooper, in a no-choice laboratory assay and their antibacterial activity against Bacillus subtilis and Escherichia coli, by the paper disc method, were tested. Ursolic acid showed more effective antifeedant activity than the betulinic acid.Citation266
7.81.Brine shrimp lethality of ursolic acid from Phryma leptostachya (L.).Citation267 See supplementary material 7.81
7.82.Extract and identify ursolic acid from Ligustrum lucidum Ait and study its effect to periodontal pathogen.Citation268 See supplementary material 7.82
7.83.Ursolic acid as a trypanocidal constituent in rosemary
Ursolic acid stopped the movement of all T. cruzi epimastigotes at the minimum concentration (MC (100) of 40 µg/mL (88 µM) after 48 h of incubation.Citation269
7.84.Triterpenes ursolic acid and phytosterols as human leukocyte elastase inhibitorsCitation270
7.85.Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT-15.Citation271 See supplementary material 7.85
7.86.Ursolic acid of Origanum majorana (L.) reduces αβ-induced oxidative injury.Citation272 See supplementary material 7.86
7.87.Inhibitory effect of ursolic acid purified from Origanum majorana (L.) on the acetylcholinesterase.Citation273 See supplementary material 7.87
7.88.Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells.Citation274 See supplementary material 7.88
7.89.In vitro anti-inflammatory activity of triterpenoid compounds isolated from Phillyrea latifolia (L.).Citation275,Citation276 See supplementary material 7.89
7.90.Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase.Citation277 See supplementary material 7.90
7.91.Ursolic acid was also isolated from Shandanshaoyao DecoctionCitation278
The crude dichloromethane bark extract of Pilidiostigma tropicum from north Queensland, Australia, shows antibacterial and cytotoxic activity. Bioactivity-directed separation led to the isolation of ursolic acid-3-p-coumarate as the biologically active materials.Citation279
7.92.Cytotoxic triterpenes from stem bark of Physocarpus intermedius. See supplementary material 7.92
Ursolic acid was responsible for the cytotoxicity against five cultured human tumor cell lines, i.e., A549 (non-small cell lung), SK-OV-3, SK-MEL-2, XF498, and HCT-15 (colon), in vitro.Citation280
7.93.Cytotoxic triterpenes from Crataegus pinnatifida.Citation281 See supplementary material S-7.93
7.94.Anti-AIDS agents. 30. Anti-HIV Activity of oleanolic acid, pomolic acid, and structurally related triterpenoids
Ursolic acid did show anti-HIV activity (EC50 2.0 µg/mL), it was slightly toxic (IC50 6.5 µg/mL, T. I. 3.3). A new triterpene (25) was also isolated from the CHCl3-soluble fraction of Rosa woodsii, though it showed no anti-HIV activity. Pomolic acid (19) demonstrated anti-HIV activity, with EC50 values of 1.4 µg/mL, respectively, and inhibited uninfected H9 cell growth with IC50 values of 23.3 µg/mL, respectively. Ursolic acid, isolated from Prosopis glandulosa, Syzygium claviflorum, and Hyptis capitata, was found to show similar anti-HIV activity, with an EC50 value of 2.0 µg/mL and IC50 values of 6.5 µg/mL (T. I. value, 3.3) ().Citation282
7.95.Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes.Citation283 See supplementary material 7.95
7.96.Ursolic acid inhibits COX-2 transcription in human mammary epithelial cells.Citation284 See supplementary material 7.96
7.97.Cyclic adenosine monophosphate inhibits ursolic acid-induced apoptosis via activation of protein kinase A in human leukemic HL-60 cells.Citation285 See supplementary material 7.97
7.98.DNA polymerase β inhibitors from Baeckea gunniana.Citation286 See supplementary material 7.98
7.99.Antimalarial activity of four plants used in traditional medicine in Mali
Ursolic acid, purified from the hydromethanol extract of Muricopsis inermis induced a significant decrease of parasite proliferation.Citation287
7.100.Chitin synthase II inhibitory activity of ursolic acid, isolated from Crataegus pinnatifida
Ursolic acid from Crataegus pinnatifida Bunge inhibits chitin synthase II from Saccharomyces cerevisiae with an IC50 value of 0.84 µg/mL.Citation288
7.101.Ursolic acid from Plantago major, a selective inhibitor of COX-2 catalyzed prostaglandin biosynthesis.Citation289 See supplementary material 7.101
7.102.Ursolic acid-induced downregulation of matrix metalloproteinase-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells.Citation290 See supplementary material 7.102
7.103.Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells.Citation291 See supplementary material S-7.103
7.104.Ursolic acid stabilize liposomal membranesCitation348
Ursolic acid on the fluidity and stability of dipalmitoyl phosphatidylcholine liposomal membrane was monitored by measuring the fluorescence polarization of 1,6-diphenyl-1, 3, 5-hexatriene labeled in the liposomal membrane and the leakage of calcein from the probe-encapsulated liposomes. The experiments with the liposomes made of dipalmitoyl phosphatidylcholine and oleanolic acid or ursolic acid showed that oleanolic acid and ursolic acid exhibited a moderate fluidity-modulating effect for the liquid-crystalline liposomal membrane, and a strong condensing effect for both crystalline and liquid-crystalline liposomal membranes. These result showed that their fluidity-modulating and condensing effects might have some implications in their biological functions.Citation348
7.105.Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice.Citation292 See supplementary material 7.105
7.106.Inhibitory effect of ursolic acid on B16 proliferation through cell-cycle arrest.Citation293 See supplementary material 7.106
7.107.Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 in HT1080 human fibrosarcoma cells.Citation294 See supplementary material 7.107
7.108.Antitumor effect in vitro and immuno-response in vivo of Fructus mume
The antitumor action of ursolic acid on HIMeg and HL-60 cells in vitro was tested. The immuno-response in rats was also studied. The Fructus mume had inhibiting effect on proliferation of HIMeg and HL-60 cells.Citation295
7.109.Ursolic acid inhibits aflatoxin B1-induced mutagenicity in a Salmonella assay system
Ursolic acid significantly decreased the numbers of Salmonella typhimurium TA100 revertants per plate, thus showing antimutagenic activity.Citation296
7.110.Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid.Citation297 See supplementary material 7.110
7.111.Search for possible antitumor promoters by inhibition of TPA-induced Epstein–Barr virus activation.Citation6 See supplementary material 7.111
7.112.Inhibitory effects of ursolic acid on skin tumor promotion.Citation298 See supplementary material 7.112
7.113.The effects of 10 triterpenoid compounds on experimental liver injury in mice.Citation299 See supplementary material 7.113
7.114.Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids.Citation300 See supplementary material 7.114
7.115.Intervention of adriamycin induced free radical damage by ursolic acid.Citation301 See supplementary material 7.115
7.116.Effect of ursolic acid from epicuticular waxes of Jacaranda decurrens on Schizaphis graminum.Citation302 See supplementary material 7.116
7.117.Inhibition of lipoxygenase activity and HL-60 leukemic cell proliferation by ursolic acid
Ursolic acid from heather flowers to be an inhibitor of both potato tuber 5-lipoxygenase and soybean 15-lipoxygenase with IC50 values of 0.3 mM. It also inhibits lipoxygenase activity in mouse peritoneal macrophages at 1 µM and HL-60 leukemic cells growth (IC50 = 0.85 µM) as well as their DNA synthesis (IC50 = 1 µM).Citation303
7.118.Characterization of ursolic acid as a lipoxygenase and COX inhibitorCitation304
7.119.Inhibition of human leukocyte elastase by ursolic acid. Evidence for a binding site for pentacyclic triterpenes.Citation305 See supplementary material 7.119
7.120.Antibiotic action of β-ursolic acid.Citation306 See supplementary material 7.120
7.121.Use of ursolic acid in skin and hair care treatment
Hair growth stimulant agent: Ursolic acid and its isomer, have been used in tonics to enhance hair growth and prevent scalp irritation. It encourage hair growth by stimulating the peripheral blood flow in the scalp and activating the hair mother cells. It also furnish alopecia-preventing and dandruff-preventing effects.Citation307
8.Derivatives
Ursolic acid derivatives, including ursane-type triterpenoid saponins, naturally occur as secondary metabolites through complex metabolic processes in different parts of the plant.Citation308,Citation309 Synthetic derivatives obtained from ursolic acid have been reported and evaluated for their pharmacological action.
8.1.Study of the trypanocidal activity of triterpene acids isolated from Miconia species.Citation310 See supplementary material 8.1
8.2.Abietane triterpenoic acids from Salvia cilicica and their antileishmanial activities
Ursolic acid was found to be potently active against amastigote IC50 values of 7–120 nM and moderately active against promastigote stages IC50 values of 51–137 nM) of the two Leishmania species.Citation311
8.3.Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivativesCitation312
8.4.Evaluation of ursolic acid and derivatives on aromatase inhibition
The inhibitory potency of ursolic acid its derivatives to inhibit aromatase activity was assessed and compared to a phytoestrogen apigenin and a steroidal aromatase inhibitor 4-hyroxyandrostenedione. Only ursolic acid showed an efficient and dose-dependent aromatase inhibition with IC50 value of 32 µM as did apigenin (IC50 = 10 nM), whereas IC50 value of 4-OHA was 0.8 nM. Results show that the incorporation of a metallocene moiety into the ursolic acid derivatives decreases the aromatase inhibition. Moreover, comparison of the structure/inhibitory potency relationship indicates that the presence of cycle A and the configuration of C3-OH and C17-COOH seems to be more favorable to recognize the active site of aromatase and to block its activity.Citation313
8.5.Antimicrobial activity of pentacyclic triterpenes and their synthesized 3-O-lipophilic chains
The metabolites of Diopsyros melanoxylon viz. ursolic acid and their lipophilic 3-O-fatty acid ester chains (C12–C18), were evaluated for their antimicrobial activity against a series of Gram-positive and Gram-negative bacteria.
Significantly these compounds were found to exhibit potent activity against Gram-negative bacteria Pseudomonas syringae and fairly good activity against Gram-positive bacteria, Bacillus sphaericus, and B. subtilis ().Citation314
8.6.Oxyfunctionalization products of terpenoids and their biological activity
Oxyfunctionalization of ursolic acid acetate and kaurenic acid, with dimethyldioxirane was investigated. Treatment of the terpenoids with dimethyldioxirane afforded a variety of oxidation and oxydegradation products to yield naturally occurring or novel compounds. The inhibitory activity of the terpenoid derivatives against α-glucosidase was investigated and ursolic acid acetate (33) and 11,12-dehydroursolic lactone, 3-O-acetyl-9,11-dehydro-12α–hydroxyoleanolic lactone (33″) were found to exhibit potent activity ().Citation315
8.7.Inhibition by boswellic acids of human leukocyte elastase
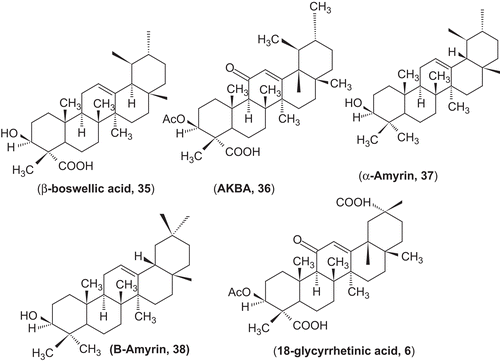
Substantial inhibition by β-boswellic acid (35), amyrin (37, 38), and ursolic acid, but not by 18β-glycyrrhetinic acid (6) was observed. The data show that the dual inhibition of 5-lipoxygenase and human leukocyte elastase is unique to boswellic acids. Other triterpenes with human leukocyte elastase inhibitory activities (ursolic acid) do not inhibit 5-lipoxygenase, and leukotriene biosynthesis inhibitors from different chemical classes do not impair human leukocyte elastase activity. Because leukotriene formation and human leukocyte elastase release are increased simultaneously by neutrophil stimulation in a variety of inflammation and hypersensitivity-based human diseases, the reported blockade of two pro-inflammatory enzymes by boswellic acids might be the rationale for the putative antiphlogistic activity of acetyl-11-keto-β-boswellic acid and derivatives ().Citation316,Citation317
8.8.Radical-scavenging activities of new hydroxylated ursane triterpenes from cv. Annurca apples.Citation318 See supplementary material 8.8
8.9.Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives
A series of derivatives of ursolic acid was assayed for structure-activity relationships studies. Ursolic acid (ED100 0.4 mg/mL) was four times more active than oleanolic acid (ED100 1.6 mg/mL). The presence of free hydroxy or carboxy groups is necessary for the trypanocidal activity as could be deduced from the effect of the acetates (39), methyl ester (40) and aldehyde derivatives ().Citation319
8.10.Beccaridiol, an unusual 28-nortriterpenoid from the leaves of Diplectria beccariana
Beccaridiol (28-nortriterpene28-nortriterpene), 2′, 4′-dihydroxy-3-(4-methoxyphenyl)-propiophenone, 4′-hydroxy-1′, 2′-dihydro-β-ionone, 4′-O-methyldavidigenin and ursolic acid, isolated from D. beccariana. All isolates were evaluated for their potential cancer chemopreventive properties utilizing a cell culture assay to determine quinone reductase induction.Citation320
8.11.The cytotoxic activity of ursolic acid derivatives
Ursolic acid and 2α-hydroxyursolic acid were found to show growth inhibitory activity against four tumor cell lines, HL-60, BGC, Bel-7402, and Hela.Citation321 See supplementary material 8.11.
8.12.Cardiotonic and antidysrhythmic effects of ursolic acid, methyl maslinate and uvaol.Citation8 See supplementary material 8.12
8.13.In vitro trypanocidal activity of triterpenes from Miconia species.Citation322 See supplementary material 8.13
8.14.Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic, and ursolic acids
Ursolic acid have been modified at the C-3 position to cinnamate-based esters (43–51) and in vitro antimycobacterial activity against M. tuberculosis H (37) Ra has been determined.
The results indicated that modification of the parent structures of ursolic acid to the p-coumarate and, the ferulate ester analogues resulted in high antimycobacterial activity (). Ursolic acid exhibited antimicobacterial activity (MIC 12.5 µg/mL), whereas its cinnamoyl and p-chlorocinnamoyl analogues 43 and 57 were inactive. The p-coumarate ester 45 was highly active, with an MIC value of 6.25 µg/mL. The corresponding acetate and methyl ether derivatives, 44 and 48, showed lower activity with MIC s of 25 and 100 µg/mL, respectively. Both the ferulate ester 49 and its acetate 48 were highly active with equal MICs of 3.13 µg/mL. However, the corresponding caffeate ester 50 and its acetate 49 were much less active, with respective MICs of 200 and 100 µg/mL.Citation323
8.15.Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana
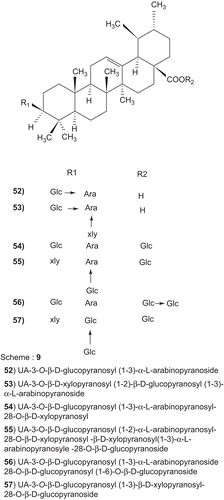
Ursolic acid could significantly inhibit the proliferation of HepG2 and its drug-resistance strain, R-HepG2 cells, but had no inhibitory effect on primarily cultured normal mouse hepatocytes whereas all the six derivatives of ursolic acid (52–57) could not inhibit the growth of all tested cell lines. Further study on mechanism demonstrated that apoptosis and G0/G1 arrest were involved in the cytotoxicity and cleavage of poly-(ADP-ribose)-polymerase. Downregulation of COX-2 protein and upregulation of heat shock protein 105 mRNA correlated to the apoptosis of HepG2 cells treated with ursolic acid. Ursolic acid is a promising antitumor agent ().Citation324
Scheme 9. (52) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside (53) UA-3-O-β-d-xylopyranosyl (1-2)-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside (54) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranosyl-28-O-β-d-xylopyranosyl (55) UA-3-O-β-d-glucopyranosyl (1-2)α-l-arabinopyranosyl-28-O-β-d-xylopyranosyl -β-d-xylopyranosyl(1-3)α-Larabinopyranosyle-28-O-β-d-glucopyranoside (56) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside 28-O-β-d-glucopyranosyl (1-6)-O-β-d-glucopyranoside (57) UA-3-O-β-d-glucopyranosyl (1-3)β-d-xylopyranosyl-28-O-β-d-glucopyranoside.
8.16.Inhibitory activity of minor polyphenolic and non-polyphenolic constituents of olive oil against in vitro LDL oxidation.Citation325 See supplementary material 8.16
8.17.Uinovic acid glycosides from Mitragyna stipulosa first examples of natural inhibitors of snake venom phosphodiesterase I
α-Amyrin, 3β-acetyl ursolic acid, and β-sitosterol glucopyranoside were isolated from the Mitragyna stipulosa bark extract, showed significant inhibitory activity against snake venom.Citation326
8.18.Abietane triterpenoic acids from Salvia cilicica and their antileishmanial activities
The triterpenoic acids were found to be potently active against amastigote (IC50 values of 7–120 nM) and moderately active against promastigote stages (IC50 values of 51–137 nM) of the two Leishmania species.Citation311
9.Structure-activity relationship of ursolic acid
9.1.Structure-activity relationship of triterpenoids isolated from Mitragyna stipulosa on cytotoxicity.Citation327 See supplementary materials 9.1
9.2.Microbial transformation of cadina-4, 10 (15)-dien-3-one, aromadendr-1(10)-en-9-one and methyl ursolate by Mucor plumbeus ATCC 4740.Citation328 See supplementary material 9.2
9.3.Activation of caspase-3 protease during the process of ursolic acid and its derivative-induced apoptosis.Citation329 See supplementary material 9.3
9.4.Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives
Several 3-O-acyl-ursolic acids were evaluated for anti-HIV activity. Ursolic acid was equipotent (EC50 4.4 µM) with oleanolic acid (EC50 3.7 µM), although it was slightly toxic (IC50 14.3 µM, therapeutic index 3.3). 3-O-Diglycoryl-ursolic acid (62) demonstrated relatively potent anti-HIV activity with an EC50 of 0.31 µM and a therapeutic index of 155.5. In contrast, 3-O-(3′, 3′-dimethylsuccinyl)-ursolic acid (60), which is analogous to the extremely potent anti-HIV betulinic acid derivatives, displayed only weak anti-HIV activity (EC50 2.1 µM) ().Citation330
9.5.Anti-inflammatory activity
Ursolic acid not only inhibits human leukocyte elastase, but also 5-lipoxygenase and COX activity. It inhibited TPA-induced mouse ear edema by 72.4% determined that 200 g and 50 g applications of ursolic acid inhibited 12-O-hexadecanoyl-16-hydroxyphorbol-13-acetate-induced inflammation by 49% and 33%. It inhibited concanavalin A induced histamine release, which can cause severe inflammation, by 95% at a concentration of 0.001 M.Citation331
9.6.Immunomodulating activity of ursolic acid derivatives, inhibitory effects of constituents from Cynomorium songaricum on HIV-1 protease
Ursolic acid and its hydrogen malonate isolated from Cynomorium songaricum Rupr as inhibitors of human immunodeficiency virus type 1 protease, with 50% inhibitory concentrations IC50 of 8 and 6 µM. Amongst various dicarboxylic acid hemiesters of related triterpenes, inhibitory activity tended to increase in the order of oxalyl, malonyl, succinyl, and glutaryl hemiesters, for ursolic acid. The most potent inhibition was observed for the glutaryl hemiesters, with an IC50 of 4 µM.Citation332
9.7. Ursolic acid: an anti-tumorigenic and chemopreventive activity. Minireview.
Ursolic acid, is of interest to scientists in the area of oncology because of its cytotoxicity, induction of differentiation, anti-mutagenic, antiviral and anti-invasive activities. Ursolic acid is capable of inducing apoptosis in tumor cells on one side and to prevent malignant transformation of normal cells on the other side. It also interferes with numerous enzymes, including the ones serving directly to DNA synthesis. This review summarizes reports on ursolic acid biological properties and to show its main anti-tumor effects and chemopreventive properties in normal cells.Citation340
9.8.Synthesis and anti-ulcer activity of new derivatives of oleanolic and ursolic acids
Among the ursolic acid derivatives, the dihemisuccinate sodium salt 72 demonstrated a good separation between anti-ulcer and mineralocorticoid activities. Nevertheless, kidney and liver toxicity was observed in the monkey thus jeopardizing its further development. Better results were obtained with the uvaol dihemiphthalate sodium salt and the diene analogue 77. In particular, 75 and 76 showed a potent anti-ulcer activity, 3- to 25-fold higher than carbenoxolone. Furthermore, compound 76 does not show signs of liver toxicity in the monkey (, ).Citation333
9.9.Antifeedant activity of some triterpene acids and their fatty acid ester analogues
The 3-O-fatty acid ester derivatives C12–C18 of ursolic acid (79–84) were screened for their antifeedant activity against the agricultural pest tobacco caterpillar larvae (Spodoptera litura F) in a no-choice laboratory study. The Urs-12-ene-28-carboxy-3β-octadecanoate and olean-12-ene-28-carboxy-3β-hexadecanoate were found to exhibit exceptionally potent antifeedant activities at 50 µg/cm2 concentration ().Citation334
9.10.Anti-AIDS agents. Anti-HIV activity of ursolic acid and structurally related triterpenoids
Oxidation of ursolic acid (1) yielded a 3-oxo- derivative (89), which was more toxic against uninfected H9 cells than its parent compound, but inhibited HIV-1 replication, with an improved EC50 value of 0.11 g/mL. Treatment of 1 with a molar equivalent of potassium hydroxide furnished the potassium salt of oleanolic acid (90). It exhibited potent anti-HIV activity, with an EC50 value of 0.5 g/mL and a T.I value of 68.6. enhanced anti-HIV activity with the potassium salt.Citation335
9.11.Cladocalol, and ursolic acid from Eucalyptus cladocalyx with cytotoxic activity.Citation336 See supplementary material 9.11
9.12.New 3-O-acyl betulinic acids and ursolic acid from Strychnos vanprukii Craib.Citation337 See supplementary material 9.12
9.13.Immunomodulating activity of ursolic acid derivatives, novel triterpenoids suppress inducible NO synthase and inducible COX in mouse macrophages.Citation338 See supplementary material 9.13
9.14.Structure-activity relationships of synthetic methyl ursolate glycosides.Citation339 See supplementary material 9.14
10.Conclusion
Ursolic acid has been found to be an active ingredient from Eriobotrya japonica and Rubus species. The literature survey reveals that it is a highly potent compound which shows variety of biological activities. It is known for their antimicrobial, hepatoprotective, anti-inflammatory, antiallergic, antiviral, and cytotoxic activities. It can be an active ingredient in treating urease, β-lactamase, acetyl cholinesterase, α-glucosidase, antimicrobial, hepatoprotective, anti-inflammatory, antipruritic effects, spasmolytic activity, anti-angiogenic activities, antiallergic, antiviral, and cytotoxic activities. In recent years, it was found that ursolic acid had marked antitumor effects. It was shown that it protects mice against the hepatotoxicity of carbon tetrachloride, acetaminophen, bromobenzene, thioacetamide, furosemide, phalloidin, colchicine, cadmium, d-galactosamine and endotoxin. It lower serum transaminase, lactic dehydrogenase, and γ-glutamyltransferase levels in the CCl4-treated rats. It enhances the bioavailability of the active ingredient of the pharmaceutical compound. On the basis of its activities, it may be possible to develop triterpenoids as useful agents for chemoprevention of cancer or other chronic diseases with an inflammatory component. Ursolic acid could provide an effective and cheap treatment of most common diseases in the world.
In the end, we can suggest that Eriobotrya japonica which is a widely grown, in many countries, could be used as a source of ursolic acid. Further studies involving crude extracts of Eriobotrya japonica containing ursolic acid would be highly economic and would account for useful consumption of the problems concerned.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Supplementary Material
Download PDF (642 KB)References
- Sultana N, Ata A. Oleanolic acid and related derivatives as medicinally important compounds. J Enzyme Inhib Med Chem 2008;23:739–756.
- Sultana N, Farheen SZ. Naturforsch 2010 (Submitted).
- Park SH, Oh SR, Jung KY, Lee IS, Ahn KS, Kim JG et al. Anticomplement activities of oleanolic acid monodesmosides and bisdesmosides isolated from Tiarella polyphylla. Arch Pharm Res 1999;22:428–431.
- Yoshimura H, Sugawara K, Saito M, Saito S, Murakami S, Miyata N et al. In vitro TGF-beta1 antagonistic activity of ursolic and oleanolic acids isolated from Clerodendranthus spicatus. Planta Med 2003;69:673–675.
- Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M et al. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem 2004;68:85–90.
- Ohigashi H, Takamura H, Koshimizu K, Tokuda H, Ito Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein–Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett 1986;30:143–151.
- Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies Africana leaves. J Ethnopharmacol 2003;84:299–305.
- Somova LI, Shode FO, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine 2004;11:121–129.
- Chaturvedula PVS, Gao Z, Hecht SM, Jones SH, Kingston DG. A new acylated oleanane triterpenoid from Couepia polyandra that inhibits the lyase activity of DNA polymerase B. J Nat Prod 2003;66:1463–5.
- Dat NT, Lee IS, Cai XF, Shen G, Kim YH. Oleanane triterpenoids with inhibitory activity against NFAT transcription factor from Liquidambar formosana. Biol Pharm Bull 2004;27:426–428.
- Giner-Larza EM, Manez S, Recio MC, Giner RM, Prieto JM, Cerda-Nicolas M, Rios J. Eur J Pharmacol 2001;28:137–43.
- Jovel EM, Zhou XL, Ming DS, Wahbe TR, Towers GH. Bioactivity-guided isolation of the active compounds from Rosa nutkana and quantitative analysis of ascorbic acid by HPLC. Can J Physiol Pharmacol 2007;85:865–871.
- Tin-Wa M, Farnsworth NR, Fong HH, Trojanek J. Catharanthus alkaloids. XXV. Isolation of leurosine and ursolic acid from C. pusillus. Lloydia 1970;33:261–263.
- Nacken M, Pachaly P, Zymalkowski F. Aminoacylation of emetine. Arch Pharm Ber Dtsch Pharm Ges 1970;303:122–133.
- Silva M. Ursolic acid in Retanilla ephedra. J Pharm Sci 1967;56:908–909.
- Brieskorn CH, Huber KK. Description and properties of ursolic acid glucoside. Arch Pharm Ber Dtsch Pharm Ges 1966;299:792–796.
- Plouvier V. Presence of ursolic acid in the blossoms of Viburnum opulus l. var. sterile dc (Caprifoliaceae). Ann Pharm Fr 1964;22:313–314.
- Cucu V, Rosca M, Grecu L, Cioaca C. Isolation of ursolic acid from Viburnum latana L. Pharmazie 1977;32:542–543.
- Takahashi T. Presence of ursolic acid in the leaves of Funtumia latifolia Stapf. Ann Pharm Fr 1961;19:520–522.
- Holtzem H. Incidence of ursolic acid in the leaves of Pyrus communis L. Arch Pharm Ber Dtsch Pharm Ges 1958;291/63:308–310.
- Hammouda Y, Le Men J. Study of the benzene extract of the leaves of little periwinkle: Vinca minor, presence of ursolic acid, ornol, beta-sitosterol and triacontane. Ann Pharm Fr 1956;14:344–347.
- Schindler H, Herb M. Chemistry of Ilex aquifolium L. I. Isolation of ursolic acid and rutin from leaves. Arch Pharm Ber Dtsch Pharm Ges 1955;288:372–377.
- Prakash Chaturvedula VS, Schilling JK, Johnson RK, Kingston DG. New cytotoxic lupane triterpenoids from the twigs of Coussarea paniculata. J Nat Prod 2003;66:419–422.
- Wu ZJ. Lipid chemical constituents of Salvia sochifolia C. Y. Wu. Zhongguo Zhong Yao Za Zhi 2001;26:264–265.
- Xu XZ, Tian X. Studies on chemical components of Gentiana tizuensis Franch. (I). Zhongguo Zhong Yao Za Zhi 2000;25:225–226.
- Riaz N, Anis I, Khan PM, Shah R, Malik A. Alysinol–a new triterpene from Alysicarpus monolifer. Nat Prod Lett 2002;16:415–418.
- Pires VS, Gosmann G, Guillaume D, Schenkel EP. Triterpenes and saponins from Ilex psammophila. Nat Prod Lett 2002;16:401–406.
- Akbar E, Malik A. Antimicrobial triterpenes from Debregeasia salicifolia. Nat Prod Lett 2002;16:339–344.
- Yang J, Song CQ, Hu ZB. Studies on constituents in Uncaria macrophylla Wall. Zhongguo Zhong Yao Za Zhi 2000;25:484–485.
- Zhang N, Li C, Yi X. Study on the chemical components of Rabdosia excisa. Zhong Yao Cai 1998;21:347–349.
- Zhang L, Feng Y, Ding A. The research on the chemical components of Schizonepeta tenuifolia Briq. Zhong Yao Cai 2001;24:183–184.
- Cheng L, Liu Y, Chen L, Luo J. Studies on the triterpenoidal saponins from flowers of Eriobotrya japonica. Hua Xi Yi Ke Da Xue Xue Bao 2001;32:283–285.
- Park JH, Son KH, Kim SW, Chang HW, Bae K, Kang SS et al. Anti-inflammatory activity of Synurus deltoides. Phytother Res 2004;18:930–933.
- Vági E, Rapavi E, Hadolin M, VásárhelyinéPerédi K, Balázs A, Blázovics A, Simándi B. Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J Agric Food Chem 2005;12:17–21.
- Urech K, Scher JM, Hostanska K, Becker H. Apoptosis inducing activity of viscin, a lipophilic extract from Viscum album L. J Pharm Pharmacol 2005;57:101–109.
- Brieskorn CH, Keskin M. Triterpenes in the bark, peel and leaf of Punica granatum L. 5. Knowledge of biochemistry of triterpenes. Pharm Acta Helv 1954;29:338–340.
- Chien MM, Svoboda GH, Schiff PL Jr, Slatkin DJ, Knapp JE. Chemical constituents of Echites hirsuta (Apocynaceae). J Pharm Sci 1979;68:247–249.
- Karikas Z, Euerby MR, Waigh RD. Chemical constituents of Rabdosia japonica var. glaucocalyx. Planta Med. 1987;12:38–9.
- Karikas GA, Euerby MR, Waigh RD. Constituents of the stems of Arbutus unedo. Planta Med 1987;53:223–224.
- Zou X, Liang J, Ding LS, Peng SL. Studies on chemical constituents of Paederia scandense. Zhongguo Zhong Yao Za Zhi 2006;31:1436–1441.
- Wang QH, Li ZY, Shen Y, Lin HW, Shu W, Zhou JB. Studies on triterpenoids from Potentilla chinensis. Zhongguo Zhong Yao Za Zhi 2006;31:1434–1436.
- Lou FM, Yang J, Bai ZC, Wu BF. Studies on chemical constituents in rhizome of Elaeagnus bockii I. Zhongguo Zhong Yao Za Zhi 2006;31:988–989.
- Zhang YH, Zhou L, Shi RB, Guo YJ, Dong Y. Studies on chemical constituents in spikes of Schizonepeta tenuifolia. Zhongguo Zhong Yao Za Zhi 2006;31:1247–1249.
- Zeng JW, Qian SH, Wu JZ, Yang NY. Studies on involatile constituents of Mentha haplocalyx. Zhongguo Zhong Yao Za Zhi 2006;31:400–402.
- Wang MY, Yang L, Tu YY. Studies on the chemical constituents from stem of Chirita longgangensis var. Hongyao. Zhongguo Zhong Yao Za Zhi 2006;31:307–308.
- Ahmed Z, Ali D, Malik A. Structure determination of ursene-type triterpenes by NMR techniques. Magn Reson Chem 2006;44:717–719.
- Zhang YH, Zhang JG, Xie JM, Chen GL, Cheng DL. Triterpenes from root of Rhaponticum uniflorum. Zhongguo Zhong Yao Za Zhi 2005;30:1833–1836.
- Wang FD, Ding L, Wang HQ. Studies on triterpenoid constituents from Rabdosia japonica var. galaucocalyx. Zhongguo Zhong Yao Za Zhi 2005;30:1929–1932.
- Yan J, Yang XW. Studies on the chemical constituents in herb of Ludwigia octovalvis. Zhongguo Zhong Yao Za Zhi 2005;30:1923–1926.
- Qi X, Yang Y, Ye Y. Study on chemical constituents from stem of Mallotus apelta. Zhong Yao Cai 2005;28:765–766.
- Yuan G, Yi Y. Studies on chemical constituents of the roots of Salacia hainanensis. Zhong Yao Cai 2005;28:27–29.
- Luo Y, Yi J, Li B, Zhang G. Novel ceramides and a new glucoceramide from the roots of Incarvillea arguta. Lipids 2004;39:907–913.
- Xiang W, Li RT, Mao YL, Zhang HJ, Li SH, Song QS et al. Four new prenylated isoflavonoids in Tadehagi triquetrum. J Agric Food Chem 2005;53:267–271.
- Tokar M, Klimek B. Isolation and identification of biologically active compounds from Forsythia viridissima flowers. Acta Pol Pharm 2004;61:191–197.
- Ma CH, Chen B, Qi HY, Li BG, Zhang GL. Two pyranocoumarins from the seeds of Calophyllum polyanthum. J Nat Prod 2004;67:1598–1600.
- Cao S, Guza RC, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DG. Cytotoxic triterpenoids from Acridocarpus vivy from the Madagascar rain forest. J Nat Prod 2004;67:986–989.
- Raju BL, Lin SJ, Hou WC, Lai ZY, Liu PC, Hsu FL. Antioxidant iridoid glucosides from Wendlandia formosana. Nat Prod Res 2004;18:357–364.
- Ragasa CY, Tiu F, Rideout JA. New cycloartenol esters from Ixora coccinea. Nat Prod Res 2004;18:319–323.
- Dallavalle S, Jayasinghe L, Kumarihamy BM, Merlini L, Musso L, Scaglioni L. A new 3,4-seco-lupane derivative from Lasianthus gardneri. J Nat Prod 2004;67:911–913.
- Jang DS, Park EJ, Kang YH, Vigo JS, Graham JG, Cabieses F et al. Phenolic compounds obtained from stems of Couepia ulei with the potential to induce quinone reductase. Arch Pharm Res 2004;27:169–172.
- Liu S, Qin Y, Du FL. Studies on chemical constituents in leafs of Ilex kudingcha. Zhongguo Zhong Yao Za Zhi 2003;28:834–836.
- Zhang PC, Wu Y, Yu DQ. Chemical constituents from the leaves of Dalbergia hainanensis. Zhongguo Zhong Yao Za Zhi 2003;28:527–530.
- Guo J, Liu LH, Mei SX, Zhao JF, Ma ZR, Li L. Studies on chemical constituents from the stems of Schisandra sphaerandra. Zhongguo Zhong Yao Za Zhi 2003;28:138–140.
- Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava. Nat Prod Res 2004;18:135–140.
- Xue PF, Qiao L, Liang H, Zhao YY. Studies on the chemical constituents from Potentilla multifida L. Beijing Da Xue Xue Bao 2004;36:21–23.
- Chang CI, Kuo CC, Chang JY, Kuo YH. Three new oleanane-type triterpenes from Ludwigia octovalvis with cytotoxic activity against two human cancer cell lines. J Nat Prod 2004;67:91–93.
- Jung HA, Chung HY, Jung JH, Choi JS. A new pentacyclic triterpenoid glucoside from Prunus serrulata var. spontanea. Chem Pharm Bull 2004;52:157–159.
- Chen WS, Jia XM, Zhang WD, Lou ZY, Qiao CZ. Chemical constituents in the roots of Salvia przewalskii Maxim. Yao Xue Xue Bao 2003;38:354–357.
- Skrzypek Z, Wysokinska H. Sterols and triterpenes in cell culture of Hyssopus officinalis L. Z Naturforsch, C, J Biosci 2003;58:308–312.
- Chen B, Zhu M, Xing WX, Yang GJ, Mi HM, Wu YT. Studies on chemical constituents in fruit of Eucalyptus globulus. Zhongguo Zhong Yao Za Zhi 2002;27:596–597.
- Ma XJ, Du CF, Zheng JH, Chen XZ. Studies on chemical constituents of Gaultheria leucocarpa var. Yunnanensis (Franch.) T. Z. Hsu R. C. & Fang. Zhongguo Zhong Yao Za Zhi 2001;26:844–845.
- Ren FZ, Luan XH, Zhao YM, Qu HH. Studies on flavonoids from leaves of Callicarpa bodinieri Lev. Zhongguo Zhong Yao Za Zhi 2001;26:841–844.
- Wang GL, Hou QY, Zhang J, Xu JM, Peng JF, Lin RC. Studies on the chemical constituents of the stems of Alyxia sinensis (I). Zhongguo Zhong Yao Za Zhi 2002;27:125–127.
- Cheng J, Bai YJ, Zhao YY, Wang B, Cheng TM. Studies on the phenylpropanoids from Eucommia ulmoides. Zhongguo Zhong Yao Za Zhi 2002;27:38–40.
- Li C, Sun YR, Sun YF. Chemical composition of fructus Liquidambaris-lulutong. Yao Xue Xue Bao 2002;37:263–266.
- Jin YR, Gui MY, Wang BZ. Studies on chemical constituents in the roots of Rabdosia japonica (Burm.f). Hara var. Glaucocalyx (Maxin) Hara. Zhongguo Zhong Yao Za Zhi 2000;25:678–679.
- Lee S, Ji J, Shin KH, Kim BK. Sessiline, a new nitrogenous compound from the fruits of Acanthopanax sessiliflorus. Planta Med 2002;68:939–941.
- Wen D, Zheng X, Inoue K. Studies on chemical constituents of Ilex Merr. Zhongguo Zhong Yao Za Zhi 1999;24:223–5, 255.
- Lee S, Kim BK, Cho SH, Shin KH. Phytochemical constituents from the fruits of Acanthopanax sessiliflorus. Arch Pharm Res 2002;25:280–284.
- Zheng X, Xu Y, Xu J. Chemical studies on Pyrrosia gralla (Gies.) Ching. Zhongguo Zhong Yao Za Zhi 1998;23:98–9, 128.
- Zhou F, Liang P, Zhou Q, Qin Z. Chemical constituents of the stem and root of Syzygium buxifolium Hook. Et Arn. Zhongguo Zhong Yao Za Zhi 1998;23:164–5, 192.
- Upadhyay RK, Pandey MB, Jha RN, Pandey VB. Eclalbatin, a triterpene saponin from Eclipta alba. J Asian Nat Prod Res 2001;3:213–217.
- Lu X, Xu W, Shen J, Han G. Chemical studies on Campylotropis hirtella (Franch. Schindl.). Zhongguo Zhong Yao Za Zhi 1997;22:680–2, 704.
- Huang H, Zhao S, Wang M, Liu Q, Sun H. Chemical constituents in the stem and leaf of Isodon oresbius. Zhongguo Zhong Yao Za Zhi 1998;23:37–8, 62.
- Huang J, Wang X, Ogihara Y, Shimizu N, Takeda T, Akiyama T. Latifolosides I and J, two new triterpenoid saponins from the bark of Ilex latifolia. Chem Pharm Bull 2001;49:239–241.
- Siddiqui BS, Sultana I, Begum S. Triterpenoidal constituents from Eucalyptus camaldulensis var. obtusa leaves. Phytochemistry 2000;54:861–865.
- Prasad D, Juyal V, Singh R, Singh V, Pant G, Rawat MS. A new secoiridoid glycoside from Lonicera angustifolia. Fitoterapia 2000;71:420–424.
- Hou AJ, Yang H, Jiang B, Zhao QS, Lin ZW, Sun HD. A new ent-kaurane diterpenoid from Isodon phyllostachys. Fitoterapia 2000;71:417–419.
- Chang CW, Wu TS, Hsieh YS, Kuo SC, Chao PD. Terpenoids of Syzygium formosanum. J Nat Prod 1999;62:327–328.
- Fullas F, Hussain RA, Chai HB, Pezzuto JM, Soejarto DD, Kinghorn AD. Cytotoxic constituents of Baccharis gaudichaudiana. J Nat Prod 1994;57:801–807.
- Shammas G, Philianos S, Verykokidou-Vitsaropoulou E. Chemical constituents of the flowers of Fraxinus ornus L. Ann Pharm Fr 1990;48:13–16.
- Isobe T, Noda Y, Ohsaki A, Sakanaka S, Kim M, Taniguchi M. Studies on the constituents of Leucoseptrum stellipillum. Yakugaku Zasshi 1989;109:175–178.
- Zeng X, Fang Z, Ma J. Chemical constituents of Osbeckia chinensis L. Zhongguo Zhong Yao Za Zhi 1991;16:99–101, 127.
- Fang Z, Yang Y, Zhou G. Isolation and identification of chemical constituents from Hedyotis chrysotricha (Palib.) Merr. Zhongguo Zhong Yao Za Zhi 1992;17:98–100, 127.
- Shi Y, Wang H. Chemical constituents of Actinidia arguta (Sieb. et Zucc.) Planch. ex Miquel. Zhongguo Zhong Yao Za Zhi 1992;17:36–8, 64.
- González AG, Barrera JB, Pérez EM, Padrón CE. Chemical Constituents of the Lichen Ramalina hierrensis. Planta Med 1992;58:214–218.
- Xu M, Xiong B, Cheng P, Guo Y. Chemical constituents of Isodon albopilosus (C.Y. Wu et H.W. Li) Hara. Zhongguo Zhong Yao Za Zhi 1992;17:228–30, 256.
- Nakanishi T, Tanaka K, Murata H, Somekawa M, Inada A. Phytochemical studies of seeds of medicinal plants. III. Ursolic acid and oleanolic acid glycosides from seeds of Patrinia scabiosaefolia Fischer. Chem Pharm Bull 1993;41:183–186.
- Bisio A, De Tommasi N, Romussi G. Triterpenoids from Salvia wagneriana. Pharmazie 2004;59:309–311.
- Ma CM, Jia SS, Sun T, Zhang YW. Triterpenes and steroidal compounds from Cynomorium songaricum. Yao Xue Xue Bao 1993;28:152–155.
- Pan WD, Li YJ, Mai LT, Ohtani KH, Kasai RT, Tanaka O et al. Studies on triterpenoid constituents of the roots of Lantana camara. Yao Xue Xue Bao 1993;28:40–44.
- Ahme R, Ifza SM, Saifuddin A, Nazeer M, Pak. Studies on triterpenoid constituents of the roots of Lantana camara. J Pharm Sci 1995;8:69–71.
- Gosmann G, Guillaume D, Taketa AT, Schenkel EP. Triterpenoid saponins from Ilex paraguariensis. J Nat Prod 1995;58:438–441.
- Miyase T, Shiokawa KI, Zhang DM, Ueno A. Araliasaponins I-XI, triterpene saponins from the roots of Aralia decaisneana. Phytochemistry 1996;41:1411–1418.
- Miyase T, Melek FR, El-Gindi OD, Abdel-Khalik SM, El-Gindi MR, Haggag MY, Hilal SH. Phytochemistry 1996;4:11175–9.
- Takeoka G, Dao L, Teranishi R, Wong R, Flessa S, Harden L et al. Identification of three triterpenoids in almond hulls. J Agric Food Chem 2000;48:3437–3439.
- Liu YQ, You S, Tashiro S, Onodera S, Ikejima T. Oridonin induced U937 cell apoptosis through ERK pathway. Zhongguo Zhong Yao Za Zhi 2005;30:1856–1859.
- Melek FR, Miyase T, el-Gindy MR, Abdel-Khalik SM, Ghaly NS, el-Kady M. Saponins from Fagonia glutinosa. Pharmazie 2000;55:772–776.
- Liu P, Duan HQ, Pan Q, Zhang YW, Yao Z. Triterpenes from herb of Potentilla chinesis. Zhongguo Zhong Yao Za Zhi 2006;31:1875–1879.
- Vági E, Rapavi E, Hadolin M, VásárhelyinéPerédi K, Balázs A, Blázovics A et al. Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J Agric Food Chem 2005;53:17–21.
- Zhao Y, Ruan JL, Wang JH, Cong Y, Song S, Cai YL et al. Chemical constituents of radix Ranunculus ternati. Nat Prod Res 2008;22:233–240.
- Jiang T, Huang BK, Zhang QY, Han T, Zheng HC, Qin LP. Studies on chemical constituents of Liriope platyphylla. Zhong Yao Cai 2007;30:1079–1081.
- Liu L, Chen YP, Wan Z, Li AL, Li RY, Tu PF. Studies on chemical constituents of in herb Pyrola calliatha. Zhongguo Zhong Yao Za Zhi 2007;32:1762–1765.
- Ding LL, Wang SC, Wang ZT. Studies on chemical constituents from root of Actinidia macrosperma. Zhongguo Zhong Yao Za Zhi 2007;32:1893–1895.
- Ji TF, Liu CH, Wang AG, Yang JB, Su YL, Yuan L et al. Studies on the chemical constituents of Urtica dioica L. grown in Tibet Autonomous Region. Zhong Yao Cai 2007;30:662–664.
- Tang LY, Luo MH, Jiang N, Lin HH. Studies on chemical constituents of Sambucus adnata. Zhong Yao Cai 2007;30:549–551.
- Huang L, Zhang RS, Wang CF, Zhang SF. Studies on chemical constituents of Patrinia villosa. Zhong Yao Cai 2007;30:415–417.
- Zhang S, Geshang SL, Dawa ZM, Zhou Y, Peng SL. Studies on chemical constituents of Eriophyton wallichii. Zhongguo Zhong Yao Za Zhi 2007;32:824–826.
- Yawer MA, Ahmed E, Malik A, Ashraf M, Rasool MA, Afza N. New lipoxygenase-inhibiting constituents from Calligonum polygonoides. Chem Biodivers 2007;4:1578–1585.
- Begum S, Ali SN, Hassan SI, Siddiqui BS. A new ethylene glycol triterpenoid from the leaves of Psidium guajava. Nat Prod Res 2007;21:742–748.
- Duan WD, Zhang J, Xie G, Zhang CZ, Li C. Chemical constituents from the flower of Paulownia fortunei (seem.) Hems. Zhong Yao Cai 2007;30:168–170.
- Wei J, Zhu HY, Shen DF, Yang B, Yang XS. Studies on the chemical constituents of Vaccinium iteophyllum. Zhong Yao Cai 2007;30:47–49.
- Yang SM, Liu XK, Qing C, Wu DG, Zhu DY. Chemical constituents from the roots of Homonoia riparia. Yao Xue Xue Bao 2007;42:292–296.
- Wu JY, Li GC, Wang DY. Chemical constituents of the non-alkaloid fraction of Uncaria macrophylla. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:226–227.
- da Silva VC, de Carvalho MG, Alves AN. Chemical constituents from leaves of Palicourea coriacea (Rubiaceae). J Nat Med 2008;62:356–357.
- Qi SH, Wu DG, Zhang S, Luo XD. Constituents of Carapa guianensis Aubl. (Meliaceae). Pharmazie 2004;59:488–490.
- Huang H, Chao QR, Tan RX, Sun HD, Wang DC, Ma J et al. A new rosmarinic acid derivative from Isodon oresbius. Planta Med 1999;65:92–93.
- Liu RH, Yu BY. Study on the chemical constituents of the leaves from Crataegus pinnatifida Bge. var. major N. E. Br. Zhong Yao Cai 2006;29:1169–1173.
- Moghaddam FM, Farimani MM, Salahvarzi S, Amin G. Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. Evid Based Complement Alternat Med 2007;4:95–98.
- Khaliq S, Volk FJ, Frahm AW. Phytochemical investigation of Perovskia abrotanoides. Planta Med 2007;73:77–83.
- Liao QF, Xie SP, Chen XH, Bi KS. Study on the chemical constituents of Sambucus chinensis Lindl. Zhong Yao Cai 2006;29:916–918.
- Gören AC, Zhou BN, Topçu G, Kökdil G, Kingston DG. DNA-damaging activities of methanol extract of Ajuga postii and iridoid glucoside reptoside. Nat Prod Res 2005;19:457–460.
- Wang L, Wang HQ, Chen RY. Studies on chemical constituents from bark of Morus nigra. Zhongguo Zhong Yao Za Zhi 2007;32:2497–2499.
- Jiang X, Zhang JC, Liu YW, Fang Y. Studies on chemical constituents of Valeriana officinalis. Zhong Yao Cai 2007;30:1391–1393.
- Zhao DB, Yang YX, Liu XH, Wang HQ. Studies on chemical constituents in herb of Chondrilla piptocoma. Zhongguo Zhong Yao Za Zhi 2005;30:588–590.
- Wang B, Liu P, Shen YM, Dai C. Studies on the chemical constituents from herb of Rhodobryum roseum. Zhongguo Zhong Yao Za Zhi 2005;30:895–897.
- Rana VS, Rawat MS. A new xanthone glycoside and antioxidant constituents from the rhizomes of Swertia speciosa. Chem Biodivers 2005;2:1310–1315.
- Khan MI, Ahmed N, Haqqani MH. Xanthones and ursolic acid from Swertia species. Planta Med 1977;32:280–281.
- Said IM, Chun NC, Houghton PJ. Ursolic acid from Mitragyna speciosa. Planta Med 1991;57:398.
- Fang X, Nanayakkara NP, Phoebe CH Jr, Pezzuto JM, Kinghorn AD, Farnsworth NR. Plant Anticancer agents XXXVII. Constituents of Amanoa oblongifolia1. Planta Med 1985;51:346–347.
- Lee I, Lee J, Lee YH, Leonard J. Ursolic acid-induced changes in tumor growth, O2 consumption, and tumor interstitial fluid pressure. Anticancer Res 2001;21:2827–2833.
- Zheng QF, Sun HX, He QJ, Ye YP. Induction of apoptosis in HeLa cells by 3beta-hydroxyurs-12-en-27-oic acid. Chem Biodivers 2006;3:742–753.
- Tokuda H, Ohigashi H, Koshimizu K, Ito Y. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett 1986;33:279–285.
- Yarosh DB, Both D, Brown D. Liposomal ursolic acid (Merotaine) increases ceramides and collagen in human skin. Horm Res 2000;54:318–321.
- Both DM, Goodtzova K, Yarosh DB, Brown DA. Liposome-encapsulated ursolic acid increases ceramides and collagen in human skin cells. Arch Dermatol Res 2002;293:569–575.
- Shin KM, Kim RK, Azefack TL, David L, Luc SB, Choudhary MI et al. In vitro anti-inflammatory activity of 23-hydroxyursolic acid isolated from Cussonia bancoensis in murine macrophage RAW 264.7 cells. Planta Med 2004;70:803–807.
- Yim EK, Lee MJ, Lee KH, Um SJ, Park JS. Antiproliferative and antiviral mechanisms of ursolic acid and dexamethasone in cervical carcinoma cell lines. Int J Gynecol Cancer 2006;16:2023–2031.
- Liu Z, Ma R, Wang Y. Study on the extracting of ursolic acid by uniform design. Zhong Yao Cai 2005;28:425–427.
- Brieskorn CH, Hofmann H. On the quantitative determination of ursolic acid and oleanolic acid by means of the Liebermann–Burchard reaction. Arch Pharm 1962;295/67:505–509.
- Shi HM, Long BS, Cui XM, Min ZD. A new bisabolane sesquiterpenoid from Euphorbia chrysocoma. J Asian Nat Prod Res 2005;7:857–860.
- Zhou C, Chen K, Sun C, Chen Q, Zhang W, Li X. Determination of oleanolic acid, ursolic acid and amygdalin in the flower of Eriobotrya japonica Lindl. by HPLC. Biomed Chromatogr 2007;21:755–761.
- Chen JH, Xia ZH, Tan RX. High-performance liquid chromatographic analysis of bioactive triterpenes in Perilla frutescens. J Pharm Biomed Anal 2003;32:1175–1179.
- Yang P, Li Y, Liu X, Jiang S. Determination of free isomeric oleanolic acid and ursolic acid in Pterocephalus hookeri by capillary zone electrophoresis. J Pharm Biomed Anal 2007;43:1331–1334.
- Ding H, Wang Y, Wang S, You W. Quantitative determination of ursolic acid in Herba cynomorii by ultraviolet spectrophotometry. Zhongguo Zhong Yao Za Zhi 1998;23:102–3, inside back cover.
- Song LL, Fan BY, Xu XJ, Lu PW, Xiang BR. Determination of ursolic acid of Liuwei Dihuangwan simulation samples by NIR. Zhongguo Zhong Yao Za Zhi 2006;31:1590–1593.
- Guo Y, Cui Y, Zhou J, Yan Y, Yuan P. Quantitative determination of ursolic acid in Folium ilicis cornutae (gouguye) gathered in different and periods. Zhongguo Zhong Yao Za Zhi 1995;20:591–2, 638.
- Jia YY, Yan XS, Nie K. Comparative analysis of ursolic acid in Crataegus pinnatifida Bge. var. major N.E. Br. before and after processing. Zhongguo Zhong Yao Za Zhi 1989;14:466–8, 510.
- Jung SH, Ha YJ, Shim EK, Choi SY, Jin JL, Yun-Choi HS et al. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J 2007;403:243–250.
- Novotny L, Abdel-Hamid ME, Hamza H, Masterova I, Grancai D. Development of LC-MS method for determination of ursolic acid: application to the analysis of ursolic acid in Staphylea holocarpa Hemsl. J Pharm Biomed Anal 2003;31:961–968.
- Liao Q, Yang W, Jia Y, Chen X, Gao Q, Bi K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional Chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi 2005;125:509–515.
- Lee WS, Im KR, Park YD, Sung ND, Jeong TS. Human ACAT-1 and ACAT-2 inhibitory activities of pentacyclic triterpenes from the leaves of Lycopus lucidus TURCZ. Biol Pharm Bull 2006;29:382–384.
- Huang L, Chen T, Ye Z, Chen G. Use of liquid chromatography-atmospheric pressure chemical ionization-ion trap mass spectrometry for identification of oleanolic acid and ursolic acid in Anoectochilus roxburghii (wall.) Lindl. J Mass Spectrom 2007;42:910–917.
- Gu JQ, Wang Y, Franzblau SG, Montenegro G, Timmermann BN. Dereplication of pentacyclic triterpenoids in plants by GC-EI/MS. Phytochem Anal 2006;17:102–106.
- Klimek B, Modnicki D. Terpenoids and sterols from Nepeta cataria L. var. citriodora (Lamiaceae). Acta Pol Pharm 2005;62:231–235.
- Wang HB, Zhang ZY, Zia ZX, Su Z, Li CK. Qualitative analysis of Chinese drug xiakucao (Prunella). Zhongguo Zhong Yao Za Zhi 1993;7:702.
- Gu BP, Zhu L, Ke WZ, Chen JW. Comparative study of the spectral characteristics of ursolic acid between laser Raman spectra and IR spectrum. Guang Pu Xue Yu Guang Pu Fen Xi 2002;22:970–972.
- Fang X, Nanayakkara NP, Phoebe CH Jr, Pezzuto JM, Kinghorn AD, Farnsworth NR. Plant Anticancer agents XXXVII. Constituents of Amanoa oblongifolia1. Planta Med 1985;51:346–347.
- Trumbull ER, Bianchi E, Eckert DJ, Wiedhopf RM, Cole JR. Tumor inhibitory agents from Vauquelinia corymbosa (Rosaceae). J Pharm Sci 1976;65:1407–1408.
- Shen YM, Zhu X, Zhang KH, Xie Y, Chen J, Dai Y et al. Effect of ursolic acid on proliferation and apoptosis of hepatic stellate cells in vitro. Zhonghua Gan Zang Bing Za Zhi 2008;16:298–301.
- Kang WY, Du ZZ, Yang XS, Hao XJ. A new triterpene from Luculia pinciana Hook. J Asian Nat Prod Res 2005;7:91–94.
- Miceli N, Taviano MF, Giuffrida D, Trovato A, Tzakou O, Galati EM. Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham. J Ethnopharmacol 2005;97:261–266.
- Chattopadhyay D, Arunachalam G, Mandal SC, Bhadra R, Mandal AB. CNS activity of the methanol extract of Mallotus peltatus (Geist) Muell Arg. leaf: an ethnomedicine of Onge. J Ethnopharmacol 2003;85:99–105.
- Chattopadhyay D, Dungdung SR, Mandal AB, Majumder GC. A potent sperm motility-inhibiting activity of bioflavonoids from an ethnomedicine of Onge, Alstonia macrophylla Wall ex A. DC, leaf extract. Contraception 2005;71:372–378.
- Zhang P, Li X, Li N, Xu J, Li ZL, Wang Y et al. Antibacterial constituents from fruit bodies of Ascomyce Bulgaria inquinans. Arch Pharm Res 2005;28:889–891.
- Chattopadhyay D, Arunachalam G, Ghosh L, Rajendran K, Mandal AB, Bhattacharya SK. Antipyretic activity of Alstonia macrophylla Wall ex A. DC: an ethnomedicine of Andaman Islands. J Pharm Pharm Sci 2005;8:558–564.
- Ali H, Houghton PJ, Soumyanath A. alpha-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006;107:449–455.
- Chaturvedula VS, Gao Z, Jones SH, Feng X, Hecht SM, Kingston DG. A new ursane triterpene from Monochaetum vulcanicum that inhibits DNA polymerase beta lyase. J Nat Prod 2004;67:899–901.
- Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC. Antiviral activities of extracts and ursolic acid constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol. 2005;32:811–816.
- Cipak L, Grausova L, Miadokova E, Novotny L, Rauko P. Dual activity of triterpenoids: apoptotic versus antidifferentiation effects. Arch Toxicol 2006;80:429–435.
- Chiang YM, Chang JY, Kuo CC, Chang CY, Kuo YH. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005;66:495–501.
- Gnoatto SC, Dassonville-Klimpt A, Da Nascimento S, Galéra P, Boumediene K, Gosmann G, Sonnet P, Moslemi S. Antioxidant, anti-inflammatory, and anticancer activities of extracts from Ledum groenlandicum Retzius. Eur J Med Chem 2007;7.
- Muto Y, Ninomiya M, Fujiki H. Present status of research on cancer chemoprevention in Japan. Jpn J Clin Oncol 1990;20:219–224.
- Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res 2008;52:26–42.
- Lim SW, Hong SP, Jeong SW, Kim B, Bak H, Ryoo HC et al. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-alpha. J Dermatol 2007;34:625–634.
- Yin MC, Chan KC. Non-enzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J Agric Food Chem 2007;55:7177–7181.
- Andersson D, Cheng Y, Duan RD. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats. J Cancer Res Clin Oncol 2008;134:101–107.
- Vidya SM, Krishna V, Manjunatha BK, Mankani KL, Ahmed M, Singh SD. Indian Evaluation of hepatoprotective activity of Clerodendrum serratum L., Indian J Exp Biol 2007;45:538–542.
- Balanehru S, Nagarajan B. Protective effect of oleanolic acid and ursolic acid against lipid peroxidation. Biochem Int 1991;24:981–990.
- Ovesná Z, Kozics K, Slamenová D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat Res 2006;600:131–137.
- Sultana N, Choudhary MI, Khan A. Protein glycation inhibitory activities of Lawsonia inermis and its active principles. J Enzyme Inhib Med Chem 2009;24:257–261.
- Vidya SM, Krishna V, Manjunatha BK, Mankani KL, Ahmed M, Singh SD. Evaluation of hepatoprotective activity of Clerodendrum serratum L. Indian J Exp Biol 2007;45:538–542.
- Senthil S, Chandramohan G, Pugalendi KV. Isomers (oleanolic and ursolic acids) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. Int J Cardiol 2007;119:131–133.
- Achiwa Y, Hasegawa K, Udagawa Y. Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci Biotechnol Biochem 2007;71:31–37.
- Kassi E, Papoutsi Z, Pratsinis H, Aligiannis N, Manoussakis M, Moutsatsou P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J Cancer Res Clin Oncol 2007;133:493–500.
- He X, Liu RH. Cranberry phytochemicals: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem 2006;54:7069–7074.
- Es-Saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C. MCF-7 cell-cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer Res 1996;16:481–486.
- Saravanan R, Pugalendi V. Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart. Pharmacol Rep 2006;58:41–47.
- Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol 2007;74:1078–1090.
- Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J Agric Food Chem 2006;54:243–248.
- Lee CJ, Seok JH, Hur GM, Lee JH, Park JS, Seol IC et al. Effects of ursolic acid, betulin and sulfur-containing compounds on mucin release from airway goblet cells. Planta Med 2004;70:1119–1122.
- Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS et al. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci 2004;74:2769–2779.
- Harmand PO, Duval R, Liagre B, Jayat-Vignoles C, Beneytout JL, Delage C et al. Ursolic acid induces apoptosis through caspase-3 activation and cell-cycle arrest in HaCaT cells. Int J Oncol 2003;23:105–112.
- Lauthier F, Taillet L, Trouillas P, Delage C, Simon A. Ursolic acid triggers calcium-dependent apoptosis in human Daudi cells. Anticancer Drugs 2000;11:737–745.
- Martín-Cordero C, Reyes M, Ayuso MJ, Toro MV. Cytotoxic triterpenoids from Erica andevalensis. Z Naturforsch, C, J Biosci 2001;56:45–48.
- Hsu YL, Kuo PL, Lin CC. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci 2004;75:2303–2316.
- Achiwa Y, Hasegawa K, Komiya T, Udagawa Y. Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep 2005;13:51–57.
- Yim EK, Lee KH, Namkoong SE, Um SJ, Park JS. Proteomic analysis of ursolic acid-induced apoptosis in cervical carcinoma cells. Cancer Lett 2006;235:209–220.
- Achiwa Y, Hasegawa K, Udagawa Y. Molecular mechanism of ursolic acid-induced apoptosis in poorly differentiated endometrial cancer HEC108 cells. Oncol Rep 2005;14:507–512.
- Tang X, Gao J, Chen J, Fang F, Wang Y, Dou H et al. Inhibition by (corrected) ursolic acid of (corrected) calcium-induced mitochondrial permeability transition and release of two pro-apoptotic proteins. Biochem Biophys Res Commun 2005;337:320–324.
- Harmand PO, Duval R, Delage C, Simon A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int J Cancer 2005;114:1–11.
- Choi YH, Baek JH, Yoo MA, Chung HY, Kim ND, Kim KW. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int J Oncol 2000;17:565–571.
- Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Chung HY et al. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int J Cancer 2000;87:629–636.
- Zhang YY, Deng T, Hu ZF, Zhang QP, Zhang J, Jiang H. Mechanisms of inhibiting proliferation and inducing apoptosis of human gastric cancer cell line SGC7901 by ursolic acid. Ai Zheng 2006;25:432–437.
- Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res 2007;5:943–955.
- Martin-Aragón S, de las Heras B, Sanchez-Reus MI, Benedi J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol 2001;53:199–206.
- Liu J, Liu Y, Mao Q, Klaassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam Appl Toxicol 1994;22:34–40.
- Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS, Kim TS et al. Antiallergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med 2000;66:358–360.
- Min BS, Jung HJ, Lee JS, Kim YH, Bok SH, Ma CM et al. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med 1999;65:374–375.
- Liu XS, Jiang J. Induction of apoptosis and regulation of the MAPK pathway by ursolic acid in human leukemia K562 cells. Planta Med 2007;73:1192–1194.
- Slamenová D, Horváthová E, Bartková M, Krajcovicová Z, Lábaj J, Kosíková B et al. Reduction of DNA-damaging effects of anti-HIV drug 3′-azido-3′-dideoxythymidine on human cells by ursolic acid and lignin biopolymer. Neoplasma 2006;53:485–491.
- Steele JC, Warhurst DC, Kirby GC, Simmonds MS. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res 1999;13:115–119.
- Paik KJ, Jeon SS, Chung HY, Lee KH, Kim KW, Chung JK et al. Induction of differentiation of the cultured rat mammary epithelial cells by triterpene acids. Arch Pharm Res 1998;21:398–405.
- Tan GT, Lee S, Lee IS, Chen J, Leitner P, Besterman JM et al. Natural-product inhibitors of human DNA ligase I. Biochem J 1996;314 (Pt 3):993–1000.
- Sohn KH, Lee HY, Chung HY, Young HS, Yi SY, Kim KW. Anti-angiogenic activity of triterpene acids. Cancer Lett 1995;94:213–218.
- Cárdenas C, Quesada AR, Medina MA. Effects of ursolic acid on different steps of the angiogenic process. Biochem Biophys Res Commun 2004;320:402–408.
- Jeong GS, An RB, Pae HO, Oh GS, Chung HT, Kim YC. Heme oxygenase-1 inducing constituent of Prunella vulgaris in HepG2 cells. Biol Pharm Bull 2008;31:531–533.
- Bonaccorsi I, Altieri F, Sciamanna I, Oricchio E, Grillo C, Contartese G et al. Endogenous reverse transcriptase as a mediator of ursolic acid’s antiproliferative and differentiating effects in human cancer cell lines. Cancer Lett 2008;263:130–139.
- Ramos AA, Lima CF, Pereira ML, Fernandes-Ferreira M, Pereira-Wilson C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: evaluation by the comet assay. Toxicol Lett 2008;177:66–73.
- Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol Pharm Bull 2007;30:1147–1149.
- Steinkamp-Fenske K, Bollinger L, Völler N, Xu H, Yao Y, Bauer R et al. Ursolic acid from the Chinese herb danshen (Salvia miltiorrhiza L.) upregulates eNOS and downregulates Nox4 expression in human endothelial cells. Atherosclerosis 2007;195:e104–e111.
- Huang J, Sun Y, Lu S. Experimental study on apoptosis induced by ursolic acid isolated from asparagus in HL-60 cells. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19:296–298.
- Andersson D, Liu JJ, Nilsson A, Duan RD. Ursolic acid inhibits proliferation and stimulates apoptosis in HT-29 cells following activation of alkaline sphingomyelinase. Anticancer Res 2003;23:3317–3322.
- Nataraju A, Raghavendra Gowda CD, Rajesh R, Vishwanath BS. Group IIA secretory PLA2 inhibition by ursolic acid: a potent anti-inflammatory molecule. Curr Top Med Chem 2007;7:801–809.
- Ikeda Y, Murakami A, Fujimura Y, Tachibana H, Yamada K, Masuda D et al. Aggregated ursolic acid, a natural triterpenoid, induces IL-1beta release from murine peritoneal macrophages: role of CD36. j Immunol 2007;178:4854–4864.
- Lai MY, Leung HW, Yang WH, Chen WH, Lee HZ. Up-regulation of matrix metalloproteinase family gene involvement in ursolic acid-induced human lung non-small carcinoma cell apoptosis. Anticancer Res 2007;27:145–153.
- Ali MS, Ibrahim SA, Jalil S, Choudhary MI. Ursolic acid: a potent inhibitor of superoxides produced in the cellular system. Phytother Res 2007;21:558–561.
- Jedinák A, Mucková M, Kost’álová D, Maliar T, Masterova I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z Naturforsch, C, J Biosci 2006;61:777–782.
- Heo HJ, Kim C, Lee HJ, Kim YS, Kang SS, Seo UK et al. Carbenoxolone and triterpenoids inhibited mucin secretion from airway epithelial cells. Phytother Res 2007;21:462–465.
- Ikeda Y, Murakami A, Nishizawa T, Ohigashi H. Ursolic acid enhances cyclooxygenases and tumor necrosis factor-alpha expression in mouse skin. Biosci Biotechnol Biochem 2006;70:1033–1037.
- Aguirre-Crespo F, Vergara-Galicia J, Villalobos-Molina R, Javier López-Guerrero J, Navarrete-Vázquez G, Estrada-Soto S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci 2006;79:1062–1068.
- Moon HK, Yang ES, Park JW. Protection of peroxynitrite-induced DNA damage by dietary antioxidants. Arch Pharm Res 2006;29:213–217.
- Thuong PT, Min BS, Jin W, Na M, Lee J, Seong R et al. Anti-complementary activity of ursane-type triterpenoids from Weigela subsessilis. Biol Pharm Bull 2006;29:830–833.
- Zhang GP, Lu YY, Lv JC, Ou HJ. Effect of ursolic acid on caspase-3 and PARP expression of human MCF-7 cells. Zhongguo Zhong Yao Za Zhi 2006;31:141–144.
- Na M, Yang S, He L, Oh H, Kim BS, Oh WK et al. Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata. Planta Med 2006;72:261–263.
- Ahmad SF, Khan B, Bani S, Suri KA, Satti NK, Qazi GN. Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol Res 2006;53:233–240.
- Oh CJ, Kil IS, Park CI, Yang CH, Park JW. Ursolic acid regulates high glucose-induced apoptosis. Free Radic Res 2007;41:638–644.
- Ikeda Y, Murakami A, Ohigashi H. Ursolic acid promotes the release of macrophage migration inhibitory factor via ERK2 activation in resting mouse macrophages. Biochem Pharmacol 2005;70:1497–1505.
- Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 2005;32:811–816.
- Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci 2006;78:713–718.
- Ren D, Zuo R, González Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol 2005;71:4022–4034.
- Saeidnia S, Gohari AR, Uchiyama N, Ito M, Honda G, Kiuchi F. Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. Chem Pharm Bull 2004;52:1249–1250.
- Rocha AD, de Oliveira AB, de Souza Filho JD, Lombardi JA, Braga FC. Antifungal constituents of Clytostoma ramentaceum and Mansoa hirsuta. Phytother Res 2004;18:463–467.
- Gu JQ, Wang Y, Franzblau SG, Montenegro G, Yang D, Timmermann BN. Antitubercular constituents of Valeriana laxiflora. Planta Med 2004;70:509–514.
- Shih YH, Chein YC, Wang JY, Fu YS. Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci Lett 2004;362:136–140.
- Jin JL, Lee YY, Heo JE, Lee S, Kim JM, Yun-Choi HS. Anti-platelet pentacyclic triterpenoids from leaves of Campsis grandiflora. Arch Pharm Res 2004;27:376–380.
- Murakami S, Takashima H, Sato-Watanabe M, Chonan S, Yamamoto K, Saitoh M et al. Ursolic acid, an antagonist for transforming growth factor (TGF)-beta1. FEBS Lett 2004;566:55–59.
- Soo Lee Y, Jin DQ, Beak SM, Lee ES, Kim JA. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur J Pharmacol 2003;476:173–178.
- Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase-2, matrix metalloproteinase-9, and cyclin D1. Cancer Res 2003;63:4375–4383.
- Li Y, Ishibashi M, Satake M, Chen X, Oshima Y, Ohizumi Y. Sterol and triterpenoid constituents of Verbena littoralis with NGF-potentiating activity. J Nat Prod 2003;66:696–698.
- Satomi Y, Nishino H, Shibata S. Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res 2005;25:4043–4047.
- Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papapeorgiou VP. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother Res 2003;17:501–507.
- Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003;10:115–121.
- Chiang LC, Chiang W, Chang MY, Ng LT, Lin CC. Antileukemic activity of selected natural products in Taiwan. Am J Chin Med 2003;31:37–46.
- Jiwajinda S, Santisopasri V, Murakami A, Kim OK, Kim HW, Ohigashi H. Suppressive effects of edible thai plants on superoxide and nitric oxide generation. Asian Pac J Cancer Prev 2002;3:215–223.
- Tapondjou LA, Lontsi D, Sondengam BL, Choi J, Lee KT, Jung HJ et al. In vivo anti-nociceptive and anti-inflammatory effect of the two triterpenes, ursolic acid and 23-hydroxyursolic acid, from Cussonia bancoensis. Arch Pharm Res 2003;26:143–146.
- Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res 2003;17:129–134.
- Lee S, Min B, Kho Y. Brine shrimp lethality of the compounds from Phryma leptostachya L. Arch Pharm Res 2002;25:652–654.
- Wang Q, Fan M, Bian Z, Nie M, Chen Z. Extract and identify ingredient from Ligustrum Lucidum Ait and study its effect to periodontal pathogen. Zhonghua Kou Qiang Yi Xue Za Zhi 2002;37:388–390.
- Abe F, Yamauchi T, Nagao T, Kinjo J, Okabe H, Higo H et al. Ursolic acid as a trypanocidal constituent in rosemary. Biol Pharm Bull 2002;25:1485–1487.
- Mitaine-Offer AC, Hornebeck W, Sauvain M, Zèches-Hanrot M. Triterpenes and phytosterols as human leucocyte elastase inhibitors. Planta Med 2002;68:930–932.
- Li J, Guo WJ, Yang QY. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT-15. World J Gastroenterol 2002;8:493–495.
- Heo HJ, Cho HY, Hong B, Kim HK, Heo TR, Kim EK et al. Ursolic acid of Origanum majorana L. reduces Abeta-induced oxidative injury. Mol Cells 2002;13:5–11.
- Chung YK, Heo HJ, Kim EK, Kim HK, Huh TL, Lim Y et al. Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetylcholinesterase. Mol Cells 2001;11:137–143.
- Hollósy F, Mészáros G, Bökönyi G, Idei M, Seprödi A, Szende B et al. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res 2000;20:4563–4570.
- Díaz AM, Abad MJ, Fernández L, Recuero C, Villaescusa L, Silván AM et al. In vitro anti-inflammatory activity of iridoids and triterpenoid compounds isolated from Phillyrea latifolia L. Biol Pharm Bull 2000;23:1307–1313.
- Begum S, Farhat F, Sultana I, Siddiqui BS, Shaheen F, Gilani AH. Spasmolytic constituents from Eucalyptus camaldulensis var. obtusa leaves. J Nat Prod 2000;63:1265–1268.
- Mizushina Y, Iida A, Ohta K, Sugawara F, Sakaguchi K. Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase. Biochem J 2000;350 Pt 3:757–763.
- Wen ZM, Xu LX. Isolation and elucidation of chemical constituents of Shandanshaoyao Decoction(I). Zhongguo Zhong Yao Za Zhi 2001;26:406–408.
- Setzer WN, Rozmus GF, Setzer MC, Schmidt JM, Vogler B, Reeb S et al. Bioactive principles in the bark of Pilidiostigma tropicum. J Mol Model 2006;12:703–711.
- Kim YK, Yoon SK, Ryu SY. Cytotoxic triterpenes from stem bark of Physocarpus intermedius. Planta Med 2000;66:485–486.
- Min BS, Kim YH, Lee SM, Jung HJ, Lee JS, Na MK et al. Cytotoxic triterpenes from Crataegus pinnatifida. Arch Pharm Res 2000;23:155–158.
- Vasconcelos MA, Royo VA, Ferreira DS, Crotti AE, Andrade e Silva ML, Carvalho JC et al. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae). Z Naturforsch, C, J Biosci 2006;61:477–482.
- Saraswat B, Visen PK, Agarwal DP. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother Res 2000;14:163–166.
- Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res 2000;60:2399–2404.
- Choi BM, Park R, Pae HO, Yoo JC, Kim YC, Jun CD et al. Cyclic adenosine monophosphate inhibits ursolic acid-induced apoptosis via activation of protein kinase A in human leukaemic HL-60 cells. Pharmacol Toxicol 2000;86:53–58.
- Deng JZ, Starck SR, Hecht SM. DNA polymerase beta inhibitors from Baeckea gunniana. J Nat Prod 1999;62:1624–1626.
- Traore-Keita F, Gasquet M, Di Giorgio C, Ollivier E, Delmas F, Keita A et al. Antimalarial activity of four plants used in traditional medicine in Mali. Phytother Res 2000;14:45–47.
- Jeong TS, Hwang EI, Lee HB, Lee ES, Kim YK, Min BS et al. Chitin synthase II inhibitory activity of ursolic acid, isolated from Crataegus pinnatifida. Planta Med 1999;65:261–263.
- Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod 1998;61:1212–1215.
- Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M et al. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene 1998;16:771–778.
- Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC et al. Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer 1997;73:725–728.
- Hsu HY, Yang JJ, Lin CC. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett 1997;111:7–13.
- Es-saady D, Simon A, Ollier M, Maurizis JC, Chulia AJ, Delage C. Inhibitory effect of ursolic acid on B16 proliferation through cell-cycle arrest. Cancer Lett 1996;106:193–197.
- Cha HJ, Bae SK, Lee HY, Lee OH, Sato H, Seiki M et al. Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Res 1996;56:2281–2284.
- Shen H, Cheng T, Qiao C, Su Z, Li C. Antitumor effect in vitro and immuno-response in vivo of Fructus mume. Zhongguo Zhong Yao Za Zhi 1995;20:365–8, inside back cover.
- Young HS, Chung HY, Lee CK, Park KY, Yokozawa T, Oura H. Ursolic acid inhibits aflatoxin B1-induced mutagenicity in a Salmonella assay system. Biol Pharm Bull 1994;17:990–992.
- Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 1994;54:701–708.
- Tokuda H, Ohigashi H, Koshimizu K, Ito Y. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett 1986;33:279–285.
- Liu J, Liu Y, Mao Q, Klaassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam Appl Toxicol 1994;22:34–40.
- Lee HY, Chung HY, Kim KH, Lee JJ, Kim KW. Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids. J Cancer Res Clin Oncol 1994;120:513–518.
- Balanehru S, Nagarajan B. Intervention of adriamycin induced free radical damage. Biochem Int 1992;28:735–744.
- Varanda EM, Zúñiga GE, Salatino A, Roque NF, Corcuera LJ. Effect of ursolic acid from epicuticular waxes of Jacaranda decurrens on Schizaphis graminum. J Nat Prod 1992;55:800–803.
- Simon A, Najid A, Chulia AJ, Delage C, Rigaud M. Inhibition of lipoxygenase activity and HL-60 leukemic cell proliferation by ursolic acid isolated from heather flowers (Calluna vulgaris). Biochim Biophys Acta 1992;1125:68–72.
- Najid A, Simon A, Cook J, Chable-Rabinovitch H, Delage C, Chulia AJ et al. Characterization of ursolic acid as a lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and differentiated HL-60 leukemic cells. FEBS Lett 1992;299:213–217.
- Ying QL, Rinehart AR, Simon SR, Cheronis JC. Inhibition of human leucocyte elastase by ursolic acid. Evidence for a binding site for pentacyclic triterpenes. Biochem J 1991;277 (Pt 2):521–526.
- Kowalewski Z, Kortus M, Kedzia W, Koniar H. Antibiotic action of beta-ursolic acid. Arch Immunol Ther Exp (Warsz) 1976;24:115–119.
- Kikuko T, Shigemi S, Masahiro S, and Tatsu M. 1993; Hair-raising cosmetic. Japanese Patent no. 05286835.
- Sultana N, Saleem M, Iqbal J, Iqbal L, Afza N. Isolation and structure characterization of three new triterpenes from alstonia scholaris flowers. Z Naturforsch 2010 (Submitted).
- Sultana N, Saleem M. Phytochemical studies on Alstonia scholaris Z Naturforsch B 2010;65b:203–210.
- Cunha WR, Crevelin EJ, Arantes GM, Crotti AE, Andrade e Silva ML, Furtado NA et al. A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phytother Res 2006;20:474–478.
- Tan N, Kaloga M, Radtke OA, Kiderlen AF, Oksüz S, Ulubelen A et al. Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 2002;61:881–884.
- Chadalapaka G, Jutooru I, McAlees A, Stefanac T, Safe S. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett 2008;18:2633–2639.
- Dufour D, Pichette A, Mshvildadze V, Bradette-Hébert ME, Lavoie S, Longtin A et al. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Ledum groenlandicum Retzius. J Ethnopharmacol 2007;111:22–28.
- Mallavadhani UV, Mahapatra A, Jamil K, Reddy PS. Antimicrobial activity of some pentacyclic triterpenes and their synthesized 3-O-lipophilic chains. Biol Pharm Bull 2004;27:1576–1579.
- Ogawa S, Hosoi K, Ikeda N, Makino M, Fujimoto Y, Iida T. Oxyfunctionalization products of terpenoids with dimethyldioxirane and their biological activity. Chem Pharm Bull 2007;55:247–250.
- Hasan S, Beatrice R, Eckart-roderich S, Hermann PTA. Inhibition by boswellic acids of human leukocyte elastase. The journal of pharmacology and experimental therapeutics 1997;281:460–463.
- Syrovets T, Büchele B, Gedig E, Slupsky JR, Simmet T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIalpha. Mol Pharmacol 2000;58:71–81.
- D’Abrosca B, Fiorentino A, Monaco P, Pacifico S. Radical-scavenging activities of new hydroxylated ursane triterpenes from cv. Annurca apples. Chem Biodivers 2005;2:953–958.
- Leite JP, Oliveira AB, Lombardi JA, Filho JD, Chiari E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol Pharm Bull 2006;29:2307–2309.
- Jang DS, Su BN, Pawlus AD, Kang YH, Kardono LB, Riswan S et al. Beccaridiol, an unusual 28-nortriterpenoid from the leaves of Diplectria beccariana. Phytochemistry 2006;67:1832–1837.
- Ma CM, Cai SQ, Cui JR, Wang RQ, Tu PF, Hattori M et al. The cytotoxic activity of ursolic acid derivatives. Eur J Med Chem 2005;40:582–589.
- Cunha WR, Martins C, da Silva Ferreira D, Crotti AE, Lopes NP, Albuquerque S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med 2003;69:470–472.
- Tanud T, John BB, Ratchanaporn C, Apichart S. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic, and ursolic acids Chem Pharm Bull 2008;56:194–198.
- Tian Z, Lin G, Zheng RX, Huang F, Yang MS, Xiao PG. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J Gastroenterol 2006;12:874–879.
- Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papageorgiou VP. Inhibitory activity of minor polyphenolic and non-polyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J Med Food 2002;5:1–7.
- Fatima N, Tapondjou LA, Lontsi D, Sondengam BL, Atta-Ur-Rahman, Choudhary MI. Quinovic acid glycosides from Mitragyna stipulosa — first examples of natural inhibitors of snake venom phosphodiesterase I. Nat Prod Lett 2002;16:389–393.
- Tapondjou LA, Lontsi D, Sondengam BL, Choudhary MI, Park HJ, Choi J et al. Structure-activity relationship of triterpenoids isolated from Mitragyna stipulosa on cytotoxicity. Arch Pharm Res 2002;25:270–274.
- Collins DO, Ruddock PL, Chiverton de Grasse J, Reynolds WF, Reese PB. Microbial transformation of cadina-4,10(15)-dien-3-one, aromadendr-1(10)-en-9-one and methyl ursolate by Mucor plumbeus ATCC 4740. Phytochemistry 2002;59:479–488.
- Hollósy F, Idei M, Csorba G, Szabó E, Bökönyi G, Seprödi A, Mészáros G, Szende B, Kéri G. Activation of caspase-3 protease during the process of ursolic acid and its derivative-induced apoptosis. Anticancer Res 2001;2:13485–13491.
- Kashiwada Y, Nagao T, Hashimoto A, Ikeshiro Y, Okabe H, Cosentino LM et al. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J Nat Prod 2000;63:1619–1622.
- Suruga T, Chun Y, Ebizuka Y, Sankawa U. Biologically active constituents of Melaleuca leucadendron: inhibitors of induced histamine release from rat mast cells. Chem Pharm Bull 1999;139:3276–3278.
- Ma C, Nakamura N, Miyashiro H, Hattori M, Shimotohno K. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem Pharm Bull 1999;47:141–145.
- Farina C, Pinza M, Pifferi G. Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Farmaco 1998;53:22–32.
- Mallavadhani UV, Mahapatra A, Raja SS, Manjula C. Antifeedant activity of some pentacyclic triterpene acids and their fatty acid ester analogues. J Agric Food Chem 2003;51:1952–1955.
- Kashiwada Y, Wang HK, Nagao T, Kitanaka S, Yasuda I, Fujioka T et al. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod 1998;61:1090–1095.
- Benyahia S, Benayache S, Benayache F, León F, Quintana J, López M et al. Cladocalol, a pentacyclic 28-nor-triterpene from Eucalyptus cladocalyx with cytotoxic activity. Phytochemistry 2005;66:627–632.
- Nguyen QC, Nguyen VH, Santarsiero BD, Mesecar AD, Nguyen MC, Soejarto DD et al. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J Nat Prod 2004;67:994–998.
- Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res 1998;58:717–723.
- Takechi M, Tanaka Y. Structure-activity relationships of synthetic methyl ursolate glycosides. Phytochemistry 1993;34:675–677.
- Ovesná Z, Vachálková A, Horváthová K, Tóthová D. Pentacyclic triterpenoic acids: new chemoprotective compounds. Mini review. Neoplasma 2004;51:327–333.
- Liao LP, Li SL, Li P. Simultaneous determination of seven triterpenoids and triterpenoid saponins in Folium llicis Purpureae by high performance liquid chromatography coupled with evaporative light scattering detection. J Sep Sci 2005;28:2061–2066.
- Yan H, Zhao L, Zhu D, Ding L. Determination of oleanolic acid and ursolic acid in Spica prunellae by derivative GC method. Zhongguo Zhong Yao Za Zhi 1999;24:744–5, 764.
- Kriwacki RW, Pitner TP. Current aspects of practical two-dimensional (2D) nuclear magnetic resonance (NMR) spectroscopy: applications to structure elucidation. Pharm Res 1989;6:531–554.
- Lopes SO, Moreno PR, Henriques AT. Growth characteristics and chemical analysis of Psychotria carthagenensis cell suspension cultures. Enzyme Microb Technol 2000;26:259–264.
- He X, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J Agric Food Chem 2007;55:4366–4370.
- Sultana N, Khalid A. A new fatty ester and a new triterpene from Skimmia laureola. Nat Prod Res 2008;22:37–47.
- Sultana N, Khalid A. A new fatty ester and a new triterpene from Skimmia laureola. Nat Prod Res 2008;22:37–47.
- Han SK, Ko YI, Park SJ, Jin IJ, Kim YM. Oleanolic acid and ursolic acid stabilize liposomal membranes. Lipids 1997;32:769–773.
- Lin CN, Lu CM, Cheng MK, Gan KH, Won SJ. The cytotoxic principles of Solanum incanum. J Nat Prod 1990;53:513–516.
- Kozai K, Miyake Y, Kohda H, Kametaka S, Yamasaki K, Suginaka H et al. Inhibition of glucosyltransferase from Streptococcus mutans by oleanolic acid and ursolic acid. Caries Res 1987;21:104–108.