Abstract
Context: Development of inexpensive and safe enzymatic assays to screen for putative neuraminidase inhibitors.
Objective: Validate the use of recombinant neuraminidase expressed in baculovirus located on the viral surface capsule to develop a neuraminidase inhibitor screening assay.
Materials and methods: Recombinant baculovirus particles displaying neuraminidase N1 and N3 were used as enzyme sources. The assay set-up required the use of 2′-(4-methylumbelliferyl)-α-D-acetyl neuraminic acid as substrate and oseltamivir carboxylate as benchmark inhibitor.
Results: The assay was set up in a standard 96-well plate. The within- and between-assay coefficients of variation were, on average, less than 10%. The 50% inhibitory concentration values of the inhibitor were in good agreement with those determined by independent kinetic experiments.
Discussion and conclusions: The assay showed satisfactory within- and between-assay repeatability. The obtained results suggest that recombinant baculovirus expressing neuraminidase located on the virus membrane capsule can be used to set up affordable and reliable neuraminidase inhibitors screening assays.
Introduction
Avian influenza infection in humans and, above all, the problem of H5N1 possible re-assortment in suitable “mixing vessels” generating highly infective new viral strains are the main concern for the public health. Although the cumulative number of confirmed patients remains relatively small, the scientific community agrees in considering the emergence of a new highly pathogenic H5N1 influenza pandemic as a real concernCitation1–3. So far, the only drugs approved to treat avian flu infected patients are the neuraminidase inhibitors Tamiflu (oseltamivir) and Zanamivir (relenza)Citation4. These drugs target the enzyme neuraminidase that is responsible for allowing budding progeny virus to be released from the surface of infected cells by cleaving the terminal neuraminic acid residues from the glycan structures on the cell surface. Blocking neuraminidase action by specific inhibitors prevents the release of newly formed viral particles thus blocking virus spreading to neighbouring cells. The confirmed occurrence of oseltamivir resistant avian influenza virusCitation5–7 calls for the development of new, more active, inhibitors. On the other hand, the availability of efficient, affordable and reliable methods for screening the activity of novel synthetic compounds as well as the activity of natural products potentially endowed with antiviral activity is also urgently needed. This goal can only be achieved by identifying inexpensive sources of neuraminidase in order to set up suitable enzyme-based assay systems with medium- to high-throughput screening capacity.
Many reports in the literature describe neuraminidase inhibitor assays relying on the cumbersome use of the influenza virus preparationsCitation1,Citation8–12. This procedure is risky, costly and inconvenient for the set-up of a screening assay. In other cases, studies aimed to screen putative neuraminidase inhibitors employed commercially available neuraminidase, for instance from Clostridium perfringensCitation13,Citation14, or secreted recombinant neuraminidase from H1N1 expressed in baculovirusCitation15. These sources are safe but still costly for applications in medium- to high-throughput screening. Recently, a recombinant influenza A virus H5N1 neuraminidase has been expressed in Pichia pastorisCitation16. This system allows a cost-effective expression of functional enzymes since yeast can be rapidly grown on simple growth media giving high levels of secreted recombinant proteinsCitation17.
Aiming to develop an inexpensive and safe but functional and reliable assay to screen neuraminidase inhibitors, we focussed on the use of recombinant neuraminidase expressed in baculovirus where the enzyme is located on the membrane capsule of the virusCitation18,Citation19. This crude source does not require extensive and costly purification procedures and is safe since baculovirus is not infectious to vertebrates. The neuraminidase preparations used in this study were originally developed by the Office International des Epizooties—Food and Agriculture Organization (OIE-FAO) to be applied in the program of “DIVA” (Differentiating Infected from Vaccinated Animals) vaccination strategyCitation18,Citation19. The functional activity of this neuraminidase preparation was not a primary concern or requirement for the success of the vaccination strategy. However, we found that these recombinant enzyme preparations displayed a sufficient enzymatic activity allowing us to set up a convenient enzyme-based assay for screening the activity of novel neuraminidase inhibitors.
As a proof of principle, we used neuraminidase molecules obtained from H7N1 (A/Turkey/Italy/4426/2000 LPAI) and H7N3 (A/ty/Italy/8000/02). The neuraminidase N1 used in this study displays a 95% sequence homology with the sequence of neuraminidase N1 from H5N1Citation3 recently used for the in silico design of novel oseltamivir analoguesCitation20 and was considered a good model to assess the feasibility of this approach. The experimental design required the following: (A) a preliminary characterization of the enzymatic activities of the two enzyme preparations; and (B) the assay set-up and its validation by using oseltamivir carboxylate as benchmark compound.
Methods
Chemicals
Oseltamivir free acid was purchased from Toronto Research Chemical Inc (Ontario, Canada). The compound was dissolved in distilled water to get a 17.58-mM stock solution. The stock solution was aliquoted and stored at −20°C. The substrate 2′-(4-methylumbelliferyl)-α-D-acetyl neuraminic acid (MUNANA) was purchased from Gold-Biotechnology Inc (Saint Luis, Missouri, USA). 4-Methylumbelliferone (4-MU) was purchased from Alfa Aesar GmbH & Co KG (Karlsruhe, Germany). 2-N-morpholino-ethanesulfonic acid (MES) and Glycine were purchased from Sigma (Milan, Italy), CaCl2 was purchased from Sigma Aldrich (Milan, Italy). Recombinant neuraminidases were obtained from the OIE, FAO and National Reference Laboratory for Avian Influenza and Newcastle Disease, Istituto Zooprofilattico Sperimentale delle Venezie according to published proceduresCitation18–19. N1 and N3 antigens were expressed using a Bac-to-Bac baculovirus expression system (Invitrogen Life Technologies, Milan, Italy) according to the manufacturer’s instructions. N1 gene was isolated from H7N1 A/Turkey/Italy/4426/2000 LPAI, whereas N3 was isolated from H7N3 A/ty/Italy/8000/02 LPAI. Proteins were found on the viral membrane capsule, baculovirus supernatants were provided as lyophilized materials and kept at −80°C for long-term storage, and the viral tieter of the starting material was in the range of 106 PFU/ml.
Preliminary assessment of neuraminidase N1 and N3 activities
The lyophilized supernatants containing the baculovirus particles carrying recombinant neuraminidase N1 or N3 were reconstituted with 1.0 ml of double distilled water. Stock solutions of the recombinant neuraminidases were prepared by diluting the crude reconstituted supernatants in 33 mM MES pH 6.5 containing 4 mM CaCl2. The N1 containing supernatant was diluted 10-fold, whereas the N3 supernatant was diluted 80-fold; different dilutions were necessary because of the different intrinsic reactivity of the N1 and N3 preparations. These stock solutions were used throughout the study. To measure the specific activity of the neuraminidase preparations, a 25-µl aliquot of these stock solutions was mixed with 90 µl of 33 mM MES pH 6.5 containing 4 mM CaCl2 and incubated at 37°C with 25 µl of 500 µM MUNANA in a transparent, medium binding ELISA plate (GREINER, Frickenhausen, Germany). After 5 h of incubation, the reaction was terminated by adding 100 µl of 0.1 M Glycine pH 10.7 containing 25% ethanol. The fluorescence of each reaction mixture was then recorded by using the VictorCitation3 multi-label counter (Perkin Elmer, Monza, Italy) with the excitation and emission wavelengths set at 355 and 460 nM, respectively. The amount of released 4-methylumbelliferone was determined by interpolation of the measured fluorescence intensity with a calibration curve constructed by measuring the fluorescence of a series of solutions with different 4-MU concentrations.
Determination of the Michaelis–Menten constants of neuraminidase N1 and N3 against 4-MUNANA
The Michaelis–Menten constants (Km) of neuraminidase N1 and N3 against 4-MUNANA were determined by incubating increasing amounts of the substrate with N1 and N3; the final concentration of MUNANA spanned the range 0–2000 µM. Practically, a set of reaction mixtures was prepared in a microtitre plate by mixing 25 µl of the supernatants’ stock solutions containing the recombinant enzyme with 90 µl of 33 mM MES pH 6.5 containing 4 mM CaCl2. To each well, 25 µl of MUNANA solution were added to initiate the reaction; after 30 min in the first well, the reaction was arrested by addition of the stop solution and the fluorescence was read; after additional 30 min, the reaction was arrested in the second well and the fluorescence was read; after additional 30 min, the reaction was arrested in the third well and the fluorescence was read and so forth. At the end of the experiment, the initial velocity of the reaction at a given concentration of 4-MUNANA could be obtained by linear fitting of the fluorescence data as a function of time. By repeating the same procedure using different concentrations of 4-MUNANA a set of different initial velocities (V0) as function of the substrate concentration (S0) could be obtained. By plotting 1/V0 (reciprocal of initial velocity) against 1/S0 (reciprocal of substrate concentration), a well-behaved Lineweaver–Burk plot could be obtained, and linear fitting of the data allowed the determination of the Michaelis–Menten constant KmCitation21.
Determination of the IC50 of oseltamivir carboxylate against neuraminidase N1
The 50% inhibitory concentration (IC50) determination was carried out by using a 96-well microtitre plate as an array of reaction vessels. In the first well, a 0.1-mM solution of oseltamivir carboxylate was prepared by mixing 90 µl of 33 mM MES pH 6.5 containing 4 mM CaCl2 and 10 µl of a 1-mM oseltamivir carboxylate stock solution. Starting from the solution contained in the first well, an in-plate 10-fold serial dilution was performed using 33 mM MES pH 6.5 containing 4 mM CaCl2 as dilution buffer obtaining a 7 points dilution curve, the last well in the vertical lane was used to obtain a negative control (no oseltamivir was added). At the end of the dilution process, each well contained 90 µl of solution. To each well, 25 µl of the enzyme stock solution were mixed with the oseltamivir solution and incubated for 2 h at 37°C. After the incubation time, 25 µl of 20 µM MUNANA were added. In the reaction mixtures, the final concentration of oseltamivir carboxylate spanned in the ranges 0.064 nM–64.2 µM. After incubating the plate for 5 h at 37°C, 50 µl of stop solution (0.1 M Glycine, pH 10.7 containing 25% ethanol) were added. The fluorescence of the solutions contained in each well was read with the VictorCitation3 multi-label counter. The IC50 was determined from dose-response curve using GraphPad Prism 5; GraphPad, San Diego, CA).
Determination of the IC50 of oseltamivir carboxylate against neuraminidase N3
For the determination of the of oseltamivir carboxylate against neuraminidase N3, the same procedure described above was used, except for the concentration of the oseltamivir carboxylate stock solution which was 10 µM. In the reaction mixtures, the final concentration of oseltamivir carboxylate spanned in the ranges 0.64 pM–642 nM.
Within- and between-assay reproducibility in the IC50 determination of oseltamivir carboxylate against neuraminidase N1 and N3
The within-assay reproducibility of the fluorescence readings was assessed by repeating five times the IC50 determination reported in the preceding paragraph. All the assays were run on the same plate. The between-assay reproducibility was assessed by repeating three to four times the IC50 determination following the procedure reported in the preceding paragraph. The assays were performed in different days using freshly prepared reagents solutions.
Kinetic determination of the inhibition constant (Ki) of oseltamivir carboxylate for neuraminidases N1 and N3
General procedure
The determination of Ki according to the Dixon methodCitation20 requires the measurements of reaction initial velocity in the presence of increasing amounts of inhibitor. The pertinent kinetic data were obtained by running enzyme kinetics using a 96-well (12 × 8) microtitre plate as an array of reaction vessels as described for the determination of the Km values for the two enzymes. The concentrations of enzyme and substrate were kept constant in all of the wells, while the concentration of oseltamivir carboxylate was varied in the range 10–643 nM for N1 and 0.1–6.43 nM for N3. The reaction rates for a given concentration of inhibitor were determined by linear fitting of the fluorescence intensities measured during the enzymatic reaction. The fluorescence intensities were recorded every 30 min for a total time of 180 min.
Ki determination for neuraminidase N1
The determination of the pertinent kinetic parameters used for the calculation of Ki was performed according to the general procedure outlined above. Practically in a microtitre plate, 90 µl of 33 mM MES pH 6.5 containing 4 mM CaCl2 were dispensed in each well, except for the first well of each lane. In the first well of each vertical lane, 18 µl of 0.01 mM oseltamivir were mixed with 162 µl of 33 mM MES pH 6.5 containing 4 mM CaCl2. The solution was thoroughly mixed to obtain a 1-µM oseltamivir carboxylate solution. For each assay, a 90-µl aliquot of this solution was withdrawn from the first well and added to the second well in the same vertical lane obtaining a 2-fold diluted solution of oseltamivir. The 2-fold dilution process was continued up to the seventh well, while the eighth well used as blank (no inhibitor was added). At the end of the dilution process, each well contained 90 µl of solution. To each well, 25 µl of enzyme stock solution were added. After an incubation time of 2 h, 25 µl of MUNANA were added to each well, the final concentrations of oseltamivir carboxylate spanned the range 10–643 nM for N1 assay. After 30 min of incubation, 50 µl of stop solution were added to the first vertical lane, and the fluorescence was recorded. After an additional 30 min (60 min total), the stop solution was added to the second vertical lane and the fluorescence recorded. After additional 30 min (90 min total), the stop solution was added to the third vertical lane and the fluorescence recorded and so on. The experiment was performed in duplicate. The initial velocities of the reactions were plotted against the concentration of inhibitor and the data were analysed according to DixonCitation22.
Ki determination for neuraminidase N3
For the determination of oseltamivir carboxylate Ki against N3, the same procedure described for N1 was used, except for the concentration of oseltamivir carboxylate stock solution which was 0.1 µM.
Results
Enzyme characterization
The development of the screening assay for neuraminidase inhibitors required the preliminary assessment of the enzymatic activity of the crude preparations. This was achieved by fluorimetric methods exploiting the enzymatic reaction of MUNANA and neuraminidase. The amount of 4-methylumbelliferone released by the enzymatic reaction was then quantitated by comparison with a calibration curve. The specific activity of neuraminidase N3 in the crude preparation was about 0.4 mU/mg, while that of neuraminidase N1 was about 0.04 mU/mg. One unit of enzyme activity was defined as the amount of active neuraminidase enzyme required to convert one micromole of substrate per minute at 37°C.
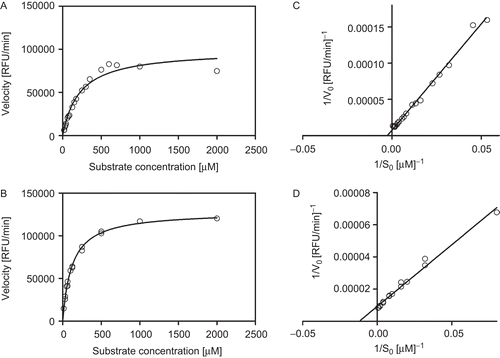
The enzymes were further characterized by determining their Michaelis–Menten constant (Km) against MUNANA. The enzyme preparations were incubated with increasing amounts of substrate spanning the range 0–2000 µM. The initial rate of the enzymatic reaction was then plotted against the substrate concentrations. As displayed in and , for both neuraminidase preparations a well-behaved Michaelis–Menten plot could be obtained. The experimental data were used to construct the pertinent Lineweaver–Burk plots by reporting 1/V0 (reciprocal of the initial reaction rate) against 1/S0 (reciprocal of the substrate concentration). The Michaelis–Menten constants were calculated by least squares linear fitting of the data reported in the Lineweaver–Burk plots, and . The Michaelis–Menten constants for N1 resulted 400 ± 60 µM, and and , whereas the Michaelis–Menten constant for N3 was 87 ± 4 µM, and and .
Table 1. Data obtained in the enzymatic characterization of recombinant neuraminidase N1 and N3.
Figure 1. Michaelis–Menten and Lineweaver–Burk plots of recombinant neuraminidase: (A, C) subtype 1, and (B, D) subtype 3. The concentration of fluorogenic substrate (MUNANA) ranged from 0 µM to 2000 µM. The kinetics were run at 37°C. The fluorescence was recorded every 30 min by using the VictorCitation3 multi-label counter (Perkin Elmer) with excitation and emission wavelengths of 355 and 460 nM, respectively. The reported data were obtained in triplicate experiments.
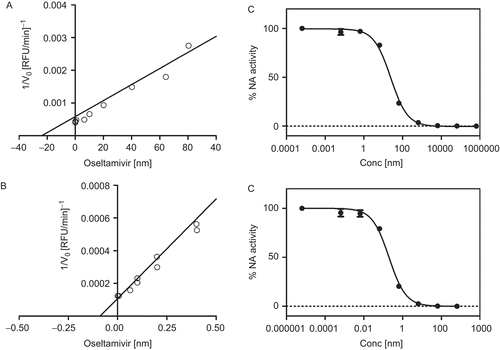
To further characterize the enzyme activity in the crude preparation, a series of kinetic experiments were carried out in order to determine the inhibition constants, Ki, of the benchmark inhibitor oseltamivir carboxylate. By plotting the initial velocity of the reaction against the concentration of oseltamivir carboxylate at a fixed concentration of substrate, a linear Dixon plot was obtained for both enzymes as shown in and . The Ki values for N1 and N3 were found to be (23.71 ± 7.2) nM and (0.12 ± 0.11) nM, respectively (). Based on these data, the expected IC50 of oseltamivir carboxylate could be calculated using the following equation IC50 = KI(1+[S]/Km), obtaining the values of 23.5 nM and 0.13 nM for N1 and N3, respectively.
Assay set-up and validation
In setting up the neuraminidase inhibition assay, we used a 96-well microtitre plate as an array of reaction vessels. Each well could be used to test a single compound at a given concentration. Practically, each test compound is incubated for 2 h in the presence of the recombinant enzyme, either N1 or N3 according to the intended experiment, afterwards the substrate is added. The reaction mixture is then incubated for 5 h at 37°C; after the incubation time, the reaction is arrested by adding the proper amount of the stop solution. The fluorescence intensity in each well is read by setting the excitation and emission wavelengths to 355 and 460 nM, respectively.
To validate the assay set-up and performances, we used the specific neuraminidase inhibitor oseltamivir carboxylate as benchmark compound. This species was used at different concentrations in order to assess the sensitivity and the repeatability of the analytical method in the whole range of possible fluorescence readings comprised between the conditions of no-inhibition (absence of inhibitor) and complete inhibition (excess inhibitor). This approach allowed the determination of the IC50 values of oseltamivir carboxylate against the two enzymes by best fitting of the IC curve reported in and , obtaining the values of (25 ± 4) nM for N1 and (0.2 ± 0.02) nM for N3. The experimental values of the IC50 were in good agreement with those calculated by using the Ki values determined kinetically, 23.9 nM and 0.12 nM for N1 and N3, respectively, . The IC50 values calculated by the two different methods were found to be identical within the experimental uncertainty.
The reproducibility of the assay was evaluated by determining the within- and between-assay coefficient of variations for the determination of the IC50 of oseltamivir carboxylate against the two enzymes in repeated analyses, . From the data reported in , it can be concluded that the within-assay coefficient of variations for the IC50 determination of oseltamivir carboxylate against N1 and N3 are 10% and 8%, respectively. The between-assay coefficient of variations are instead 5% and 20% for N1 and N3, respectively.
Table 2. Within- and between-assay determinations of IC50 of oseltamivir carboxylate against neuraminidase N1 and N3.
To run a total number of 96 tests, the developed assay requires as little as 240 µl of reconstituted N1 supernatant displaying a reactivity of 0.04 mU/mg. The N3 test required the use of 30 µl of reconstituted neuraminidase supernatant displaying a reactivity of 0.4 mU/mg.
Discussion
Highly pathogenic avian influenza remains a problematic issue in certain regions of the world, and the development of affordable and reliable methods for screening new antiviral compounds with potent inhibitory activity on the neuraminidase represents an urgent need. To this aim, the availability of affordable sources of enzymatically active neuraminidase is mandatory. Among the various sources reported, recombinant neuraminidases N1 and N3, obtained from baculovirus carrying the neuraminidase gene propagated in insect cells and located on the membrane capsule of the virusCitation18,Citation19, may be of particular interest because these preparations displayed a significant enzymatic activity even without any purification step. These recombinant virus particles were originally used to elicit protecting antibodies in poultryCitation18,Citation19. Although the success of vaccination strategy did not require any enzyme activity, our preliminary study interestingly showed that these preparations of N1 and N3 remained enzymatically active. This opened the possibility of obtaining a convenient source of the two neuraminidases to develop an enzyme-based bioassay for the screening of putative inhibitors.
Our results showed that the supernatants consisting of baculovirus particles carrying the recombinant neuraminidases N1 and N3 displayed a specific enzyme activities of 0.04 and 0.4 mU/mg of total protein, respectively. Although these values are not as high as those reported for the enzyme preparations from other sourcesCitation16,Citation23,Citation24, the measured neuraminidase activity levels were sufficient to set up a sensitive and reproducible fluorimetric assay. The enzymes have been characterized by determining their Michaelis–Menten constants against MUNANA as well as by determining the inhibition constant (Ki) of oseltamivir carboxylate against the two species.
As summarized in , for recombinant N1 a Km value of 400 ± 60 µM was determined, which is almost 5 times higher than the Km of N3, 87 ± 4 µM, suggesting that N3 can catalyze the reaction under low substrate concentration, whereas N1 needs high substrate concentration. Comparatively, the values of Km and IC50 (oseltamivir) of N1 obtained from this study appear to be higher than those reported previouslyCitation16,Citation25,Citation26. These differences may be due to the different strains (clades) of avian influenza viruses used in the various studies. In fact, the values of Km of neuraminidase N1 against MUNANA have been reported to span in the range 15–359 µM, depending on the specific nature of the neuraminidase considered. For the IC50 values, the observed higher sensitivity of N3 than N1 towards oseltamivir might be explained by the fact that the original design of the oseltamivir molecule was against the neuraminidase subgroup 2 structures (comprising N2, N3, N6, N7 and N9), not the subgroup 1 structures (comprising N1, N4, N5 and N8)Citation3. In addition, it has been reported that the clade 1 H5N1 (China, South East Asia) is intrinsically more sensitive to oseltamivir than the clade 2 H5N1 (Europe, Africa, Indonesia, China, South East Asia)Citation2.
The inhibition constants obtained by kinetic experiments were used to calculate the IC50 of oseltamivir carboxylate against the two enzymes obtaining the values of 23.9 nM and 0.12 nM for neuraminidase N1 and N3, respectively. These values were in rather good agreement with those determined by fitting the inhibition-dose curve reported in . The agreement of these values, obtained in two independent manners, supports a rather good performance of the developed assay.
The determination of the between- and within-assay repeatability of the assay was performed by using different concentrations of oseltamivir carboxylate in order to span the whole range of fluorescence intensities attainable in the assay. The within-assay coefficient of variations on the determination of the IC50 of oseltamivir carboxylate against neuraminidase N1 and N3 were 10% and 8%, respectively, and the between-assay coefficients of variations were 5% for N1 and 20% for N3. Given the rather simple set-up of the developed assay, the performance was considered to be satisfactory.
Conclusions
In summary, the findings reported in this work suggest that a crude preparations of recombinant neuraminidase obtained from a baculovirus expression system where the enzyme is located on the membrane capsule of the virus could be effectively used to set up cost-effective, medium-throughput screening assays to assess the value of putative neuraminidase inhibitors. This approach was preliminarily validated by using crude supernatants of neuraminidase N1 and N3 obtained from H7N1 (A/Turkey/Italy/4426/2000 LPAI) and H7N3 (A/ty/Italy/8000/02), respectively. The performance of the developed assay was assessed by using the benchmark neuraminidase inhibitor oseltamivir carboxylate, the within- and between-assay repeatability was satisfactory. The IC50 values of oseltamivir carboxylate against the two enzymes analysed as determined by the developed assay were in good agreement with the values obtained by independent kinetic experiments, supporting the robustness of the assay. Moreover, the use of these sources of neuraminidase avoids the need of expensive purified recombinant neuraminidase or the costs associated to the safety standards required for handling avian influenza viruses. In fact, materials containing baculovirus particles are safer to work with than most mammalian viruses because they are non-infectious to vertebrates.
Acknowledgments
J. K. would like to thank Chulalongkorn University and The Embassy of Italy in Thailand for supporting her Ph.D. scholarship.
Declaration of interest
This work was supported by the Joint Project between the International Centre for Science and High Technology (UNIDO) Trieste, Italy and the Office of the Higher Education Commission, Bangkok, Thailand.
References
- Eichelberger MC, Hassantoufighi A, Wu M, Li M. Neuraminidase activity provides a practical read-out for a high throughput influenza antiviral screening assay. Virol J 2008;5:109.
- Taylor WR, Burhan E, Wertheim H, Soepandi PZ, Horby P, Fox A, Benamore R, de Simone L, Hien TT, Chappuis F. Avian influenza—A review for doctors in travel medicine. Trav Med Inf Dis 2009;20:1–12.
- Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM et al. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006;443:45–49.
- Schünemann HJ, Hill SR, Kakad M, Bellamy R, Uyeki TM, Hayden FG et al.; WHO Rapid Advice Guideline Panel on Avian Influenza. WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect Dis 2007;7:21–31.
- de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005;353:2667–2672.
- Earhart KC, Elsayed NM, Saad MD, Gubareva LV, Nayel A, Deyde VM et al. Oseltamivir resistance mutation N294S in human influenza A(H5N1) virus in Egypt. J Infect Public Health 2009;2:74–80.
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH et al. Avian flu: Isolation of drug-resistant H5N1 virus. Nature 2005;437:1108.
- Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res 2005;68:66–74.
- Liu AL, Liu B, Qin HL, Lee SM, Wang YT, Du GH. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med 2008;74:847–851.
- Liu AL, Wang HD, Lee SM, Wang YT, Du GH. Structure activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg Med Chem 2008;16:7141–7147.
- Miki K, Nagai T, Suzuki K, Tsujimura R, Koyama K, Kinoshita K et al. Anti-influenza virus activity of biflavonoids. Bioorg Med Chem Lett 2007;17:772–775.
- Guo CT, Takahashi T, Bukawa W, Takahashi N, Yagi H, Kato K et al. Edible bird’s nest extract inhibits influenza virus infection. Antiviral Res 2006;70:140–146.
- Ryu YB, Curtis-Long MJ, Kim JH, Jeong SH, Yang MS, Lee KW et al. Pterocarpans and flavanones from Sophora flavescens displaying potent neuraminidase inhibition. Bioorg Med Chem Lett 2008;18:6046–6049.
- Ryu YB, Curtis-Long MJ, Lee JW, Kim JH, Kim JY, Kang KY et al. Characteristic of neuraminidase inhibitory xanthones from Cudrania tricuspidata. Bioorg Med Chem 2009;17:2744–2750.
- Jeong HJ, Ryu YB, Park SJ, Kim JH, Kwon HJ, Kim JH et al. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med Chem 2009;17:6816–6823.
- Yongkiettrakul S, Boonyapakron K, Jongkaewwattana A, Wanitchang A, Leartsakulpanich U, Chitnumsub P et al. Avian influenza A/H5N1 neuraminidase expressed in yeast with a functional head domain. J Virol Methods 2009;156:44–51.
- Verma R, Boleti E, George AJT. Antibody engineering: Comparison of bacterial, yeast, insect and mammalian expression systems. J Immunol Methods 1998;216:165–181.
- Cattoli G, Terregino C, Brasola V, Rodriguez JF, Capua I. Development and preliminary validation of an ad hoc N1-N3 discriminatory test for the control of avian influenza in Italy. Avian Dis 2003;47:1060–1062.
- Cattoli G, Milani A, Bettini F, Serena Beato M, Mancin M, Terregino C et al. Development and validation of an anti-N3 indirect immunofluorescent antibody test to be used as a companion diagnostic test in the framework of a “DIVA” vaccination strategy for avian influenza infections in poultry. Avian Pathol 2006;35:154–159.
- Rungrotmongkol T, Frecer V, De-Eknamkul W, Hannongbua S, Miertus S. Design of oseltamivir analogs inhibiting neuraminidase of avian influenza virus H5N1. Antiviral Res 2009;82:51–58.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:568.
- Dixon M. The determination of enzyme inhibitor constants. Biochem J 1953;55:170–171.
- Dalakouras T, Smith BJ, Platis D, Cox MM, Labrou NE. Development of recombinant protein-based influenza vaccine expression and affinity purification of H1N1 influenza virus neuraminidase. J Chromatogr A 2006;1136:48–56.
- Tanimoto T, Nishimura Y, Matsuura Y, Fuke I, Ishikawa T, Yamanishi K, Tamura S. Expression of influenza neuraminidase in CHO-K1 cells. International Congress Series 2004;1263:568–572.
- Yen HL, Ilyushina NA, Salomon R, Hoffmann E, Webster RG, Govorkova EA. Neuraminidase inhibitor-resistant recombinant A/Vietnam/1203/04(H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo J Virol 2007;81:12418–12426.
- Rameix-Welti MA, Agou F, Buchy P, Mardy S, Aubin JT, Véron M, van der Werf S, Naffakh N. Natural variation can significantly alter the sensitivity of influenza A (H5N1) viruses to oseltamivir. Antimicrob Agents Chemother 2006;50:3809–3815.