Abstract
DDAH inhibition presents a novel promising pharmaceutical strategy to lower NO formation. To date, several potent DDAH inhibitors have been published, most of them representing analogues of l-arginine. While inhibitory effects on NOSs have already been considered, selectivity over arginase has been neglected so far. In our view, the latter selectivity is more important since an additional inhibition of arginase decreases the desired effects on NO levels. Thus, we particularly focus on selectivity over arginase. We present a comprehensive selectivity profile of known DDAH inhibitors by covering their inhibitory potency on arginase. Among the studied compounds, Nω-(2-methoxyethyl)-l-arginine (2a, L-257) that is already selective over NOSs also only modestly affected arginase activity and is thus far the most suitable DDAH inhibitor for pharmacological studies.
| Abbreviations | ||
| ADMA, | = | asymmetric Nω,Nω-dimethyl-l-arginine |
| DDAH, | = | dimethylarginine dimethylaminohydrolase |
| NMMA, | = | Nω-monomethyl-l-arginine |
| NO, | = | nitric oxide |
| NOS, | = | nitric oxide synthase |
| SARs, | = | structure activity relationships |
Introduction
Nitric oxide (NO) is formed via nitric oxide synthases (NOSs) from l-arginine. Physiologically, NOSs activity is regulated by the endogenous, competitive inhibitors asymmetric Nω,Nω-dimethyl-l-arginine (ADMA) and Nω-monomethyl-l-arginine (NMMA)Citation1. The major physiological route of NMMA and ADMA degradation is via dimethylarginine dimethylaminohydrolase (DDAH) to l-citrulline and either dimethylamine or methylamine (see )Citation2. To date, two different isoforms have been described: DDAH-1 is mainly expressed in liver and kidney, whereas DDAH-2 is located in the vascular endothelium, heart, placenta and kidneyCitation3. Thus, there is a colocalization of DDAH-1/nNOS and DDAH-2/eNOS, indicating an isoform-specific regulation of NOS activityCitation4.
Figure 1. Overview on the NO generating system and its physiological regulation. N-reduction, microsomal/mitochondrial reduction23. DDAH, dimethylarginine dimethylaminohydrolase; NOS, nitric oxide synthase; PRMT, protein arginine methyltransferase.
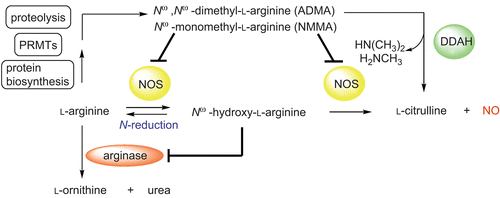
Accordingly, DDAH inhibition represents a promising pharmaceutical strategy to indirectly affect NO formation by elevating Nω-methylated l-arginine levelsCitation5. This hypothesis was supported by former studies showing that pharmacological inhibition of DDAH results in an accumulation of ADMA and subsequently decreased NO formationCitation6,Citation7. Thus, DDAH inhibition might be useful for the therapy of pathophysiological conditions associated with NO overproduction such as cardiogenic shock, pain, migraine, neurodegenerative diseases (Alzheimer’s disease), or it might be a pharmaceutical strategy to reduce angiogenesis associated with tumours, arthritis and diabetic retinopathyCitation8,Citation9.
At present, several l-arginine and N5-(1-imino-alk(en)yl)-l-ornithine derivatives have been identified as DDAH inhibitors, with N5-(1-iminobut-3-enyl)-l-ornithine (1a) and Nω-(2-methoxyethyl)-l-arginine (2a, L-257) as the most potent representatives. However, most of these l-arginine analogues additionally affect NOSs activityCitation7,Citation10–12. In fact, it is still a matter of debate whether selectivity over NOSs is essentially needed, considering that inhibition of both NOSs and DDAH represents a dual mode of action and should increase potency of these compoundsCitation9,Citation11. However, taken into account that all direct NOS inhibitors failed in clinical trials, we think that selectivity over NOSs is desirable or at least inhibitory effects on NOS should be minimized. Further studies (preferably in vivo) are needed to show whether or not a highly specific DDAH inhibitor is superior over a dual DDAH/NOS inhibitor.
With regards to enzyme selectivity most published works dealing with DDAH inhibitors merely considered selectivity over NOS isoforms, whereas selectivity over arginase has never been investigated at all. However, arginases and NOSs are the predominant enzymes in l-arginine metabolism and compete for their common substrate l-arginine (see )Citation13. Hence, arginase inhibition elevates NO formation by augmenting the l-arginine substrate pool for NOSs. Meanwhile, several studies confirmed this reciprocal interaction between both pathways under physiological conditionsCitation9,Citation14,Citation15. Additionally, taken the different Km-values for NOS (~5 µM) and arginase (~5 mM) for l-arginine into account, inhibitors showing comparable Ki-values for these enzymes can be assumed to mainly affect arginase activityCitation15,Citation16. Thus, we assume that selectivity over arginase is even more important than selectivity over NOSs, since an additional inhibition of arginase would lower the desired pharmacological effect of DDAH inhibitionCitation9. In the presented work, we addressed this selectivity issue for a selection of DDAH inhibitors as this has been so far neglected in the development of such pharmacological tools or drug candidates.
Methods
General
Commercially available materials were purchased from either Sigma-Aldrich (Seelze, Germany), Fluka (Buchs, Switzerland), Merck (Darmstadt, Germany), Alexis Biochemicals (Lausen, Switzerland) or Roth (Karlsruhe, Germany) unless otherwise stated. NMMA, 1a, 2b were purchased from Alexis Biochemicals. 1d and 1e were purchased from Cayman chemicals (Ann Arbor, Michigan, USA). Nω-substituted L-arginine derivatives except for 2b and 2g were synthesized according to Schade et al.Citation17. 1b and 1c were obtained from BogaR Laboratories LLC (Alpharetta, Georgia, USA).
Expression and purification of 6xHis-tagged hDDAH-1
Human DDAH-1 was expressed in Escherichia coli BL21 and purified via Ni-NTA-agarose as described previouslyCitation18.
In vitro hDDAH-1 assay
A colorimetric assay was carried out in 150 µL 50 mM potassium phosphate buffer pH 7.4 containing 4 µg of recombinant hDDAH-1 and varying concentrations of NMMA and inhibitor. NMMA was applied at 50, 100, 300, 700 and 1250 µM. Each dilution was incubated each inhibitor in five different concentrations and one without inhibitor as a control. Samples were incubated in a 96-well microplate at 37°C for 30 min and the reaction was stopped by addition of 200 µL Colder reagentCitation19. Microplates were sealed with sealing tape, incubated at 95°C for 20 min and then read at 540.5 nm. Subsequent, Ki-values were calculated using SigmaPlot 8.0 (SPSS Inc.) and Microsoft Excel.
In vitro NOS assay
Inhibition studies were carried out as described previouslyCitation10.
In vitro arginase assay
A colorimetric assay was carried out with 0.3 µg of bovine liver arginase in 150 µL of 50 mM Tris buffer pH 7.4 containing 100 µM MnCl2, and 100 µM maleic acid and 7 mM l-arginine. Inhibitors were applied at concentrations of 100 µM and 1 mM. Samples were incubated in a 96-well microplate at 37°C for 30 min and the reaction was stopped by addition of 200 µL Colder reagentCitation19. Microplates were sealed with sealing tape, incubated at 95°C for 20 min and then read at 526 nm.
Results
In vitro DDAH-assay
Initially, we investigated biochemical properties of our recombinantly expressed hDDAH-1 and determined the Km-value for NMMA (133 ± 75 μM) and Vmax (210 ± 14 nmol × min−1 × mg−1), which lies in the same range as published values of other groupsCitation16.
We previously reported of several novel DDAH-1 inhibitorsCitation10. In this work, we determined Ki-values for all these compounds for hDDAH-1. 1a, 1b, 1c, 2a, and 2e showed highest potency ().
Table 1. Inhibition data of N5-(1-imino-alk(en)yl)-l-ornithine (1) and Nω-substituted l-arginine (2) derivatives. NOS and DDAH inhibition data is taken from a former publicationCitation10. Data stated in brackets are inhibition data at 1 mM.
In vitro arginase assay
We established an easy plate reader assay format to test the effects on arginase. Our study revealed that most potent DDAH-1 inhibitors additionally inhibit arginase activity (). In particular, the most potent DDAH-1 inhibitor 1a known so far, turned out to be a moderately potent inhibitor of arginase, as well. Other compounds moderately affecting arginase activity are 2b and 2d. Compound 2a (L-257) turned out to be not only selective over NOS but also over arginase. Regarding compounds of series 1 it is observable that 1c has minimal side effects on arginase activity.
Discussion
We previously reported several novel human DDAH inhibitors of which 1a, 1b, 1c, 2a, and 2e showed highest potency. Results of the herein presented arginase assay revealed that most potent DDAH inhibitors additionally inhibit arginase activity. In particular, the most potent DDAH inhibitor known so far 1a, turned out to be a moderately potent inhibitor of arginase as well, limiting its applicability in further studies. In contrast, 1c has minimal side effects on arginase activity, whereas it is not entirely selective over NOSs and about 2.5-fold less potent than 2a (L-257). Nevertheless, this compound seems to be suitable for further studies. Interestingly, 1b which differs from 1a in a double-bond exhibits less effects on arginase activity. Within series 2, compound 2a (L-257) turned out to be not only exceptionally selective over NOS but also over arginase. Additionally, 2c and 2e represent potent DDAH inhibitors possessing a moderate selectivity over arginase; however, as for 1b these compounds are not selective over NOSs. Nevertheless, these compounds might represent interesting model compounds since other authors claim the dual inhibition of NOSs and DDAH as a promising pharmaceutical strategyCitation11. 2b and 2d are other compounds that slightly more potently affected arginase activity limiting their applicability in further studies. In particular, 2d originally was deemed to be a promising lead for further studies because of its high selectivity over NOSs.
The most potent arginase inhibitors known so far (i.e. the boronic acid and N-hydroxylated l-arginine derivatives) mimic the tetrahedral intermediate of l-arginine in arginase catalysisCitation20,Citation21. In contrast, other l-arginine derivatives turned out to be much less effective. However, at present our knowledge about structure activity relationships (SARs) of moderately potent arginase inhibitors such as 1a, 2b, and 2d is still very restricted and we cannot easily predict their inhibitory potency, but rather have to rely on empirical knowledge based on our in vitro selectivity profile studies. Nevertheless, with the herein presented data we tried to further deduce SARs for DDAH inhibitors under consideration of arginase cross-inhibition, but:
In respect of the Nω- (l-arginines/guanidines) or N5- (amidines) substituent length, compounds showing greatest side effects on arginase are 1a, 2b, and 2d which all differ in substituent length.
When comparing some alkyl derivatives with their corresponding alkenyl derivatives for compounds 1a/1b the alkenyl derivative more potently inhibits arginase. However, for compound pair 2b/2c the alkyl derivative is much less selective over arginase. Thus, regarding the influence of multiple bonds no general conclusions can be drawn. Hence, at this point no general conclusions regarding SARs for the development of arginase selective DDAH inhibitors can be drawn, underlining the necessity of investigating arginase selectivity for each newly developed DDAH inhibitor.
Conclusions
In summary, the present study underlines the importance to more enlighten the influence of potential DDAH inhibitors on arginase activity, aside from other enzymes involved in NO-related metabolism. Thus, investigating selectivity over arginase should be performed as a standard assay in all further studies dealing with the development of DDAH inhibitors.
Essentially, 2a (L-257) developed by the Leiper group represents the most promising DDAH inhibitor known so far due to its good selectivity profile over the other l-arginine converting enzymesCitation7,Citation8. This compound turned out to be not only selective over NOSs—as already known—but also over arginase. Although, we think that DDAH inhibitors should be selective for this enzyme, other authors claim a dual DDAH/NOS inhibition as promisingCitation11. Based on this aspect, 1c, 2c, and 2e may also be useful in future studies.
The herein presented study reveals the necessity of addressing arginase selectivity issues, since 1a, the most potent DDAH inhibitor known so far, and 2d also effectively inhibit arginase, and thus, despite their potency on DDAH cannot be considered as suitable candidates for studies that are aimed at investigating DDAH specific (patho)physiological effects. Nevertheless, our knowledge about the broad spectrum of DDAH (patho)physiological functions is still rather low at presentCitation9. In particular, more studies are needed to investigate the in vivo effects of an additional arginase inhibition in order to support or possibly disprove our assumption that selectivity over arginase is more important than selectivity over NOS. Additionally, isoform selectivity of DDAH inhibitors requires further investigations. But recombinant expression of DDAH-2 is a challenging task, and to date, no working group succeeded in recombinant expression and isolation of this isoform. Therefore, current inhibition data is exclusively available for DDAH-1. However, due to the different tissue distribution isoform-specific inhibitors bear the potential to affect DDAH activity—and consequently NOS activity—in specific tissuesCitation22.
Declaration of interest
The authors report no conflicts of interest.
References
- Knipp M. How to control NO production in cells: N(omega),N(omega)-dimethyl-L-arginine dimethylaminohydrolase as a novel drug target. Chembiochem 2006;7:879–889.
- Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem 1989;264:10205–10209.
- Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 1999;43:542–548.
- Tran CT, Fox MF, Vallance P, Leiper JM. Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 2000;68:101–105.
- Vallance P, Leiper J. Blocking NO synthesis: How, where and why? Nat Rev Drug Discov 2002;1:939–950.
- MacAllister RJ, Parry H, Kimoto M, Ogawa T, Russell RJ, Hodson H et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol 1996;119:1533–1540.
- Rossiter S, Smith CL, Malaki M, Nandi M, Gill H, Leiper JM et al. Selective substrate-based inhibitors of mammalian dimethylarginine dimethylaminohydrolase. J Med Chem 2005;48:4670–4678.
- Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med 2007;13:198–203.
- Schade D, Kotthaus J, Clement B. Modulating the NO generating system from a medicinal chemistry perspective: Current trends and therapeutic options in cardiovascular disease. Pharmacol Ther 2010;126:279–300.
- Kotthaus J, Schade D, Muschick N, Beitz E, Clement B. Structure-activity relationship of novel and known inhibitors of human dimethylarginine dimethylaminohydrolase-1: Alkenyl-amidines as new leads. Bioorg Med Chem 2008;16:10205–10209.
- Wang Y, Monzingo AF, Hu S, Schaller TH, Robertus JD, Fast W. Developing dual and specific inhibitors of dimethylarginine dimethylaminohydrolase-1 and nitric oxide synthase: Toward a targeted polypharmacology to control nitric oxide. Biochemistry 2009;48:8624–8635.
- Lluis M, Wang Y, Monzingo AF, Fast W, Robertus JD. Characterization of C-alkyl amidines as bioavailable covalent reversible inhibitors of human DDAH-1. Chemmedchem 2011;6:81–88.
- Boucher JL, Moali C, Tenu JP. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci 1999;55:1015–1028.
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003;108:2000–2006.
- Durante W, Johnson FK, Johnson RA. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 2007;34:906–911.
- Forbes SP, Druhan LJ, Guzman JE, Parinandi N, Zhang L, Green-Church KB et al. Mechanism of 4-HNE mediated inhibition of hDDAH-1: Implications in no regulation. Biochemistry 2008;47:1819–1826.
- Schade D, Kotthaus J, Clement B. Efficient synthesis of optically pure Nω-alkylated L-arginines. Synthesis 2008;15:2391–2397.
- Kotthaus J, Schade D, Töpker-Lehmann K, Beitz E, Clement B. N(delta)-methylated L-arginine derivatives and their effects on the nitric oxide generating system. Bioorg Med Chem 2008;16:2305–2312.
- Knipp M, Vasák M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 2000;286:257–264.
- Custot J, Boucher J-L, Vadon S, Guedes C, Dijols S, Dealforge M et al. Nω-hydroxyamino-α-amino acids as a new class of very strong inhibitors of arginases. J Biol Inorg Chem 1996;1:73–82.
- Colleluori DM, Ash DE. Classical and slow-binding inhibitors of human type II arginase. Biochemistry 2001;40:9356–9362.
- Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 1999;343 Pt 1:209–214.
- Kotthaus J, Wahl B, Havemeyer A, Kotthaus J, Schade D, Garbe-Schönberg D et al. Reduction of Nω-hydroxy-L-arginine by the mitochondrial amidoxime reducing component (mARC). Biochem J 2010;433:383–391.
