Abstract
The effects of ketotifen, meloxicam, phenyramidol–HCl and gadopentetic acid on the enzyme activity of GR were studied using human erythrocyte glutathione reductase (GR) enzymes in vitro. The enzyme was purified 209-fold from human erythrocytes in a yield of 19% with 0.31 U/mg. The purification procedure involved the preparation of haemolysate, ammonium sulphate precipitation, 2′′,5′-ADP Sepharose 4B affinity chromatography and Sephadex G-200 gel filtration chromatography. Purified enzyme was used in the in vitro studies. In the in vitro studies, IC50 values and Ki constants were 0.012 mM and 0.0008 ± 0.00021 mM for ketotifen; 0.029 mM and 0.0061 ± 0.00127 mM for meloxicam; 0.99 mM and 0.4340 ± 0.0890 mM for phenyramidol–HCl; 138 mM and 28.84 ± 4.69 mM for gadopentetic acid, respectively, showing the inhibition effects on the purified enzyme. Phenyramidol–HCl showed competitive inhibition, whereas the others showed non-competitive inhibition.
Keywords::
Introduction
Oxidative stress, which refers to the unusually high presence of molecules with a high potency to abstract electrons from biomolecules, plays an important role in the pathogenesis of various diseasesCitation1,Citation2. The undesirable biological effects of these highly reactive molecules are eliminated by enzymatic and nonenzymatic antioxidant defence systems. Enzymatic defence is provided by many enzyme systems such as superoxide dismutase, catalase, glutathione peroxidase, glutathione S-transferase, glutathione reductase (GR), aldoketoreductases and DNA repair enzymes. Nonenzymatic antioxidant defence systems include many different agents, such as transferrin, ceruloplasmin, lactoferrin, vitamins (e.g. A, E and C), uric acid, taurine, glutathione (GSH), cysteamine and cysteine. The GSH and GSH-related enzymes responsible for glutathione metabolism make up one of the most important protective systems in cellsCitation3.
Glutathione reductase (glutathione:NADP+ oxidoreductase, EC 1.8.1.7; GR) plays a central role in glutathione metabolism. This flavin enzyme is essential for reducing glutathione disulphide (GSSG) to the tripeptide form. GSH not only counteracts oxidative agents but also protects the sulphydryl groups of intracellular proteins in the erythrocytes. The GSH concentration needs to be about 2 mM to keep red blood cells alive and not being haemolyzedCitation4. On the other hand, a high GSSG concentration inhibits a number of important enzyme systems including protein synthesisCitation5. Decreased GSH levels have also been reported in several diseases, such as acquired immune deficiency syndrome (AIDS)Citation6, adult respiratory distress syndromeCitation7, Parkinson’s diseaseCitation8 and diabetesCitation9. Many drugs have activation or inhibition effects on the body’s enzyme systemsCitation10–12. Many drugs may show the same effects under both in vivo and in vitro conditions; however, some of them may not show the same effects on enzymesCitation13.
In the present study, ketotifen, meloxicam, phenyramidol–HCl and gadopentetic acid were selected. Since the effects of these drugs on human erythrocyte GR enzyme activity has not been investigated, these drugs are used very often for medical treatments. Considering the study from this direction, it is thought that different class and chemical structures of drugs are important to add a better sense on GR enzyme activity.
Materials and methods
Materials
2′,5′-ADP Sepharose 4B was purchased from Pharmacia (Germany). Sephadex G-200, NADPH, GSSG, the protein assay reagent and the chemicals for electrophoresis were purchased from Sigma (Germany). All other chemicals used were of analytical grade and purchased from either Sigma or Fluka (Germany).
Preparation of the haemolysate
Fresh human blood from one single subject was collected into a tube containing 0.1 M Na citrate, 0.16 M glucose, 0.016 M Na-phosphate and 2.59 mM adenine for anticoagulation. The sample was centrifuged at 3000 g for 15 min and the plasma and leucocyte coat were removed. The erythrocytes were washed three times with isotonic NaCl solution including 1 mM EDTA, the samples being centrifuged at 3000 g each time and the supernatants were removed. The washed erythrocytes were haemolyzed with five volumes of ice-cold distilled water containing 2.7 mM EDTA and 0.7 mM β-mercaptoethanol and then centrifuged at 4°C, 20,000 g for 30 min to remove residual intact cells and membranesCitation14.
Ammonium sulphate fractionation and dialysis
Ammonium sulphate precipitation was done in the haemolysate at 30–70% saturation. Ammonium sulphate was added slowly to the haemolysate, followed by stirring until it dissolved completely. The mixture was centrifuged at 5000 g for 15 min. The precipitate was dissolved in 50 mM of phosphate buffer including 1 mM EDTA (pH 7.0). Precipitates in the range (30–70%) were collected and dialyzed at 4°C in the same buffer for 2 h with two changes of bufferCitation15,Citation16.
2′,5′-ADP sepharose 4B affinity chromatography
Dry 2′,5′-ADP Sepharose 4B (2 g) was washed and swelled in distilled and deionized water. After removal of the air in the gel, it was resuspended in 0.1 M potassium acetate/0.1 M potassium phosphate buffer, pH 6.0, and packed into a small column (1 × 10 cm). After precipitation of the gel, it was equilibrated with the same buffer by means of a peristaltic pump with the flow rate adjusted to 20 mL/h. The dialyzed sample obtained from ammonium sulphate precipitation was loaded onto the column, and washed with 25 mL of 0.1 M potassium acetate + 0.1 M potassium phosphate, pH 6 and 25 mL of 0.1 M potassium acetate + 0.1 M potassium phosphate, pH 7.85. Washing continued with 50 mM of potassium phosphate including 1 mM EDTA (pH 7.0) until the final absorbance difference was 0.05 at 280 nm. Proteins bound on the gel were eluted with a gradient of 0–0.5 mM GSH and 0–1 mM NADPH in 50 mM potassium phosphate, containing 1 mM EDTA (pH 7.0). Active fractions were collected and dialyzed with 50 mM of potassium phosphate including 1 mM EDTA (pH 7.0) at 4°CCitation15–19.
Sephadex G-200 gel filtration chromatography
Dry Sephadex G-200 (5 g) was incubated in distilled water at 90°C for 5 h. After removal of the air in the gel, it was loaded onto a column (2 × 50 cm). The flow rate was adjusted to 15 mL/h by means of a peristaltic pump. The column was equilibrated with 50 mM Tris–HCl, and 50 mM KCl buffer (pH 7.0) until the final absorbance difference became zero at 280 nm and the pH was the same as that of the equilibration buffer. The dialysate from the affinity chromatography column was mixed with glycerol in a proportion of 5%. The final sample was loaded onto the column and 2 mL of each elution was collected. The absorbance values were determined at 280 and 340 nm in each fraction. Active fractions were lyophilized and stored at −85°C for use in the in vitro studies.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
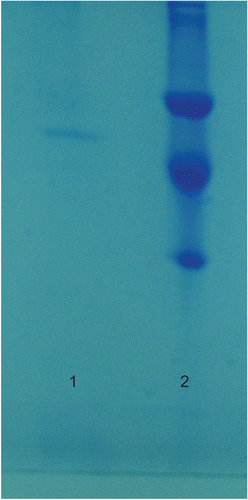
To determine the purity of the enzyme, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli’s methodCitation20. The acryl amide concentration of the stacking and separating gels was 3% and 8%, respectively, and 1% SDS was also added to the gel solution. The gel was stained for 2 h in 0.1% Coomassie Brilliant Blue R-250 containing 50% methanol, 10% acetic acid and 40% distilled water, then destained with many changes of the same solvent without dye. Cleared protein bands were photographed ().
Activity assay
GR activity was determined by the method of Carlberg and MannervikCitation21 with a Shimadzu Spectrophotometer UV-(1208) at 25°C. The assay system contained 40 mM Tris–HCl buffer, pH 8.0, including 0.8 mM EDTA, 1 mM GSSG and 0.1 mM NADPH in 1 mL total reaction volume. The activity was measured by monitoring the decrease in absorbance at 340 nm due to the oxidation of NADPH at 25°C. One enzyme unit is defined as the oxidation of 1 mmol NADPH per min under the assay conditions.
Protein determination
Quantitative protein determination was performed spectrophotometrically at 595 nm according to Bradford’s methodCitation22, using bovine serum albumin as a standard. Qualitative protein determination was also performed spectrophotometrically at 280 nm according to Segel’s methodCitation23.
In vitro drug studies
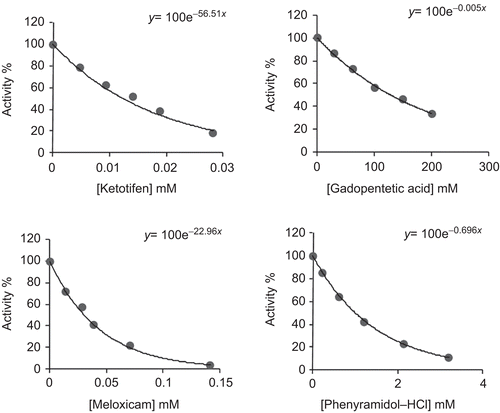
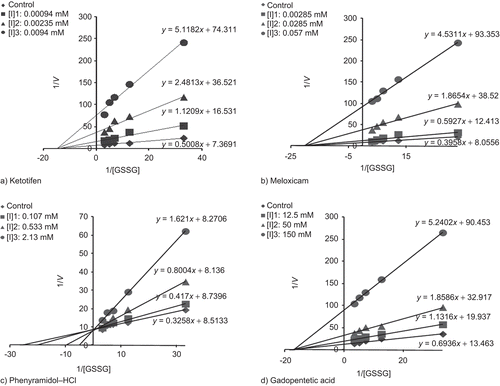
Ketotifen, meloxicam, phenyramidol–HCl and gadopentetic acid were tested to determine their effects on human erythrocyte GR activity. Assays were performed in cuvette concentrations of 0.0047–0.0282 mM ketotifen; 0.01425–0.142 mM meloxicam; 0.107–3.20 mM phenyramidol–HCl; 12.5–200 mM gadopentetic acid. The cuvette GR activity in the absence of drug was taken as 100%. For each drug, a activity% − [drug] graph was drawn (), and drug concentrations producing 50% inhibition (IC50) were graphically calculated for ketotifen, meloxicam, phenyramidol–HCl and gadopentetic acid. In the media with or without inhibitor, five substrate (GSSG) concentrations (0.03, 0.08, 0.14, 0.20 and 0.30 mM for drugs) were used. Three different fixed concentrations of inhibitor solution selected from activity% − [drug] graphs were added to the reaction medium in a total reaction volume of 1 mL for each fixed inhibitor concentration. Regression analysis provided the equations to be used for drawing the Lineweaver–Burk graphs () using 1/V and 1/[S] values. The Ki values were graphically calculated for these drugs.
Results
Human erythrocyte GR was purified 209-fold with a yield of 19% using 2′,5′-ADP Sepharose 4B affinity chromatography and Sephadex G-200 gel filtration chromatography after ammonium sulphate precipitation of the haemolysate (). The purity of the enzyme was determined by SDS-PAGE and a single band was observed on the gel after the final chromatographic step (). In vitro studies showed that ketotifen, meloxicam, phenyramidol–HCl and gadopentetic acid inhibited enzyme activity. IC50 values and Ki constants were 0.012 mM and 0.0008 ± 0.00021 mM for ketotifen; 0.029 mM and 0.0061 ± 0.00127 mM for meloxicam; 0.99 mM and 0.4340 ± 0.0890 mM for phenyramidol–HCl; 138 mM and 28.84 ± 4.69 mM for gadopentetic acid, respectively ().
Table 1. Purification scheme of glutathione reductase (GR) from human erythrocyte.
Table 2. Ki values obtained from Lineweaver–Burk graphs for glutathione reductase (GR) in the presence of three fixed inhibitors and five substrate concentrations for different drugs.
Inhibition types were determined to be non-competitive for ketotifen, gadopentetic acid and meloxicam, but competitive for phenyramidol–HCl ().
Discussion
GR is essential for the maintenance of cellular glutathione in its reduced form, which is highly nucleophilic for many reactive electrophilsCitation24. GSH is either involved as a substrate in the cytosolic GSH redox cycle, or able to directly inactivate free radicals and reactive oxygen species (ROS), which are known to be effective stress agentsCitation25. Many chemicals and drugs are known to have adverse or beneficial effects on human enzyme and metabolic events and the effects can be dramatic and systemicCitation26. The inhibition of some important enzymes plays a key role in a metabolic pathway, for example, some metabolic diseases such as diabetes mellitus are affected by enzyme activityCitation27. Similarly, acetazolamide has an inhibitory effect on the carbonic anhydrase (CA) enzyme leading to diuresisCitation28. Additionally, epiandrosterone was found to inhibit red blood cell glucose 6-phosphate dehydrogenase (G6PD) uncompetitively and suppress hexose monophosphate shunt activity by >95%Citation29. Some chemicals and drugs, such as nitrofurazone, nitrofurantoin, 5-nitroindol, 5-nitro-2-furoic acid, 2,4,6-trinitrobenzene sulphonate (TNBS)Citation30 and polyphenolic compounds, also inhibit GR enzyme activityCitation31. Furthermore, it is reported that human erythrocyte CA-I and CA-II are inhibited by ampicillinCitation13, human erythrocyte G6PD by netilmicin, gentamicin, streptomycin, ampicillinCitation32, metamizol and magnesium sulphateCitation33, and bovine and human erythrocyte GR by some drugsCitation15,Citation16. Many drugs are commonly used in the therapy of human diseases, but we have not encountered any investigations of their inhibition or activation effects on GR enzyme in the literature. In the present article, the effects of some used drugs on human erythrocyte GR enzyme were investigated, and IC50 values and Ki constants were estimated for the drugs showing inhibition effects.
Ki constants show that ketotifen had the highest inhibitory effect, followed by meloxicam, phenyramidol–HCl and gadopentetic acid, respectively. IC50 values showed the same trend (). In this investigation, by using the obtained Ki constants and IC50 values, undesirable side effects of these drugs on GR activity and body metabolism and antioxidative capacity can be reduced. shows that the enzyme is mostly inhibited by ketotifen and meloxicam drugs. The chemical structures of ketotifen and meloxicam contain an active group of sulphur that appears to be different from the other two drugs.
It was determined that drugs such as tenoxicam, lornoxicam, thiocolchicoside and olanzapine, containing sulphur groups, efficiently inhibit GR enzyme activityCitation34,Citation35. Similar results were obtained in the present study for ketotifen and meloxicam. Probably, the sulphur group binds to outside of the active region of GR enzyme (non-competitive) that inhibits GR activity by changing the enzyme’s three-dimensional structure. Phenyramidol–HCl and gadopentetic acid have a less inhibitory effect on the enzyme’s activity, we anticipated both drugs contain carbonyl, nitrogen and carboxyl groups, but do not contain sulphur groups.
In this investigation, by using the obtained Ki and IC50 values, undesirable side effects of these drugs on GR activity and body metabolism and fatty acid synthesis can be reduced. According to this data, if it is required to give ketotifen and meloxicam to patients, its dosage should be very well-controlled to decrease haemolytic and other side effects due to possible inhibition of GR.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Serban MG, Tanaseanu S, Bâra C. Oxidant stress and antioxidant protection in lupus nephropathy. Rom J Intern Med 1996;34:105–109.
- Ruiz C, Alegría A, Barberá R, Farré R, Lagarda MJ. Lipid peroxidation and antioxidant enzyme activities in patients with type 1 diabetes mellitus. Scand J Clin Lab Invest 1999;59:99–105.
- Knapen MF, Zusterzeel PL, Peters WH, Steegers EA. Glutathione and glutathione-related enzymes in reproduction. A review. Eur J Obstet Gynecol Reprod Biol 1999;82:171–184.
- Kondo T, Dale GL, Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci USA 1980;77:6359–6362.
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol 1989;257:L163–L173.
- Akerlund B, Tynell E, Bratt G, Bielenstein M, Lidman C. N-Acetylcysteine treatment and the risk of toxic reactions to trimethoprim–sulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIV-infected patients. J Infect 1997;35:143–147.
- Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest 1991;100:1397–1403.
- Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol 1998;44:S72–S84.
- Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med 1996;13:715–719.
- Edward E, Morse MD. Toxic effects of drugs on erythrocytes. Ann Clin Sci 1988;18:13–18.
- Jacobasch G, Rapoport SM. Hemolytic anemias due to erythrocyte enzyme deficiencies. Mol Aspects Med 1996;17:143–170.
- Erat M, Sakiroglu H, Ciftci M. Effects of some antibiotics on glutathione reductase from bovine erythrocytes activity. Vet Med Czech 2003;48:305–312.
- Beydemir S, CIftçi M, Ozmen I, Buyukokuroglu ME, Ozdemir H, Küfrevioglu OI. Effects of some medical drugs on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 2000;42:187–191.
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. Orlando: Grune and Stratton Inc.; 1984, pp. 134–135.
- Erat M, Sakiroglu H, Ciftci M. Purification and characterization of glutathione reductase from bovine erythrocytes. Prep Biochem Biotechnol 2003;33:283–300.
- Erat M, Sakiroglu H, Ciftçi M. Effects of some antibiotics on glutathione reductase activities from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:69–74.
- Boggaram V, Brobjer T, Larson K, Mannervik B. Purification of glutathione reductase from porcine erythrocytes by the use of affinity chromatography on 2′,5′-ADP-Sepharose 4B and crystallization of the enzyme. Anal Biochem 1979;98:335–340.
- Carlberg I, Mannervik B. Purification and characterization of glutathione reductase from calf liver. An improved procedure for affinity chromatography on 2′,5′-ADP-Sepharose 4B. Anal Biochem 1981;116:531–536.
- Le Trang N, Bhargava KK, Cerami A. Purification of glutathione reductase from gerbil liver in two steps. Anal Biochem 1983;133:94–99.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Carlberg I, Mannervik B. Methods Enzymology. Orlando FL: Academic Press; 1985, Vol. 113, pp. 484–495.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Segel IH. Biochemical Calculation. New York: John Wiley & Sons; 1968, p. 403.
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 1975;250:5475–5480.
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem 1988;263:17205–17208.
- Christensen GM, Olson D, Riedel B. Chemical effects on the activity of eight enzymes: a review and a discussion relevant to environmental monitoring. Environ Res 1982;29:247–255.
- Gupta BL, Nehal M, Baquer NZ. Effect of experimental diabetes on the activities of hexokinase, glucose-6-phosphate dehydrogenase and catecholamines in rat erythrocytes of different ages. Indian J Exp Biol 1997;35:792–795.
- Warnock DG. Diuretic agent. In: Katzung BG, ed. Basic, and Clinical Pharmacology, 4th ed. USA: Appleton and Lange; 1989, p. 183.
- Grossman S, Budinsky R, Jollow D. Dapsone-induced hemolytic anemia: role of glucose-6-phosphate dehydrogenase in the hemolytic response of rat erythrocytes to N-hydroxydapsone. J Pharmacol Exp Ther 1995;273:870–877.
- McCallum MJ, Barrett J. The purification and properties of glutathione reductase from the cestode Moniezia expansa. Int J Biochem Cell Biol 1995;27:393–401.
- Zhang K, Yang EB, Tang WY, Wong KP, Mack P. Inhibition of glutathione reductase by plant polyphenols. Biochem Pharmacol 1997;54:1047–1053.
- Ciftci M, Küfrevioglu ÖI, Gündogdu M, Özmen II. Effects of some antibiotics on enzyme activity of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 2000;41:107–111.
- Ciftçi M, Ozmen I, Büyükokuroglu ME, Pençe S, Küfrevioglu OI. Effects of metamizol and magnesium sulfate on enzyme activity of glucose 6-phosphate dehydrogenase from human erythrocyte in vitro and rat erythrocyte in vivo. Clin Biochem 2001;34:297–302.
- Senturk M, Irfan Kufrevioglu O, Ciftci M. Effects of some analgesic anaesthetic drugs on human erythrocyte glutathione reductase: an in vitro study. J Enzyme Inhib Med Chem 2009;24:420–424.
- Akkemik E, Senturk M, Ozgeris FB, Taser P, Ciftci M. In vitro effects of some drugs on human erythrocyte glutathione reductase. Turk J Med Sci 2011;41:235–241.