Abstract
In the present study, 5-substituted-1,3,4-oxadiazolin-2-thiones (1a–b) were synthesized via the ring closure reactions of appropriate acid hydrazides with carbon disulphide. N-(Benzothiazol-2-yl)-2-[[5-substituted-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide derivatives (3a–j) were obtained by the nucleophilic substitution reactions of 5-substituted-1,3,4-oxadiazolin-2-thiones (1a–b) with N-(benzothiazol-2-yl)-2-chloroacetamides. The chemical structures of the compounds were elucidated by IR, 1H NMR, 13C NMR and FAB+-MS spectral data and elemental analyses. The synthesized compounds were screened for their antimicrobial activities against Micrococcus luteus, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Listeria monocytogenes and Candida albicans. All compounds except compound 3h exhibited the highest antibacterial activity against P. aeruginosa. Among all compounds (3a–j), the compounds bearing 4-methoxyphenoxymethyl moiety on oxadiazole ring (3a–e) exhibited the highest inhibitory activity against C. albicans. Although compound 3j did not possess 4-methoxyphenoxymethyl moiety on oxadiazole ring, this derivative also exhibited the same level of anti-candidal activity. The compounds were also investigated for their cytotoxic effects using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Compound 3a exhibited the highest cytotoxic activity, whereas compound 3g possessed the lowest cytotoxic activity against NIH/3T3 cells.
Introduction
Nosocomial infections have emerged as important causes of morbidity and mortality due to the fact that hospitalized patients tend to be more susceptible to infections because of their severe underlying disease conditions. If the patient is immunocompromised, micro-organisms that are not normally pathogenic are also capable of causing disease. Furthermore, hospital conditions contribute to the acquisition of drug resistance, which complicates the treatment of infections due to drug-resistant pathogensCitation1.
The development of resistance to antimicrobial agents has led to the failure of the treatment of bacterial and fungal infections, which are responsible for the bulk of nosocomial infections. In order to overcome this serious problem, which is considered as the inevitable consequence of the widespread use of these drugs, the development of new effective antimicrobial agents has gained great importanceCitation1–7.
Oxadiazoles are widely used and studied pharmacophores in medicinal chemistry due to their broad spectrum and metabolic profile. Some studies have confirmed that oxadiazole derivatives possess antimicrobial activity. Furamizole, which is a nitrofuran derivative bearing oxadiazole nucleus, is an example of promising antibacterial agentsCitation8–17.
Among oxadiazole derivatives, 1,3,4-oxadiazolin-2-thiones, which are synthesized by the ring closure reactions of various acid hydrazides with carbon disulphide, are versatile reaction intermediates in synthetic chemistry owing to the fact that the thiol group on oxadiazole ring acts as a strong nucleophileCitation18,Citation19.
Medicinal chemists have carried out considerable research for novel antimicrobial agents that possess amide moiety. The prominent drugs bearing amide group are penicillins and cephalosporins, all of which are widely used as antibiotics for the treatment of systemic infections. Penicillins and cephalosporins, which are also classified as β-lactam antibiotics, possess cyclic amide as the main scaffold and acetamide moiety as the side chainCitation20–24.
Compounds bearing benzothiazole moiety have also been reported to exhibit a wide spectrum of biological effects including antimicrobial activityCitation25–27.
In this study, we described the synthesis of some new oxadiazole derivatives, which possess two important functional moieties, namely amide and benzothiazole and focused on their potential antimicrobial and cytotoxic effects.
Methods
Chemistry
All reagents were used as purchased from commercial suppliers without further purification. Melting points were determined using an Electrothermal 9100 digital melting point apparatus and were uncorrected (Electrothermal, Essex, UK). The compounds were checked for purity by thin-layer chromatography on silica gel 60 F254. Spectroscopic data were recorded on the following instruments: IR, Shimadzu 435 IR spectrophotometer (Shimadzu, Tokyo, Japan); 1H NMR, Bruker 400 MHz NMR spectrometer (Bruker Bioscience, Billerica, MA) and 13C NMR, Bruker Avance II 100 MHz NMR spectrometer (Bruker Bioscience, Billerica, MA) in DMSO-d6 using tetramethylsilane as internal standard; MS-FAB, VG Quattro mass spectrometer (Fisons Instruments Vertriebs GmbH, Mainz, Germany), elemental analyses were performed on a Perkin-Elmer EAL 240 elemental analyser (Perkin-Elmer, Norwalk, CT).
General procedure for the synthesis of the compounds
5-Substituted-1,3,4-oxadiazolin-2-thiones (1a–b)
The acid hydrazide (5 mmol) was dissolved in a solution of potassium hydroxide (5 mmol) in ethanol (50 mL). Carbon disulfide (5 mL) was then added while stirring and the reaction mixture was heated under reflux for 10 h. The solution was cooled and acidified to pH 5–6 with hydrochloric acid solution and crystallised from ethanolCitation28.
N-(benzothiazol-2-yl)-2-chloroacetamides (2a–e)
Chloroacetyl chloride (5 mmol) was added dropwise with stirring to a mixture of 2-aminobenzothiazole (5 mmol) and triethylamine (2 mL) in toluene (50 mL) at 0–5°C. The solvent was evaporated under reduced pressure. The residue was washed with water and crystallized from ethanolCitation29.
N-(benzothiazol-2-yl)-2-[[5-substituted-1,3,4-oxadiazol-2-yl]sulfanyl]acetamides (3a–j)
A mixture of 1,3,4-oxadiazolin-2-thione (1) (2 mmol) and appropriate N-(benzothiazol-2-yl)-2-chloroacetamide (2) (2 mmol) in acetone was stirred at room temperature for 8 h in the presence of potassium carbonate and filtered. The residue was washed with water and crystallized from ethanol.
N-(benzothiazol-2-yl)-2-[[5-(4-methoxyphenoxymethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3a)
IR (KBr) νmax (cm−1): 3206 (amide N–H), 1681 (amide C=O), 1605, 1551, 1441 (C=N and C=C), 1206 (C–O).
1H NMR (400 MHz, DMSO-d6): 3.69 (3H, s), 4.41 (2H, s), 5.32 (2H, s), 6.86 (2H, d, J = 8.9 Hz), 6.95 (2H, d, J = 8.9 Hz), 7.30 (1H, t, J = 7.1 Hz), 7.43 (1H, t, J = 7.1 Hz), 7.75 (1H, d, J = 7.9 Hz), 7.99 (1H, d, J = 7.8 Hz), 12.71 (1H, br).
13C NMR (100 MHz, DMSO-d6): 35.57 (CH2), 55.59 (CH3), 60.15 (CH2), 104.99 (CH), 113.95 (2CH), 115.25 (CH), 116.18 (2CH), 121.14 (CH), 131.31 (CH), 151.22 (C), 154.31 (C), 155.79 (C), 156.45 (C), 164.19 (C), 164.20 (C), 166.04 (C), 180.06 (C).
For C19H16N4O4S2 calculated: 53.26% C, 3.76% H, 13.08% N; found: 53.30% C, 3.82% H, 13.10% N.
MS (FAB) [M+1]+: m/z 429.
N-(6-chlorobenzothiazol-2-yl)-2-[[5-(4-methoxyphenoxymethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3b)
IR (KBr) νmax (cm−1): 3210 (amide N–H), 1685 (amide C=O), 1615, 1560, 1445 (C=N and C=C), 1211 (C–O).
1H NMR (400 MHz, DMSO-d6): 3.70 (3H, s), 4.40 (2H, s), 5.35 (2H, s), 6.88 (2H, d, J = 9.0 Hz), 6.99 (2H, d, J = 9.0 Hz), 7.45 (1H, dd, J = 8.6, 2.4 Hz), 7.76 (1H, d, J = 8.6 Hz), 8.13 (1H, d, J = 2.4 Hz), 12.79 (1H, br).
13C NMR (100 MHz, DMSO-d6): 35.56 (CH2), 55.69 (CH3), 60.05 (CH2), 104.86 (CH), 114.55 (2CH), 115.15 (CH), 116.10 (2CH), 121.04 (CH), 127.62 (C), 151.13 (C), 154.20 (C), 155.70 (C), 156.21 (C), 164.15 (C), 163.91 (C), 166.02 (C), 180.02 (C).
For C19H15ClN4O4S2 calculated: 49.30% C, 3.27% H, 12.10% N; found: 49.35% C, 3.29% H, 12.14% N.
MS (FAB) [M+1]+: m/z 463.
N-(6-Methylbenzothiazol-2-yl)-2-[[5-(4-methoxyphenoxymethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3c)
IR (KBr) νmax (cm−1): 3201 (amide N–H), 1676 (amide C=O), 1601, 1566, 1435 (C=N and C=C), 1228 (C–O).
1H NMR (400 MHz, DMSO-d6): 2.41 (3H, m), 3.71 (3H, s), 4.42 (2H, s), 5.33 (2H, s), 6.87 (2H, d, J = 9.1 Hz), 6.97 (2H, d, J = 9.1 Hz), 7.25 (1H, d, J = 8.2 Hz), 7.68 (1H, d, J = 8.2 Hz), 7.75 (1H, m), 12.64 (1H, br).
13C NMR (100 MHz, DMSO-d6): 20.95 (CH3), 35.56 (CH2), 55.60 (CH3), 60.17 (CH2), 104.75 (CH), 114.60 (2CH), 115.05 (CH), 116.18 (2CH), 121.20 (CH), 132.71 (C), 151.22 (C), 154.29 (C), 155.72 (C), 156.29 (C), 164.05 (C), 164.09 (C), 165.87 (C), 179.89 (C).
For C20H18N4O4S2 calculated: 54.29% C, 4.10% H, 12.66% N; found: 54.32% C, 4.11% H, 12.69% N.
MS (FAB) [M+1]+: m/z 443.
N-(6-methoxybenzothiazol-2-yl)-2-[[5-(4-methoxyphenoxymethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3d)
IR (KBr) νmax (cm−1): 3221 (amide N–H), 1685 (amide C=O), 1621, 1552, 1415 (C=N and C=C), 1230 (C–O).
1H NMR (400 MHz, DMSO-d6): 3.69 (3H, s), 3.81 (3H, s), 4.44 (2H, s), 5.30 (2H, s), 6.84 (2H, d, J = 9.0 Hz), 6.97 (2H, d, J = 9.0 Hz), 7.05 (1H, dd, J = 8.8, 2.6 Hz), 7.59 (1H, d, J = 2.6 Hz), 7.67 (1H, d, J = 8.8 Hz), 12.66 (1H, br).
13C NMR (100 MHz, DMSO-d6): 35.57 (CH2), 55.32 (CH3), 55.62 (CH3), 60.18 (CH2), 104.75 (CH), 114.62 (2CH), 115.04 (CH), 116.15 (2CH), 121.29 (CH), 132.77 (C), 151.15 (C), 154.25 (C), 155.51 (C), 156.27 (C), 164.01 (C), 164.04 (C), 165.86 (C), 179.88 (C).
For C20H18N4O5S2 calculated: 52.39% C, 3.96% H, 12.22% N; found: 52.41% C, 3.91% H, 12.15% N.
MS (FAB) [M+1]+: m/z 459.
N-(6-ethoxybenzothiazol-2-yl)-2-[[5-(4-methoxyphenoxymethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3e):
IR (KBr) νmax (cm−1): 3218 (amide N–H), 1682 (amide C=O), 1619, 1541, 1401 (C=N and C=C), 1218 (C–O).
1H NMR (400 MHz, DMSO-d6): 1.35 (3H, t, J = 7.0 Hz), 3.68 (3H, s), 4.07 (2H, q, J = 7.0 Hz), 4.44 (2H, s), 5.30 (2H, s), 6.84 (2H, d, J = 9.1 Hz), 6.96 (2H, d, J = 9.1 Hz), 7.03 (1H, dd, J = 8.8, 2.5 Hz), 7.56 (1H, d, J = 2.5 Hz), 7.66 (1H, d, J = 8.8 Hz), 12.63 (1H, br).
13C NMR (100 MHz, DMSO-d6): 14.63 (CH3), 35.55 (CH2), 55.31 (CH3), 60.17 (CH2), 63.62 (CH2), 105.39 (CH), 114.62 (2CH), 115.41 (CH), 116.15 (2CH), 121.28 (CH), 132.75 (C), 148.94 (C), 151.13 (C), 154.25 (C), 155.49 (C), 164.01 (C), 164.04 (C), 165.86 (C), 179.02 (C).
For C21H20N4O5S2 calculated: 53.38% C, 4.27% H, 11.86% N; found: 53.43% C, 4.35% H, 11.90% N.
MS (FAB) [M+1]+: m/z 473.
N-(benzothiazol-2-yl)-2-[[5-(2-cyclohexylethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3f):
IR (KBr) νmax (cm−1): 3217 (amide N–H), 2922, 2848 (aliphatic C–H), 1683 (amide C=O), 1600, 1554, 1446 (C=N and C=C).
1H NMR (400 MHz, DMSO-d6): 0.73–0.89 (2H, m), 0.97–1.26 (4H, m), 1.46–1.74 (7H, m), 2.81 (2H, t, J = 7.5 Hz), 4.38 (2H, s), 7.32 (1H, t, J = 7.0 Hz), 7.45 (1H, t, J = 7.0 Hz), 7.77 (1H, d, J = 8.0 Hz), 7.98 (1H, d, J = 7.8 Hz), 12.71 (1H, bs).
13C NMR (100 MHz, DMSO-d6): 21.90 (CH2), 24.60 (2CH2), 27.10 (CH2), 32.05 (2CH2), 33.61 (CH2), 35.18 (CH2), 38.27 (CH), 122.06 (CH), 132.61 (CH), 152.21 (CH), 154.68 (CH), 155.96 (C), 157.12 (C), 163.98 (C), 164.11 (C), 166.54 (C), 182.11 (C).
For C19H22N4O2S2 calculated: 56.69% C, 5.51% H, 13.92% N; found: 56.73% C, 5.52% H, 13.96% N.
MS (FAB) [M+1]+: m/z 403.
N-(6-chlorobenzothiazol-2-yl)-2-[[5-(2-cyclohexylethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3g)
IR (KBr) νmax (cm−1): 3201 (amide N–H), 2922, 2850 (aliphatic C–H), 1683 (amide C=O), 1604, 1556, 1442 (C=N and C=C).
1H NMR (400 MHz, DMSO-d6): 0.73–0.89 (2H, m), 0.97–1.26 (4H, m), 1.46–1.74 (7H, m), 2.81 (2H, t, J = 7.5 Hz), 4.37 (2H, s), 7.47 (1H, dd, J = 8.7, 2.3 Hz), 7.76 (1H, d, J = 8.7 Hz), 8.13 (1H, d, J = 2.3 Hz), 12.79 (1H, bs).
13C NMR (100 MHz, DMSO-d6): 21.95 (CH2), 24.43 (2CH2), 26.92 (CH2), 32.13 (2CH2), 34.01 (CH2), 35.22 (CH2), 36.92 (CH), 121.18 (CH), 126.91 (CH), 150.02 (CH), 153.99 (C), 155.63 (C), 156.91 (C), 163.15 (C), 163.91 (C), 165.90 (C), 181.04 (C).
For C19H21ClN4O2S2 calculated: 52.22% C, 4.84% H, 12.82% N; found: 52.25% C, 4.80% H, 12.83% N.
MS (FAB) [M+1]+: m/z 437.
N-(6-methylbenzothiazol-2-yl)-2-[[5-(2-cyclohexylethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3h)
IR (KBr) νmax (cm−1): 3200 (amide N–H), 2920, 2850 (aliphatic C–H), 1683 (amide C=O), 1612, 1564, 1460 (C=N and C=C).
1H NMR (400 MHz, DMSO-d6): 0.73–0.89 (2H, m), 0.97–1.26 (4H, m), 1.46-1.74 (7H, m), 2.41 (3H, m), 2.81 (2H, t, J = 7.5 Hz), 4.36 (2H, s), 7.27 (1H, d, J = 8.3 Hz), 7.65 (1H, d, J = 8.3 Hz), 7.77 (1H, m), 12.64 (1H, bs).
13C NMR (100 MHz, DMSO-d6): 21.51 (CH3), 22.32 (CH2), 23.93 (2CH2), 26.81 (CH2), 31.99 (2CH2), 33.96 (CH2), 35.41 (CH2), 37.10 (CH), 120.96 (CH), 132.11 (CH), 152.02 (CH), 153.91 (C), 155.42 (C), 156.26 (C), 163.88 (C), 164.11 (C), 164.99 (C), 180.12 (C).
For C20H24N4O2S2 calculated: 57.67% C, 5.81% H, 13.45% N; found: 57.70% C, 5.85% H, 13.49% N.
MS (FAB) [M+1]+: m/z 417.
N-(6-methoxybenzothiazol-2-yl)-2-[[5-(2-cyclohexylethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3i)
IR (KBr) νmax (cm−1): 3200 (amide N–H), 2920, 2847 (aliphatic C–H), 1681 (amide C=O), 1610, 1564, 1465 (C=N and C=C).
1H NMR (400 MHz, DMSO-d6): 0.73–0.89 (2H, m), 0.97–1.26 (4H, m), 1.46–1.74 (7H, m), 2.81 (2H, t, J = 7.5 Hz), 3.81 (3H, s), 4.36 (2H, s), 7.27 (1H, d, J = 8.3 Hz), 7.65 (1H, d, J = 8.3 Hz), 7.77 (1H, m), 12.64 (1H, bs).
13C NMR (100 MHz, DMSO-d6): 22.06 (CH2), 23.72 (2CH2), 26.65 (CH2), 32.41 (2CH2), 34.11 (CH2), 35.82 (CH2), 37.20 (CH), 55.22 (CH3), 121.11 (CH), 131.99 (CH), 152.12 (CH), 153.88 (C), 156.10 (C), 156.38 (C), 163.48 (C), 164.05 (C), 165.16 (C), 181.01 (C).
For C20H24N4O3S2 calculated: 55.53% C, 5.59% H, 12.95% N; found: 55.57% C, 5.61% H, 12.96% N.
MS (FAB) [M+1]+: m/z 433.
N-(6-ethoxybenzothiazol-2-yl)-2-[[5-(2-cyclohexylethyl)-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide (3j)
IR (KBr) νmax (cm−1): 3221 (amide N–H), 2922, 2850 (aliphatic C–H), 1676 (amide C=O), 1602, 1581, 1458 (C=N and C=C).
1H NMR (400 MHz, DMSO-d6): 0.73–0.89 (2H, m), 0.97–1.26 (4H, m), 1.38 (3H, t, J = 7.1 Hz), 1.46–1.74 (7H, m), 2.81 (2H, t, J = 7.5 Hz), 4.02 (2H, q, J = 7.1 Hz), 4.36 (2H, s), 7.27 (1H, d, J = 8.3 Hz), 7.65 (1H, d, J = 8.3 Hz), 7.77 (1H, m), 12.64 (1H, bs).
13C NMR (100 MHz, DMSO-d6): 14.55 (CH3), 21.88 (CH2), 23.46 (2CH2), 26.12 (CH2), 32.15 (2CH2), 33.98 (CH2), 35.61 (CH2), 36.95 (CH), 62.51 (CH2), 121.21 (CH), 132.09 (CH), 152.28 (CH), 153.68 (C), 155.81 (C), 156.63 (C), 162.11 (C), 164.78 (C), 165.06 (C), 182.15 (C).
For C21H26N4O3S2 calculated: 56.48% C, 5.87% H, 12.55% N; found: 56.51% C, 5.90% H, 12.56% N.
MS (FAB) [M+1]+: m/z 447.
Microbiology
Antimicrobial activity
The in vitro antimicrobial activities of the compounds (3a–j) were tested using the microbroth dilution methodCitation30. The tested micro-organism strains were Micrococcus luteus (NRLL B-4375), Bacillus subtilis (NRS-744), Pseudomonas aeruginosa (ATCC-254992), Staphylococcus aureus (NRRL B-767), Escherichia coli (ATCC-25922), Listeria monocytogenes (ATCC-7644) and Candida albicans (ATCC-22019). Microbroth dilution-susceptibility assay was used for antimicrobial evaluation of the compounds. Stock solutions of the samples were prepared in dimethyl sulfoxide. Dilution series using sterile distilled water were prepared from 1–4 mg/mL to 0.001–0.007 mg/mL in micro-test tubes that were transferred to 96-well microtitre plates. Overnight-grown bacterial and C. albicans suspensions in double-strength Mueller–Hinton broth were standardized to 108 colony-forming unit/mL using McFarland No: 0.5 standard solutions. Hundred microlitre of each micro-organism suspension was then added into the wells. The last well-chain without a micro-organism was used as a negative control. Sterile distilled water and the medium served as a positive growth control. After incubation at 37°C for 18–24 h, the first well without turbidity was determined as the minimum inhibitory concentration. Streptomycin was used as an antibacterial agent, whereas ketoconazole was used as an antifungal agent.
Toxicity
The level of cellular MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma) reduction was quantified as previously described in the literature with small modificationsCitation31,Citation32.
Cell culture and drug treatment
NIH/3T3 cells were obtained from the American Type Culture Collection (ATCC). The cells were incubated in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal calf serum (Life Technologies, UK), 100 IU/mL penicillin (Gibco, Paisley, Scotland) and 100 mg/mL streptomycin (Gibco) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Exponentially growing cells were plated at 2 × 104 cells/mL into 96-well microtitre tissue culture plates (Nunc, Denmark) and incubated for 24 h before the addition of the drugs (the optimum cell number for cytotoxicity assays was determined in preliminary experiments). Stock solutions of compounds were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Poole, UK) and further dilutions were made with fresh culture medium (the concentration of DMSO in the final culture medium was <0.1% which had no effect on the cell viability).
MTT assay for cytotoxicity of the compounds
It is widely used as a measure of cytotoxicity. After 24 h of preincubation, the tested compounds were added to give final concentration in the range 0.5–500 µg/mL and the cells were incubated for 24 h. At the end of this period, MTT was added to a final concentration of 0.5 mg/mL and the cells were incubated for 4 h at 37 °C. After the medium was removed, the formazan crystals formed by MTT metabolism were solubilised by addition of 200 µl DMSO to each well and absorbance was read at 540 nm with a microtitre plate spectrophotometer (Bio-Tek plate reader). Every concentration was repeated in three wells and IC50 values were defined as the drug concentrations that reduced absorbance to 50% of control values.
Results and discussion
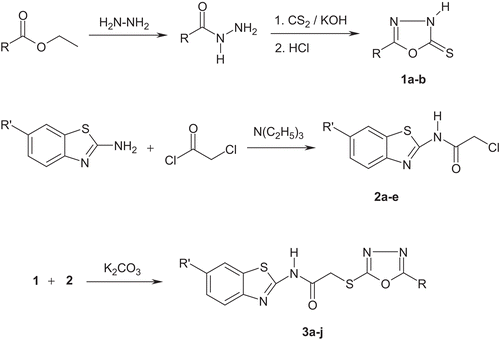
Initially, the compounds which have oxadiazoline-2-thione structure (1a–b) were obtained by the ring closure reactions of acid hydrazides with carbon disulphide. 2-Chloro-N-(benzothiazol-2-yl)acetamides (2a–e) were synthesized via the nucleophilic acyl substitution reactions of 2-aminobenzothiazoles with chloroacetyl chloride in the presence of triethylamine. The desired compounds (3a–j) were prepared by reacting 2-chloro-N-(benzothiazol-2-yl)acetamides (2a–e) with oxadiazoline-2-thiones (1a–b) under basic conditions. The presence of a base favours the thiol form, which is prone to nucleophilic substitution reactions. These reactions are summarized in and some properties of the compounds are given in .
Table 1. Some properties of the synthesized compounds (3a–j).
The structures of the compounds (3a–j) were confirmed by IR, 1H NMR, 13C NMR and FAB+-MS spectral data and elemental analyses.
In the IR spectra of all compounds (3a–j), all derivatives have a strong, characteristic band in the region 1685–1676 cm−1 due to the amide C=O stretching vibration. The amide N–H stretching vibration of the compounds (3a–j) gives rise to a band at 3221–3200 cm−1. The bands due to the C=C and C=N stretching vibrations are observed in the region 1621–1401 cm−1. In the IR spectra of the compounds (3a–e), the stretching bands for C–O group occur at 1230–1206 cm−1.
In the 1H NMR spectra of the compounds (3a–j), the signal due to the amide proton appears at 12–13 ppm. The signal due to the−S–CH2– protons gives rise to a singlet peak at 4.0–5.5 ppm. In the 1H NMR spectra of the compounds 3a–e, the O–CH2 protons are observed at 4.42–4.44 ppm as a singlet peak. The benzothiazole protons of all derivatives are observed in the region 6.8–8.2 ppm. All other aromatic and aliphatic protons were observed at expected regions.
In their 13C NMR spectra, the signal due to the−S–CH2– carbon appears at 35–36 ppm. The other aromatic and aliphatic carbons were observed at expected regions.
In the mass spectra of all compounds (3a–j), the M+1 peak is observed. All compounds gave satisfactory elemental analysis.
All compounds were tested in vitro against S. aureus, L. monocytogenes, E. coli, P. aeruginosa, M. luteus, B. subtilis and C. albicans and compared with streptomycin and ketoconazole, respectively ().
Table 2. Antimicrobial activities of the compounds (3a–j) (μg/mL).
All compounds (3a–j) showed less antimicrobial potency than streptomycin and ketoconazole. The results indicate that the compounds (3a–j) exhibit the highest antimicrobial effect on P. aeruginosa and C. albicans.
Among these compounds (3a–j), the compounds bearing 2-cyclohexylethyl moiety on oxadiazole ring (3f–j) exhibit the highest inhibitory activity against S. aureus. It is clear that there is a positive correlation between antibacterial activity against S. aureus and aliphatic group on oxadiazole ring.
The compounds that possess 4-methoxyphenoxymethyl moiety on oxadiazole ring (3a–e) are the most effective derivatives against L. monocytogenes and E. coli. It is apparent that 4-methoxyphenoxymethyl moiety on oxadiazole ring has an important impact on antibacterial activity against L. monocytogenes and E. coli.
All compounds except compound 3h are equally active against P. aeruginosa. Compounds 3b and 3c exhibit the lowest antibacterial activity against M. luteus.
The compounds possessing 2-cyclohexylethyl moiety on oxadiazole ring (3f–j) except 3j are more effective than the compounds possessing 4-methoxyphenoxymethyl moiety on oxadiazole ring (3a–e) against B. subtilis.
The compounds that possess 4-methoxyphenoxymethyl moiety on oxadiazole ring (3a–e) exhibit the highest inhibitory activity against C. albicans. This outcome confirms that the 4-methoxyphenoxymethyl group on oxadiazole ring may have a considerable influence on antifungal activity. Although compound 3j does not possess 4-methoxyphenoxymethyl moiety on oxadiazole ring, this derivative also exhibits the same level of antifungal activity.
All compounds were also evaluated for their cytotoxic properties using MTT assay. The biological study indicated that compound 3a possessed the highest cytotoxicity, whereas compound 3g exhibited the lowest cytotoxicity against NIH/3T3 cells among the title compounds ().
Table 3. In vitro cytotoxicity of the compounds (3a–j)
Conclusions
In conclusion, we described the synthesis of new oxadiazole derivatives and evaluated their in vitro antimicrobial activities against various pathogenic bacteria and C. albicans.
5-Substituted-1,3,4-oxadiazolin-2-thiones (1a–b) were synthesized via the ring closure reactions of appropriate acid hydrazides with carbon disulphide. N-(Benzothiazol-2-yl)-2-[[5-Substituted-1,3,4-oxadiazol-2-yl]sulfanyl]acetamide derivatives (3a–j) were prepared by the nucleophilic substitution reactions of 5-substituted-1,3,4-oxadiazolin-2-thiones (1a–b) with N-(benzothiazol-2-yl)-2-chloroacetamides in the presence of potassium carbonate.
The biological results indicate that P. aeruginosa and C. albicans are more susceptible to the synthesized compounds. All compounds except compound 3h are equally active against P. aeruginosa. The compounds which possess 4-methoxyphenoxymethyl moiety on oxadiazole ring (3a–e) exhibit the highest inhibitory activity against C. albicans. This outcome confirms that 4-methoxyphenoxymethyl group on oxadiazole ring may have a considerable influence on anti-candidal activity. Compound 3j, which does not possess 4-methoxyphenoxymethyl moiety on oxadiazole ring, also exhibits the same level of antifungal activity.
The cytotoxic effects of the compounds were also investigated and compound 3a possessed the highest cytotoxicity, whereas compound 3g exhibited the lowest cytotoxicity against NIH/3T3 cells.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 1993;6:428–442.
- Gootz TD. Discovery and development of new antimicrobial agents. Clin Microbiol Rev 1990;3:13–31.
- Fluit AC, Visser MR, Schmitz FJ. Molecular detection of antimicrobial resistance. Clin Microbiol Rev 2001;14:836–871, table of contents.
- Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev 1996;9:499–511.
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev 2007;20:133–163.
- Sims CR, Ostrosky-Zeichner L, Rex JH. Invasive candidiasis in immunocompromised hospitalized patients. Arch Med Res 2005;36:660–671.
- Ghannoum MA Rice LB. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12:501–517.
- Sahin G, Palaska E, Ekizoglu M, Ozalp M. Synthesis and antimicrobial activity of some 1,3,4-oxadiazole derivatives. Farmaco 2002;57:539–542.
- Mir I, Siddiqui MT, Comrie AM. Antituberculosis agents. V: α-[5-(5-nitro-2-furyl)-1,3,4-oxadiazol-2-ylthio]acethydrazide and related compounds. J Pharm Sci 1991;80:548–550.
- Mayekar AN, Yathirajan HS, Narayana B, Sarojini BK, Suchetha Kumari N. Synthesis and antimicrobial studies on new substituted 1,3,4-oxadiazole derivatives bearing 6-bromonaphthalene moiety. Int J Chem 2010;2:38–54.
- Farshori NN, Banday MR, Ahmad A, Khan AU, Rauf A. Synthesis, characterization, and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg Med Chem Lett 2010;20:1933–1938.
- Chikhalia KH, Vashi DB, Patel MJ. Synthesis of a novel class of some 1,3,4-oxadiazole derivatives as antimicrobial agents. J Enzyme Inhib Med Chem 2009;24:617–622.
- Karabasanagouda T, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of some novel 1,3,4-oxadiazoles carrying alkylthio and alkylsulphonylphenoxy moieties. Phosphorus Sulfur Silicon 2007;182:2925–2941.
- Bakht MA, Yar MS, Abdel-Hamid SG, Al Qasoumi SI, Samad A. Molecular properties prediction, synthesis and antimicrobial activity of some newer oxadiazole derivatives. Eur J Med Chem 2010;45:5862–5869.
- Bala S, Kamboj S, Kumar A. Heterocyclic 1,3,4-oxadiazole compounds with diverse biological activities: A comprehensive review. J Pharm Res 2010;3:2993–2997.
- Padmavathi V, Sudhakar Reddy G, Padmaja A, Kondaiah P, Ali-Shazia. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur J Med Chem 2009;44:2106–2112.
- Dolman SJ, Gosselin F, O’Shea PD, Davies IW. Superior reactivity of thiosemicarbazides in the synthesis of 2-amino-1,3,4-oxadiazoles. J Org Chem 2006;71:9548–9551.
- Horning DE, Muchowski JM. Five-membered heterocyclic thiones. part i. 1,3,4-oxadiazole-2-thione. Can J Chemistry 1972;50:3079–3082.
- Mekuskiene G, Burbuliene MM, Jakubkiene V, Udrenaite E, Gaidelis P, Vainilavicius P. 5-(4,6-diphenyl-2-pyrimidinyl)-1,3,4-oxadiazole-2-thione with some C-electrophiles and N nucleophiles. Chem Heterocyc Compd 2003;39:1364–1368.
- Gupta AK, Misra HK. Synthesis and evaluation of substituted quinazolone derivatives for antibacterial, antifungal, and antiacetylcholinesterase activities. J Pharm Sci 1980;69:1313–1317.
- Misra HK. Synthesis of some new substituted 1,3,4-oxadiazoles as potential insecticidal, antibacterial and anti-acetylcholine esterase agents. Arch Pharm (Weinheim) 1983;316:487–493.
- Lemke TL, Williams DA. (2008) Foye’s Principles of Medicinal Chemistry. Baltimore and Philadelphia: Lippincott Williams & Wilkins USA, 1028–1083.
- Bhat AR, Bhat GV, Shenoy GG. Synthesis and in-vitro antimicrobial activity of new 1,2,4-triazoles. J Pharm Pharmacol 2001;53:267–272.
- Collin X, Sauleau A, Coulon J. 1,2,4-Triazolo mercapto and aminonitriles as potent antifungal agents. Bioorg Med Chem Lett 2003;13:2601–2605.
- Ryu CK, Choi KU, Shim JY, You HJ, Choi IH, Chae MJ. Synthesis and antifungal activity of 6-arylthio-/6-arylamino-4,7-dioxobenzothiazoles. Bioorg Med Chem 2003;11:4003–4008.
- Singh T, Srivastava VK, Saxena KK, Goel SL, Kumar A. Synthesis of new thiazolylthiazolidinylbenzothiazoles and thiazolylazetidinylbenzothiazoles as potential insecticidal, antifungal, and antibacterial agents. Arch Pharm (Weinheim) 2006;339:466–472.
- Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M. 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: synthesis and structure-activity relationship. Bioorg Med Chem 2008;16:3714–3724.
- Andrushko AP, Demchenko AM, Krasovskii AN, Rusanov EB, Chernega AN, Lozinskii MO. Reactions of α-halo ketones with 5-benzyl and 5-phenoxymethyl-2H,3H-1,3,4-oxadiazole-2-thiones. Russ J Gen Chem 2001;71:1754–1758.
- Bhargava PN, Sharma SC. Some possible antihistaminics and antispasmodics. II. Synthesis of mannich bases. Bull Chem Soc Jpn 1965;38:912–915.
- Winn WC, Allen SD, Janda WM, Koneman EW, Procop GW, Schreckenberger PC, Woods GL. (2006). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Baltimore and Philadelphia: Lippincott Williams & Wilkins USA:945.
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod 2000;26:288–291.