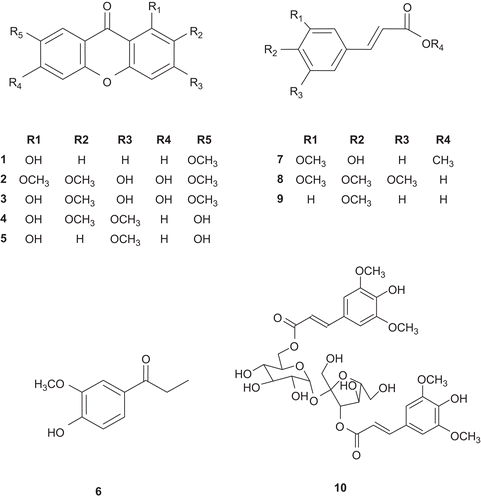
Abstract
A methanolic extract of the roots of Polygala tenuifolia (Polygalaceae) significantly attenuated nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated BV2 microglia cells. Five xanthones, 1-hydroxy-7-methoxyxanthone (1), 3,6-dihydroxy-1,2,7-trimethoxyxanthone (2), 1,3,6-trihydroxy-2,7-dimethoxyxanthone (3), 1,7-dihydroxy-2,3-dimethoxyxanthone (4) and 1,7-dihydroxy-3-methoxyxanthone (5), and five phenylpropanoids, 4-hydroxy-3-methoxypropiophenone (6), methyl 4-hydroxy-3-methoxycinnamic acid (7), 3,4,5-trimethoxycinnamic acid (8), 4-methoxycinnamic acid (9) and β-d-(3-O-sinapoyl) fructofuranosyl-α-d-(6-O-sinapoyl)glucopyranoside (10), were isolated from CHCl3 fraction using bioactivity-guided fractionation. Among these compounds, compounds 1, 2, 4, 5 and 7 showed significant inhibitory effects on LPS-induced NO production in BV2 microglia cells at the concentration ranging from 10.0 to 100.0 μM.
Introduction
Nitric oxide (NO) is a small membrane-permeable gas that is produced through the oxidation of l-arginine by NO synthasesCitation1. Although NO is crucial for many physiological functions, inappropriate release of this mediator has been linked to pathogenesis in a number of neurodegenerative and neuroinflammatory diseasesCitation2–5. During our search for new regulators on NO overproduction from medicinal plants, the methanolic extract of the roots of Polygala tenuifolia (Polygalaceae) significantly inhibited lipopolysaccharide (LPS)-induced NO production. The roots of P. tenuifolia have been used as an expectorant, tonic, sedative and for preventing dementiaCitation6. Xanthones, saponins and oligosaccharide esters have been reported as the constituents of this plantCitation7. Bioassay-guided fractionation of the extract of P. tenuifolia led to the isolation of five xanthones (1–5) and five phenylpropanoids (6–10). Xanthones have extensive biological activities including antihypertensive, antidiabetic, anticancer, anti-inflammatory, hepatoprotective and immunomodulatory propertiesCitation8. However, neuroprotective activity of xanthones has not been reported yet. In this study, we reported the isolation and identification of xanthones isolated from P. tenuifolia roots and their inhibitory effects on NO production in LPS-stimulated BV2 microglia cells.
Methods and materials
Plant material
The dried whole plant of P. tenuifolia was purchased from Kyungdong traditional herbal market (Seoul, Korea) and authenticated by Dr. Jae hyun Lee, professor of Dongguk University. A voucher specimen (SNUPH-6388) has been deposited at the Herbarium of the Medicinal Plant Garden, College of Pharmacy, Seoul National University.
Isolation of NO production inhibitory compounds
The plant material (8 kg) was grounded and extracted with 80% methanol at room temperature. The methanol extract was concentrated in vacuo to give a crude extract (1.6 kg). The methanolic extract was then suspended in H2O and partitioned successively with CHCl3, n-butanol and H2O. The CHCl3 fraction (20 g) was subjected to silica gel column chromatography (CC) to yield seven fractions (C1–C7). Compounds 1 (20 mg) and 2 (38 mg) were isolated from C2 by sequentially using silica gel CC and ODS reverse phase CC. Compounds 3 (157 mg), 4 (10 mg) and 5 (15 mg) were isolated from C3 by ODS reverse phase CC and the consecutive C18 HPLC (MeOH: H2O = 3:7, 2 mL/min). Compound 6 (22 mg) was isolated from C1 by recrystallization with methanol. Compounds 7 (13 mg), 8 (230 mg) and 9 (64 mg) were isolated from C4 and C6, respectively, by ODS reverse phase CC and Sephadex LH-20. Compound 10 (28 mg) was isolated from C7 using silica gel CC followed by ODS reverse phase CC.
Cell cultures
BV2 mouse microglia cells originally developed by Dr. Bocchini at University of Perugia (Perugia, Italy)Citation9 was generously provided by Dr. Sun-yeou Kim at Kyunghee University (Suwon, Korea). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum (FBS) with penicillin (100 IU/mL) and streptomycin (10 mg/mL) at 37.8°C in a humidified atmosphere of 95% air with 5% CO2.
Evaluation of inhibitory effect on NO production in LPS-stimulated BV2 microglia cells
Fractions and compounds to be tested were dissolved in dimethyl sulphoxide (DMSO) (final concentration in cultures, 0.1%). To remove any trace of phenol red, the cell cultures were washed and the medium was replaced with phenol red-free DMEM. Then BV2 microglia cells (2 × 105 cells/well in 96-well plates) were treated with test samples for 1 h before exposure to 100 ng/mL of LPS. After 24 h incubation, nitrite in culture media was measured to assess NO production in BV2 cells using Griess reagent. In 96-well plate, 100 µL of sample aliquots were mixed with 100 µL of Griess reagent (1% sulphanilamide, 0.1% naphtylethylenediamine dihydrochloride, 2% phosphoric acid) and incubated at room temperature for 15 min. The absorbance (abs) at 550 nm was measured on a microplate reader. The concentration was determined using nitrite standard curve. After 100 µL of sample aliquots were collected for Griess assay, MTT (0.2 mg/mL) was directly added to cultures, followed by incubation at 37.8°C for 3 h. The supernatant was then aspirated and 100 µL of DMSO was added to dissolve the formazan. The absorbance (abs) at 540 nm was measured using a microplate reader. Data were expressed as percent cell viability relative to control cultures.
NAME (ω-nitro-l-arginine methyl ester; Sigma) was used as a positive control in our study.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). The evaluation of statistical significance was determined by a ‘one-way ANOVA’ test using computerized statistical package. The data were considered to be statistically significant if the probability had a value of 0.05 or less.
Results and discussion
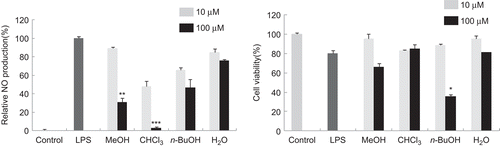
The relevance of NO in both physiological and pathological scenarios is crucial; however, the excessive NO has been linked to neurodegenerative disorders including Alzheimer’s disease (AD) and Parkinson’s disease (PD)Citation10,Citation11. Thus, we tried to obtain anti-inflammatory compounds from medicinal plants modulating NO production using LPS-stimulated BV2 microglia cells. In this screening system, the methanolic extract of P. tenuifolia roots significantly inhibited LPS-induced NO production. The methanolic extract was suspended in H2O and partitioned successively with CHCl3 and n-butanol. As shown in , the CHCl3 fraction effectively inhibited LPS-induced NO production without cytotoxicity in BV2 microglia cells. Through bioactivity-guided fractionation and isolation using a variety of chromatographic methods, five xanthones (1–5) such as 1-hydroxy-7-methoxyxanthone (1), 3,6-dihydroxy-1,2,7-trimethoxyxanthone (2), 1,3,6-trihydroxy-2,7-dimethoxyxanthone (3), 1,7-dihydroxy-2,3-dimethoxyxanthone (4) and 1,7-dihydroxy-3-methoxyxanthone (5), and five phenylpropanoids including 4-hydroxy-3-methoxypropiophenone (6), methyl 4-hydroxy-3-methoxycinnamic acid (7), 3,4,5-trimethoxycinnamic acid (8), 4-methoxycinnamic acid (9) and β-d-(3-O-sinapoyl) fructofuranosyl-α-d-(6-O-sinapoyl)glucopyranoside (10) were obtained from the CHCl3 fraction. The chemical structures of these compounds were determined by comparison of their spectroscopic data with those previously reportedCitation12–16 (). Inhibitory effects of these compounds on NO production were evaluated at the concentration ranging from 10.0 to 100.0 µM. As shown in , it was found that compounds 1, 2, 4, 5 and 7 had significant inhibitory effects on LPS-induced NO production in BV2 microglia cells. To further investigate the cytotoxicity of these compounds, cell viability was measured by employing MTT assay. The isolated compounds showed no toxicity at the concentration of 100.0 µM (). Among the isolated xanthones (1–5), compounds 1 and 5 showed the most potent inhibitory activity at the concentration over 50.0 µM. Compounds 2–4 that have ortho-substituted aromatic ring showed the moderate activity, suggesting that the oxygenation pattern in aromatic ring might be important for the inhibitory activity on LPS-induced NO production in BV2 microglia cells. To determine more relevant structure–activity relationships, more xanthones should be evaluated for their inhibitory activity on NO production. On the basis of above results, it could be postulated that xanthones isolated from P. tenuifolia have potential for prevention or treatment of inflammation-related neurodegenerative disorders.
Table 1. Effect of the isolated compounds from the roots of P. tenuifolia on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in BV2 microglia.
Figure 1. (A) Effect of the methanolic extract and the fractions of P. tenuifolia on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in BV2 microglia. BV2 cells were washed with phenol red-free Dulbecco’s modified Eagle’s medium (DMEM) and incubated with test samples for 1 h. The cultures were then stimulated by 100 ng/mL of LPS for 24 h. After incubation, NO production was measured by the Griess reaction and sodium nitrite was used as the standard. NO production (NP) of control and LPS-treated cultures were 1.0 ± 0.3 μM and 19.6 ± 2.9 μM, respectively. Relative NO production (%) was calculated as (NP of sample-treated− NP of control)/(NP of LPS-treated− NP of control) × 100 (%). LPS-stimulated value differs significantly from control at a level of P < 0.001. Results differ significantly from the LPS-treated, *P < 0.05, **P < 0.01 and ***P < 0.001, respectively. (B) Effect of the methanolic extract and the fractions of from P. tenuifolia on cell viability of BV2 cells. BV2 cells were incubated with compounds at the concentration of 10 or 100 µM for 24 h. Cell viability was measured by the MTT assay.
Acknowledgement
This research was supported by a grant (S10042CHM8900B) from Korea Food and Drug Administration funded by the Ministry of Science and Technology, Korea.
Declaration of interest
The authors report no declarations of interest.
References
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 1994;63:175–195.
- Bugnon O, Schaad NC, Schorderet M. Nitric oxide modulates endogenous dopamine release in bovine retina. Neuroreport 1994;5:401–404.
- Bolaños JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB et al. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem 1997;68:2227–2240.
- Fernández-Vizarra P, Fernández AP, Castro-Blanco S, Encinas JM, Serrano J, Bentura ML et al. Expression of nitric oxide system in clinically evaluated cases of Alzheimer’s disease. Neurobiol Dis 2004;15:287–305.
- Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson’s disease. J Neuropathol Exp Neurol 1998;57:338–342.
- Jiang Y, Tu PF. Xanthone O-glycosides from Polygala tenuifolia. Phytochemistry 2002;60:813–816.
- Sakuma S, Shoji J. Studies on the constituents of the root of Polygala tenuifolia Willdenow. 1. Isolation of saponins and the structures of Onjisaponin-G and Onjisaponin-F. Chem Pharm Bull 1981;29:2431–2441.
- Pinto MM, Sousa ME, Nascimento MS. Xanthone derivatives: new insights in biological activities. Curr Med Chem 2005;12:2517–2538.
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 1990;27:229–237.
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 2003;111:163–169.
- Norris PJ, Faull RL, Emson PC. Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer’s disease brains. Brain Res Mol Brain Res 1996;41:36–49.
- Fujita T, Liu DY, Ueda S, Takeda Y. Xanthones from Polygala tenuifolia. Phytochemistry 1992;31:3997–4000.
- Mao SL, Liao SX, Wu JH, Ling N, Chen H, Liang HQ et al. [Studies on chemical constituents of Polygala arillata buch-ham]. Yao Xue Xue Bao 1997;32:360–362.
- Ikeya Y, Sugama K, Okada M, Mitsuhashi H. 2 Xanthones from Polygala tenuifolia. Phytochemistry 1991;30:2061–2065.
- Bashir A, Hamburger M, Msonthi JD, Hostettmann K. Sinapic acid esters from Polygala virgata. Phytochemistry 1993;32:741–745.
- Pinheiro TR, Cechinel V, Santos ARS, Calixto JB, Monache FD, Pizzolatti MG, Yunes RA. Three xanthones from Polygala cyparissias. Phytochemistry 1998;48:725–728.