Abstract
New compounds incorporating with the oxindole nucleus were synthesized via the reaction of substituted isatins [5-methyl-, 5-chloro- and 1-hydroxymethyl isatins] with different nucleophiles. The structures of the newly compounds were elucidated on the basis of FTIR, 1H NMR, 13CMR spectral data, GC/MS and chemical analysis. Investigation of antimicrobial activity of the new compounds was evaluated using broth dilution technique in terms of minimal inhibitory concentration (MIC) count against four pathogenic bacteria and two pathogenic fungi. Most of the new compounds are significantly active against bacteria and fungi. MIC showed that compound (4a) possesses higher effect on Gram-positive bacteria Bacillus cereus than the selected antibacterial agent sulphamethoxazole, whereas compound (11c) possesses more activity against Gram-negative bacteria Shigella dysenterie.
Introduction
Oxindoles have a broad range of pharmacological actions, being described as anxiogenic and sedative agentsCitation1,Citation2 or antagonists of guanylate cyclase-coupled atrial natriuretic peptide receptorCitation3. Schiff bases and Mannich bases of isatin were reported to possess antibacterialCitation4–6, antifungalCitation7–9, anti-TBCitation10–12, anti-HIVCitation13–15, analgesicCitation16 antiviralCitation17 and anticancerCitation18 activities.
Spiro compounds represent an important class of naturally occurring substances characterized by interesting biological propertiesCitation19,Citation20. Furthermore, it has been reported that sharing of the indole 3-carbon atom in the formation of spiroindoline derivatives highly enhances biological activity for some Gram-positive (Bacillus subtilis and Bacillus megatherium), Gram-negative (Escherichia coli) and fungi (Aspergillus niger and Aspergillus oryzae)Citation21–23. The spirooxindole system is the core structure of many pharmacological agents and natural alkaloids such as spiroindole-thiazolidinones showed good activity against the pathogens, Rhizoctonia solani, Fusarium oxysporum and Collectotrichum capsici. Also, spirotryprostatins have been found to have anti-mitotic properties, and as such they have become of great interest as anticancer drugs and also were shown to be active as cell cycle inhibitor and pteropodine and isopteropodine, which are heteroyohimbine-type oxindole alkaloid, act as positive modulators of muscarinic M(1) and 5-HT(2) receptorsCitation24–28.
In continuation of our studies on the synthesis and evaluation of biological activity of heterocycles, we report herein the efficient synthesis of new biologically active compounds based on oxindole nucleus and evaluate its antimicrobial activity using broth dilution technique in terms of minimal inhibitory concentration (MIC) count.
Materials and methods
Chemistry
All melting points are uncorrected and were determined on Gallenkamp instrument. Infrared spectra of the new compounds were measured on Perkin-Elmer spectrophotometer model 1430 using potassium bromide pellets and frequencies are reported in cm−1. The 1H NMR were measured on Varian genini-300 MHZ spectrophotometer and chemical shifts (δ) are in ppm. The mass spectra (m/z) values were measured on mass spectrophotometer HP model GC MS-QPL000EX (Shimadzu) at 70 eV. Elemental analyses were carried out at the Microanalytical Centre, Cairo University. Antimicrobial activity evaluation were carried out at the Basic Science Department, Faculty of Applied Medical Science, October 6th University, October City, Egypt. Explorer Automated Microwave Synthesis Workstation (CEM) was used for synthesis of compounds.
Synthesis of 2-(5-substituted 2-oxindolin-3-ylideneamino) isoindolin-1,3-diones (2a,b)
General procedure
Method (A): A mixture of 5-substituted isatin 1a,b (0.01 mol) and N-aminophthalimide (0.01 mol) in absolute ethanol (30 mL) was refluxed for 30 min. The solid product formed during heating was filtered and crystallized from ethanol to give 2a,b.
Method (B): A mixture of 5-substituted isatin 1a,b (0.01 mol) and N-aminophthalimide (0.01 mol) in a little amount of ethanol made as a slurry was irradiated with MW (temperature 130°C, pressure 250 psi, time 1 min). The solid product obtained after cooling was filtered and crystallized from ethanol to give 2a,b.
2-(5-Methyl-2-oxindolin-3-ylideneamino) isoindoline-1,3-dione (2a)
Orange crystals, 86% yield, mp 269°C; IR (KBr pellet): 3275 for (NH), 1740–1720 for (C=O1,3dione, C=Ooxindole) and 1603 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 2.49 (s, 3H, CH3), 6.80–7.90 (m, 7H, 2Ar-H) and 10.9 (s, 1H, NH) ppm; Anal. Calcd. for C17H11N3O3 (305.28); C, 66.88; H, 3.63; N, 13.76; found: C, 66.65; H, 3.77; N, 13.77; m/z (305) (M+).
2-(5-Chloro-2-oxindolin-3-ylideneamino) isoindoline-1,3-dione (2b)
Orange crystals, 76.7% yield, mp 279–280°C; IR (KBr pellet): 3264 for (NH), 1730–1710 for (C=O1,3dione, C=Ooxindole) and 1602 for (C=N) cm−1. Anal. Calcd. for C16H8ClN3O3 (325.70); C, 59.00; H, 2.48; Cl, 10.88; N, 12.90; found: C, 58.85; H, 2.87; Cl, 10.85; N, 12.70; m/z 328 (M++3).
Preparation of 3-(1H-benzoimidazol-2-ylimino)-5-methyl-indolin-2-one (3)
General procedure
A mixture of 5-methylisatin 1a (0.01 mol) and 1H-benzoimidazol-2-amine (0.01 mol) was refluxed in absolute ethanol (20 mL) containing a few drops of glacial acetic acid for 8 h. After cooling to room temperature, red needles obtained were filtered and crystallized from ethanol to give compound 3.
3-(1H-Benzoimidazol-2-ylimino)-5-methyl indolin-2-one) (3)
Red crystals, 74% yield, mp 205°C, IR (KBr pellet): 3408–3235 for (NHimidazole, NHoxindole), 1710 for (C=O) and 1622, 1651 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 2.49 (s, 3H, CH3), 6.71–7.40 (m, 7H, 2Ar-H), 8.78 (s, 1H, NHimidazole) and 10.90 (s, 1H, NHoxindole) ppm; Anal. Calcd. for C16H12N4O (276.29), C, 69.55; H, 4.38; N, 20.28; found: C, 69.25; H, 4.39; N, 20.00; m/z
Preparation of N-(5-methylisoxazol-3-yl)-4-(5-substituted 2-oxindolin-3-ylideneamino) benzenesulphonamides (4a,b) and 4-[(1-hydroxymethyl)-2-oxindolin-3-ylideneamino]-N-(5-methylisoxazol-3-yl) benzenesulphonamide (4c)
General procedure
A mixture of 5-substituted isatins or 1-hydroxymethylisatin (0.01 mol) 1a–c and 4-amino-N-(5-methyl isoxazol-3-yl) benzene sulphonamide (0.01 mol) in ethanol (20 mL) was refluxed for 8 h. After cooling to room temperature, the solid product obtained was filtered and crystallized from ethanol to give 4a–c.
N-(5-Methylisoxazol-3-yl)-4-(5-methyl-2-oxindolin-3-ylideneamino) benzenesulphonamide (4a)
Red crystals, 78% yield, mp 150°C; IR (KBr pellet): 3469–3180 for (NHsulphonamide, NHoxindole), 1718 for (C=O), 1623 for (C=N) and 1362 for SO2 cm−1. 1H NMR (DMSO, 300 MHz): δ 2.47–2.48 (s, 6H, 2CH3), 6.05 (s, 1H, CH), 6.54–7.40 (m, 7H, 2Ar-H), 10.85 (s, 1H, NHoxindole) and 10.88 (s, 1H, NHsulphonamide) ppm; Anal. Calcd. for C19H16N4O4S (396.41); N, 14.13; S, 8.09; found: N, 13.90; S, 8.13; m/z 397 (M++1).
N-(5-Methylisoxazol-3-yl)-4-(5-chloro-2-oxindolin-3-ylideneamino) benzene sulphonamide (4b)
Orange crystals, 85% yield, mp 230–232°C; IR (KBr pellet): 3463–3168 for (NHsulphonamide, NHoxindole), 1708 for (C=O), 1617 for (C=N) and 1353 for (SO2) cm−1. Anal. Calcd. for C18H13ClN4O4S (416.83); C, 51.86; H, 3.14; Cl, 8.51; N, 13.44; S, 7.69; found: C, 52.00; H, 3.00; Cl, 8.81; N, 13.45; S, 7.47; m/z 417 (M+).
4-[(1-Hydroxymethyl)-2-oxindolin-3-ylideneamino]-N-(5-methylisoxazol-3-yl) benzene sulphonamide (4c)
Orange crystals, 90% yield, mp 215°C; IR (KBr pellet): 3417–3318 for (NHsulphamethoxazole, OH), 1732 for (C=O), 1610 for (C=N) and 1362 for (SO2) cm−1. 1H NMR (DMSO, 300 MHz): δ 2.50 (s, 3H, CH3), 5.13 (s, 2H, CH2 of CH2OH), 6.08 (s, 1H, CH), 6.09 (s, 1H, OH), 6.72–7.70 (m, 8H, 2Ar-H) and 11.01 (s, 1H, NHsulphonamide) ppm; Anal. Calcd. for C19H16N4O5S (412.41); C, 55.33; H, 3.91; N, 13.58; S, 7.77; found: C, 55.61; H, 4.22; N, 13.45; S, 7.75; m/z 411 (M+−1).
Preparation of 2-[(5-substituted 2-oxindolin-3-ylidene) hydrazono] carbonyl-N-propylbenzamides (6a,b)
General procedure
A mixture of 1a,b (0.01 mol) and n-propylamine (0.01 mol) in absolute ethanol (30 mL) was stirred at room temperature for 24 h. The solid product obtained was filtered off and crystallized from benzene to give 6a,b.
2-[5-Methyl-2-oxindolin-3-ylidene) hydrazono] carbonyl-N-propylbenzamide (6a)
Yellow crystals, 74% yield, mp 237–238°C; IR (KBr pellet): 3170–3136 for (NHoxindole, NHamide), 1696–1692 for (C=O) and 1605 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz); δ 2.27 (s, 3H, CH3), 2.49 (t, 3H, CH3), 3.40 (t, 2H, CH2), 3.51 (m, 2H, CH2), 6.70–7.90 (m, 7H, 2Ar-H), 8.30, 8.31 (s, 2H, 2NHamide) and 10.30 (s, 1H, NHoxindole) ppm. Anal. Calcd. for C20H20N4O3 (364.39); C, 65.92; H, 5.53;N, 15.38; found; C, 66.15; H, 5.75; N, 15.49; m/z 362 (M+−2).
2-[5-Chloro-2-oxindolin-3-yilidene) hydrazono] carbonyl-N-propylbenzamide (6b)
Yellow crystals, 76.7% yield, mp 227°C; IR (KBr pellet): 3170–3140 for (NHoxindole, NHamide), 1690–1685 for (C=O) and 1605 for (C=N) cm−1. Anal. Calcd. for C19H17ClN4O3 (384.81); C, 59.30; H, 4.45; Cl, 9.21; N, 14.56; found: C, 59.61; H, 4.51; Cl, 9.30; N, 14.32; m/z 385 (M++1).
Preparation of N-(5-substituted 2-oxindolin-3-ylidene)-2-(piperidin-1-ylcarbonyl) benzohydrazides (7a,b)
General procedure
A mixture of 1a,b (0.01 mol) and piperidine (0.01 mol) in absolute ethanol (30 mL) was stirred at room temperature for 24 h. The solid product obtained was filtered off and crystallized from benzene to give 7a,b.
N-(5-Methyl-2-oxindolin-3-ylidene)-2-(piperidin-1-ylcarbonyl) benzohydrazide (7a)
Yellow crystals, 65% yield, mp 240°C; IR (KBr pellet): 3190–3166 for (NHoxindole, NHamide), 1701 for (C=O) and 1617 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 2.49 (s, 3H, CH3), 3.30 (m, 6H, 3CH2), 3.51 (t, 4H, 2CH2), 6.90–7.90 (m, 7H, 2Ar-H), 8.29 (s, 1H, NHamide) and 10.30 (s, 1H, NHoxindole) ppm. Anal. Calcd. for C22H22N4O3 (390.43); C, 67.68; H, 5.68; N, 14.35; found: C, 67.36; H, 5.39; N, 14.09; m/z 390 (M+).
N-(5-Chloro-2-oxindolin-3-ylidene)-2-(piperidin-1-ylcarbonyl) benzohydrazide (7b)
Yellow crystals, 64% yield, mp 236–238°C; IR (KBr pellet): 3182–3150 for (NHoxindole, NHamide), 1691 for (C=O) and 1606 for (C=N) cm−1. Anal. Calcd. for C21H19ClN4O3 (410.85); C, 61.39; H, 4.66; Cl, 8.63; N, 13.64; found: C, 61.59; H, 4.56; Cl, 8.77; N, 13.21; m/z 412 (M++2).
Preparation of 3-[2-(1-phenyl-2-oxoethyl) hydrazono]-5-substituted indolin-2-ones (9b,c)
General procedure
A mixture of 8b,c (0.01 mol) and phenacyl chloride (0.01 mol) in absolute ethanol (20 mL) containing a few drops of triethylamine was refluxed for 6 h. The reaction mixture was concentrated under reduced pressure. The oily residue obtained was triturated with petroleum ether 40–60°C and then crystallized from petroleum ether 60–80°C to give 9b,c.
3-2(1-Phenyl-2-oxoethyl) hydrazono]-5-chloroindolin-2-one (9b)
Brown crystals, 50% yield, mp 236–238°C; IR (KBr pellet): 3375–3180 for (NHhydrazono, NHoxindole), 1699–1659 for (C=Oacetophenone, C=Ooxindole) and 1616 for (C=N) cm−1. Anal. Calcd. for C16H12ClN3O2 (313.74); Cl, 11.30; N, 13.39; found: Cl, 11.79; N, 13.39.
3-[2-(1-Phenyl-2-oxoethyl) hydrazono]-5H-indolin-2-one (9c)
Brown crystals, 54% yield, mp 98°; IR (KBr pellet): 3391–3221 for (NHhydrazono, NHoxindole), 1723–1687 for (C=Oacetophenone, C=Ooxindole) and 1619 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 4.79 (s, 2H, CH2), 6.80–7.99 (m, 9H, 2Ar-H), 9.70 (s, 1H, NHhydrazono) and 10.86 (s, 1H, NHoxindole) ppm. Anal. Calcd. for C16H13N3O2 (279.29); C, 68.81; H, 4.69; N, 15.05; found: C, 68.93; H, 4.69; N, 15.16 m/z 278(M+−1).
Preparation of 3-[2-(2-chloro-1-phenylethylidene) hydrazono] indolin-2-one (10)
General procedure
A mixture of 8c (0.01 mol) and phenacyl chloride (0.01 mol) in HCl (2 M, 20 mL) was refluxed for 3 h. The solid product separated while hot was filtered and crystallized from benzene to give 10.
3-[2-(2-Chloro-1-phenylethylidene) hydrazono] indolin-2-one (10)
Violet crystals, 53% yield, mp 300°C; IR (KBr pellet): 3227 for (NH), 1699 for (C=O) and 1618, 1614 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 5.10 (s, 2H, CH2), 6.82–7.60 (m, 9H, 2Ar-H) and 10.66 (s, 1H, NH) ppm. Anal. Calcd. for C16H12ClN3O (297.74); C, 64.54; H, 4.06; Cl, 11.91; N, 14.11; found: C, 64.72; H, 4.30; N, Cl, 12.11, 14.61, m/z 297 (M+).
Preparation of 3-[2-(4-oxopentan-2-ylidene) hydrazono]-5-substituted indolin-2-ones (11a–c)
General procedure
A mixture of 8a–c (0.01 mol) and acetylacetone (0.01 mol) was refluxed in absolute ethanol (20 mL) for 5 h. After cooling to room temperature, the solid product obtained was filtered and crystallized from ethanol to give 11a–c.
3-[2-(4-Oxopentan-2-ylidene) hydrazono]-5-methyl-indolin-2-one (11a)
Orange crystals, 79% yield, mp 260–262°C; IR (KBr pellet): 3180 for (NH), 1699 for (C=O) and 1590 for (C=N) cm−1; Anal. Calcd. for C14H15N3O2 (257.28) C, 65.35; H, 5.88; N, 16.33; found: C, 65.14; H, 6.12; N, 16.28; m/z 257(M+).
3-[2-(4-Oxopentan-2-ylidene) hydrazono]-5-chloroindolin-2-one (11b)
Orange crystals, 73% yield, mp 280–282°C; IR (KBr pellet): 3139 for (NH), 1701 for (C=O) and 1583 for (C=N) cm−1. Anal. Calcd. for C13H12ClN3O2 (277.70): C, 56.22; H, 4.36; Cl, 12.77; N, 15.13; found: C, 56.02; H, 4.70; Cl, 12.74; N, 15.02; m/z 277(M+).
3-[2-(4-Oxopentan-2-ylidene) hydrazono]-5H-indolin-2-one (11c)
Orange crystals, 76% yield, mp 229–230°C; IR (KBr pellet): 3206 for (NH), 1689 for (C=O) and 1625, 1590 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 2.07 (s, 3H, CH3), 2.20 (s, 3H, CH3), 5.51 (s, 2H, CH2), 6.87–7.46 (m, 4H, Ar-H) and 10.98 (s, 1H, NH) ppm. Anal. Calcd. for C13H13N3O2 (243.26); C, 64.19; H, 5.39; N, 17.27; found: C, 63.94; H, 5.64; N, 17.16, m/z 243(M+).
Preparation of 3-[2-(4-hydroxy-3-methoxybenzylidene) hydrazono]-5-substituted indolin-2-ones (12a–c)
General procedure
A mixture of 8a–c (0.01 mol) and vanillin (0.01 mol) was refluxed in absolute ethanol (20 mL) for 5 h. The solid product after cooling was filtered and crystallized from ethanol to give 12a–c.
3-[2-(4-Hydroxy-3-methoxybenzylidene) hydrazono]-5-methyl-indolin-2-one (12a)
Orange crystals, 85% yield, mp 270°C; IR (KBr pellet): 3400–3204 for (OH, NH), 1719 for (C=O) and 1615 for (C=N). Anal. Calcd. for C17H15N3O3 (309.31); C, 66.01; H, 4.89; N, 13.58; found: C, 65.90; H, 5.22; N, 13.47, m/z 311(M+2).
3-[2-(4-Hydroxy-3-methoxybenzylidene) hydrazono]-5-chloroindolin-2-one (12b)
Orange crystals, 64% yield, mp 290°C; IR (KBr pellet): 3380–3185 for (OH, NH), 1713 for (C=O) and 1613 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 3.87 (s, 3H, OCH3), 6.82–8.10 (m, 6H, 2Ar-H), 8.62 (s, 1H, CH of CH=N), 10.10 (s, 1H, OH) and 10.91 (s, 1H, NH) ppm. Anal. Calcd. for C16H12ClN3O3 (329.73); C, 58.28; H, 3.67; Cl, 10.75; N, 12.74; found: C, 58.34; H, 3.82; Cl, 10.58; N, 12.56; m/z 329(M+2).
3-[2-(4-Hydroxy-3-methoxybenzylidene) hydrazono]-5H-indolin-2-one (12c)
Orange crystals, 79% yield, mp 278°C; IR (KBr pellet): 3436–3251 for (OH, NH), 1728 for (C=O) and 1619 for (C=N) cm−1. 1H NMR (DMSO, 300 MHz): δ 3.92 (s, 3H, OCH3), 6.78–7.90 (m, 7H, 2Ar-H), 8.58 (s, 1H, CH of CH=N), 10.13 (s, 1H, OH) and 10.68 (s, 1H, NH) ppm. Anal. Calcd. for C16H13N3O3 (295.29); C, 65.08; H, 4.44; N, 14.23; found: C, 64.81; H, 4.47; N, 14.29; m/z 297 (M+).
Preparation of spirocyclic compound (13)
General procedure
Method (A): A mixture of compound 12a (0.01 mol) and thiosemicarbazide (0.01 mol) in absolute ethanol was refluxed for 6 h, the solid product after cooling was filtered and crystallized from ethanol to give 13.
Method (B): A mixture of compound 12a (0.01 mol) and thiosemicarbazide (0.01 mol) in a little amount of ethanol made as a slurry was irradiated with MW (temperature 130°C, pressure 250, time for 1 min).The solid product obtained after cooling was filtered and crystallized from ethanol to give 13.
Spiro compound (13)
Yellow crystals, 78% yield, mp 216°C; IR (KBr pellet): 3395–3225 for (OH, NH), 3216–3172 for (NH of NH2), 1690 for (C=O) and 1592 for (C=N) cm−Citation1. 1H NMR (DMSO, 300 MHz) δ 2.25 (s, 3H, CH3), 3.38 (s, 3H, OCH3), 6.70–7.17 (m, 6H, 2Ar-H), 7.70 (s, 1H, CH of CH=N), 8.68 (s, 1H, NHtriazole), 9.42, 9.46 (s, 2H, NH2), 10.15 (s, 1H, OH) and 10.54 (s, 1H, NHoxindole) ppm. 13CMR (DMSO) δ 162.9, 136.4 130.1, 129.8, 128.8, 127.5, 126.4, 123.4, 122.2, 120.7, 117.9, 109.6, 109.2, 40.3, 40.0, 39.4, 20.7. Anal. Calcd. for C18H18N6O3 (366.37); C, 59.01; H, 4.95; N, 22.94; found: C, 59.25; H, 4.74; N, 22.63; m/z 368 (M++2).
Biological activity evaluation
In vitro antimicrobial activity measurement
Primary screening
Most of the newly synthesized compounds were screened for their antibacterial and antifungal activities using the agar well diffusion techniqueCitation29. The microorganisms (reference and clinical isolates) used include Gram-negative E. coli (ATCC-25922) and Shigella dysenterie, Gram-positive Staphylococcus aureus (ATCC-25923) and Bacillus cereus, fungi Aspergillus flavus and Candida albicans (ATCC 10231). For the antibacterial assay, a standard inoculum (105 CFU/mL) was distributed on the surface of sterile nutrient agar plates by a sterile glass spreader, whereas for the antifungal assay a loopful of a particular fungal strain was transferred to 3 mL saline to get a suspension of the corresponding species; 0.1 mL of the spore suspension was distributed on the surface of sterile Sabouraud dextrose agar plates. Six millimetre diameter wells were punched in the agar media and filled with 100 µL (500 µg/mL in DMSO) of the tested chemical compounds previously sterilized through 0.45 sterile membrane filterCitation30. The plates were kept at room temperature for 1 h and then incubated at 37°C for 24 h for bacteria and 30°C for 4 days for fungi. The antimicrobial activities were evaluated by measuring the inhibition zone diameters. Commercial antibiotic discs were used as positive reference standard to determine the sensitivity of the strains ().
Table 1. Antimicrobial screening results of the tested compounds.
Determination of MIC of the synthesized compounds
Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their MIC and were determined by broth dilution techniqueCitation31. The nutrient broth and the yeast extract broth media, which contained 1 mL of different concentrations of the tested compounds (5, 10, 15, 20, 25 µg/mL), were inoculated with the microbial strains; the bacterial cultures were incubated for 24 h at 37°C, whereas the fungal ones were incubated at 30°C for 48 h; the growth was monitored spectrophotometrically. The lowest concentration required to arrest the microbial growth was regarded as MICs and are given in .
Table 2. MIC (µg/mL) results of the tested compounds.
Results and discussion
Chemistry
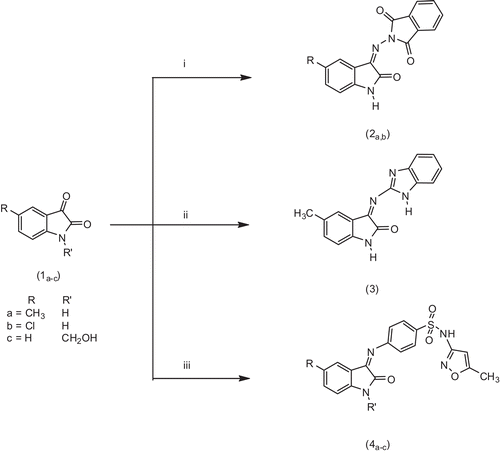
We investigated earlier the reaction of isatin, N-aminophthalimide or pyridazines as key starting materials for the synthesis of biologically active compoundsCitation32–40. In the present work, we studied the reaction of 5-substituted isatins and 1-hydroxy methylisatin (1a–c) with different amines, such as N-aminophthalimide, 1H-benzodimidazol-2-amine and sulphamethoxazole. It was reported by PoppCitation41 that the reaction of N-aminophthalimide with isatin and 5-chloro isatin gave the corresponding ylideneamino derivatives. In the present study, we reported the microwave assists reaction of 5-methyl, 5-chloro isatins (1a,b) with N-aminophthalimide to give 2-(5-substituted 2-oxindolin-3-ylideneamino) isoindolin-1,3-diones (2a,b). The structures of (2a,b) were confirmed by elemental analysis: FTIR, 1H NMR and MS (cf. Materials and methods). However, refluxing 5-methyl isatin (1a) with 3-(1H-benzoimidazol-2-amine) in ethanol led to the formation of 3-(1H-benzoimidazol-2-ylimino)-5-methyl indolin-2-one (3). Sulphamethoxazole reacted with (1a–c) in refluxing absolute ethanol to afford N-(5-methylisoxazol-3-yl)-4-(5-substituted 2-oxindolin-3 ylideneamino) benzenesulphonamides (4a,b) and 4-(1-hydroxymethyl)-2-oxindolin-3-ylideneamino)-N-(5-methylisoxazol-3-yl) benzenesulphonamide (4c). The target compounds (2–4) were prepared as depicted in .
Scheme 1. Synthesis of the target compounds 2a,b, 3 and 4a–c. (i) N-Aminophthalimide, (ii) 1H-benzoimidazol-2-amine and (iii) 4-amino-N-(5-methylisoxazol-3-yl) benzenesulphonamide.
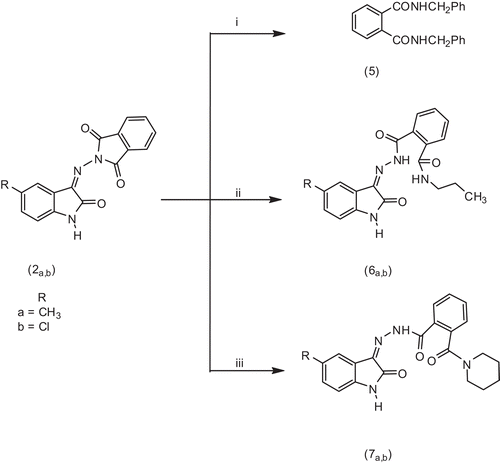
In continuation of our interest in N-aminophthalimide chemistry, with a view directed towards preparing biologically active compoundsCitation33,Citation35, in the present article, we studied the reaction of 2-(5-substituted 2-oxindolin-3-ylideneamino) isoindolin-1,3-diones (2a,b) with different amines. When 2-(5-substituted 2-oxindolin-3-ylideneamino) isoindolin-1,3-diones (2a,b) were reacted with benzylamine, they underwent ring cleavage to give N,N′-dibenzylphthalamide (5), as expected (mp, mixed melting point with authentic sample)Citation33,Citation34, in addition to 3-hydrazonoindolin-2-ones (8a,b) as by-product. The structure of (8a,b) were confirmed by mp (mixed melting point with authentic sample)Citation42–44. However, reaction of (2a,b) with n-propylamine and piperidine took place smoothly via ring opening of the phthalide moiety to give the corresponding 2-[(5-substituted 2-oxindolin-3-ylidene) hydrazino] carbonyl-N-propylbenzamides (6a,b) and N-(5-substituted 2-oxindolin-3-ylidene)-2-(piperidin-1-ylcarbonyl) benzohydrazides (7a,b), respectively. The new compounds (6a,b and 7a,b) have been characterized by standard procedures such as elemental analysis, IR and NMR spectroscopy and MS, which conformed their structure (cf. experimental part). illustrates the synthetic pathway for target compounds (5, 6 and 7).
Scheme 2. Synthetic pathway for target compounds 5, 6a,b and 7a,b. (i) Benzylamine, (ii) n-propylamine and (iii) piperidine.
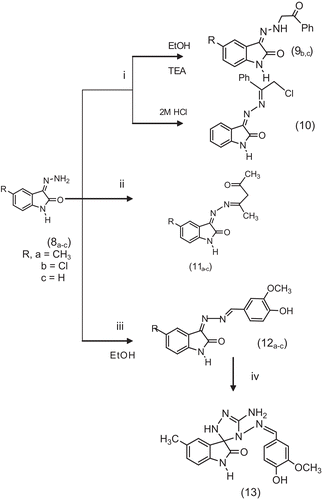
The present study was extended to synthesize new biologically active oxindole derivatives using 3-hydrazono-5-substituted indolin-2-ones (8a–c) as starting materials. Thus, reaction of (8a–c) with phenacyl chloride was investigated at various pH values. When isatin-3-hydrazones (8b,c) reacted with phenacyl chloride in boiling ethanol and in the presence of catalytic amount of triethylamine afforded 3-[2-(1-phenyl-2-oxoethyl) hydrazono]-5-substituted indolin-2-ones (9b,c), on the other hand, it has been found that isatin-3-hydrazone (8c) behaved differently on adding 2 M HCl to the reaction condition, the product was formulated as 3-[2-(2-chloro-1-phenylethylidene) hydrazono] indolin-2-one (10). Moreover, the reaction of isatin-3-hydrazones (8a–c) with dicarbonyl compound such as acetylacetone in boiling ethanol afforded 3-[2-(4-oxopentan-2-ylidene) hydrazono]-5-substituted indolin-2-ones (11a–c). Reaction of isatin-3-hydrazones (8a–c) with 3-methoxy-4-hydroxybenzaldehyde in boiling ethyl alcohol it afforded 3-[2-(4-hydroxy-3-methoxy benzylidene) hydrazono]-5-substituted indolin-2-ones (12a–c).
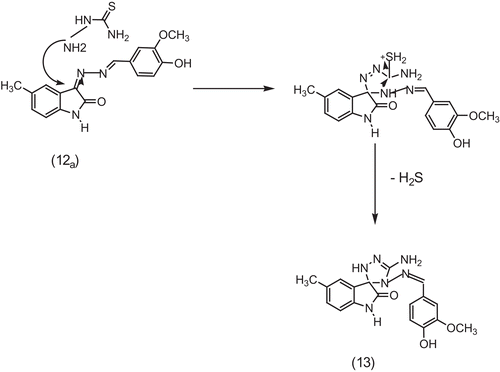
Finally, spirocyclic compound (13) was synthesized via spiro annelation reaction of 3[2-(4-hydroxy-3-methoxybenzylidene) hydrazono]-5-methyl-indolin-2-one (12a) with thiosemicarbazide. The spiro compound was achieved either by the usual thermal method or by using microwave irradiation. illustrates the synthetic pathway for target compounds (9–13).
Scheme 3. Synthetic pathway for target compounds 9b,c, 10, 11a–c, 12a,b and 13. (i) Phenacyl chloride, (ii) acetylacetone, (iii) 4-hydroxy-3-methoxybenzaldehyde (vanillin) and (iv) thiosemicarbazide.
The proposed mechanism for the synthesis of the spirocyclo derivative (13) is described in , and it involves a nucleophilic attack of the amino electrons of thiosemicarbazide at the electrophilic indolyl carbon, accompanied by the migration of a hydrogen atom to form an intermediate thiol derivative, followed by cyclization and desulphurization with the loss of H2S to give the suggested spirocyclic compound (13).
Biological evaluation (in vitro antimicrobial measurement)
Most of the synthesized compounds were tested for their in vitro antimicrobial activity by the broth dilution technique in terms of MIC. The MICs of the compounds against six pathogenic microbial species are present in . The study also included the activity of reference compounds sulphamethoxazole as antibacterial agent and fluconazole as antifungal agent. From the data of primary antimicrobial screening followed by MICs count in and , the following conclusion can be drawn:
E. coli: Compounds N,N′-dibenzylphthalamide (5) and N-(5-methylisoxazol-3-yl)-4-(5-substituted 2-oxindolin-3-ylideneamino) benzenesulphonamides (4a,b) are the most active compounds comparable with other tested compounds.
S. dysenterie: Compound 3-[2-(4-oxopentan-2-ylidene) hydrazono]-5H-indolin-2-one (11c) is the most potent compound and showed significant activity comparable with the standard sulphamethoxazole.
S. aureus: Compounds N,N′-dibenzylphthalamide (5) and N-(5-methylisoxazol-3-yl)-4-(5-methyl-2-oxindolin-3-ylideneamino) benzenesulphonamide (4a) showed the highest activity against S. aureus as well as against E. coli strains.
B. cereus: Compound N-(5-ethylisoxazol-3-yl)-4-(5-methyl-2-oxindolin-3-ylideneamino) benzenesulphonamide (4a) was the most active compound as with species (1 and 3) and more active than the standard sulphamethoxazole.
A. flavus: All the tested compounds nearly have the same activity.
C. albicans: Compounds N-(5-methylisoxazol-3-yl)-4-(5-chloro-2-oxindolin-3-ylideneamino) benzene sulphonamide (4b) and 2-[5-methyl-2-oxindolin-3-ylidene) hydrazono] carbonyl-N-propylbenzamide (6a) were the most potent as antifungal comparable with other tested compounds.
Conclusion
In this study, we reported a convenient route for the synthesis of some new compounds incorporating with the oxindole nucleus starting from substituted isatins [5-(methyl, chloro) and 1-hydroxymethyl isatins] and investigated their antimicrobial and antifungal activities.
The in vitro evaluation of their antimicrobial against several pathogenic bacterial and fungal strains revealed that compounds N-(5-methylisoxazol-3-yl)-4-(5-substituted 2-oxindolin-3-ylideneamino) benzenesulphonamides (4a,b) are more active comparable with other synthesized oxindole derivatives against bacterial strains. This higher activity may be attributed to benzenesulphonamide moiety. The data show that derivative (4b) with the chlorine atom at C-5 position in the oxindole nucleus was more potent as antifungal agent than the (4a) derivative with the methyl group. Compound (5) showed against all tested bacterial strains exerted excellent antibacterial activity. The data of MICs count also indicated that compounds (11c) and (4a) showed significant activity against S. dysenterie and B. cereus bacterial species, respectively, comparable with standard sulphamethoxazole and may serve as useful lead compounds in search for potent antibacterial agent.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Morley JE, Farr SA, Flood JF. Isatin inhibits food intake in mice. Eur J Pharmacol 1996;23:305.
- Tozawa Y, Ueki A, Manabe S, Matsushima K. Stress-induced increase in urinary isatin excretion in rats: reversal by both dexamethasone and alpha-methyl-p-tyrosine. Biochem Pharmacol 1998;56:1041–1046.
- Medvedev AE, Goodwin B, Clow A, Halket J, Glover V, Sandler M. Inhibitory potency of some isatin analogues on human monoamine oxidase A and B. Biochem Pharmacol 1992;44:590–592.
- Pandeya SN, Sriram D. Synthesis and screening for antibacterial activity of Schiff and Mannich bases of isatin derivatives. Acta Pharm Turc 1998;40:33–38.
- Sarangapani M, Reddy VM. Pharmacological evaluation of 1-(N,N-disubstituted aminoethyl)-3-imino-(2-phenyl-3,4-dihydro-4-oxo-quinazoline-3-yl)indolin-2-ones. Indian J Pharm Sci 1994;56:174–177.
- Varma RS, Nobles WL. Antiviral, antibacterial, and antifungal activities of isatin N-Mannich bases. J Pharm Sci 1975;64:881–882.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activity of Schiff and Mannich bases of isatin with N-[6-chlorobenzothiazol-2-yl]thiosemicarbazide. Indian J Pharm Sci 1999;61:358–361.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of norfloxacin Mannich bases. Sci Pharm 1999;67:103–111.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm Acta Helv 1999;74:11–17.
- Sriram D, Yogeeswari P, Gopal G. Synthesis, anti-HIV and antitubercular activities of lamivudine prodrugs. Eur J Med Chem 2005;40:1373–1376.
- Patole J, Sandbhor U, Padhye S, Deobagkar DN, Anson CE, Powell A. Structural chemistry and in vitro antitubercular activity of acetylpyridine benzoyl hydrazone and its copper complex against Mycobacterium smegmatis. Bioorg Med Chem Lett 2003;13:51–55.
- Karali N, Gürsoy A, Kandemirli F, Shvets N, Kaynak FB, Ozbey S et al. Synthesis and structure–antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg Med Chem 2007;15:5888–5904.
- Pandeya SN, Yogeeswari P, Sriram D, de Clercq E, Pannecouque C, Witvrouw M. Synthesis and screening for anti-HIV activity of some N-Mannich bases of isatin derivatives. Chemotherapy 1999;45:192–196.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of norfloxacin mannich bases. Eur J Med Chem 2000;35:249–255.
- Pandeya SN, Sriram D, Nath G, de Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole. Arzneimittelforschung 2000;50:55–59.
- Chinnasamy RP, Sundararajan R, Govindaraj S. Synthesis, characterization, and analgesic activity of novel Schiff base of isatin derivatives. J Adv Pharm Technol Res 2010;1:342–347.
- Bal TR, Anand B, Yogeeswari P, Sriram D. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives. Bioorg Med Chem Lett 2005;15:4451–4455.
- Solomon VR, Hu C, Lee H. Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 2009;17:7585–7592.
- Li JJ, Liang X-M, Jin S-H, Zhang J-J, Yuan H-Z, Qi S-H, Chen F-H, Wang D-Q. Synthesis, fungicidal activity, and structure–activity relationship of spiro-compounds containing macrolactam (Macrolactone) and thiadiazoline rings. J Agric Food Chem 2010;58:2659–2663.
- Kumar RR, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D. Discovery of antimycobacterial spiro-piperidin-4-ones: an atom economic, stereoselective synthesis, and biological intervention. J Med Chem 2008;51:5731–5735.
- Joshi KC, Chand P. Biologically active indole derivatives. Pharmazie 1982;37:1–12.
- Abdel-Rahman AH, Keshk EM, Hanna MA, el-Bady ShM. Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents. Bioorg Med Chem 2004;12:2483–2488.
- Dandia A, Singh R, Khaturia S, Mérienne C, Morgant G, Loupy A. Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro [indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3,2′-thiazolidine]-3′(1,2,4-triazol-3-yl)-2,4′(1H)-dione. Bioorg Med Chem 2006;14:2409–2417.
- Khafagy MM, Abd el-Wahab AH, Eid FA, el-Agrody AM. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 2002;57:715–722.
- Sebahar PR, Williams RM. The asymmetric total synthesis of (+)- and (−)-spirotryprostatin B. J Am Chem Soc 2000;122:5666–5667.
- Ma J, Hecht SM. Javaniside, a novel DNA cleavage agent from Alangium javanicum having an unusual oxindole skeleton. Chem Commun (Camb) 2004;10:1190–1191.
- Edmondson S, Danishefsky SJ, Sepp-lorenzinol L, Rosen N. Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J Am Chem Soc 1999;121:2147–2155.
- Ahmad S, Rathish IG, Bano S, Alam MS, Javed K. Synthesis and biological evaluation of some novel 6-aryl-2-(p-sulfamylphenyl)-4,5-dihydropyridazin-3(2H)-ones as anti-cancer, antimicrobial, and anti-inflammatory agents. J Enzyme Inhib Med Chem 2010;25:266–271.
- Karthikeyan SM, Prasad JD, Mahalinga M, Holla SB, Kumari SN. Antimirobial studies of 2,4-dihloro-5-fluorophenyl containing oxadiazoles. Eur J Med Chem 2008;43:25–31.
- Tadig H, Mohmed E, Asres K, Gebre T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol 2005;100:168–175.
- Awad WI, Ismail MF, Kandile NG. Action of Grignard reagents on N-alkylidene- and N-arylmethylene-aminophthalimides. Aust J Chem 1975;28:1621.
- Ismail MF, Kandile NG. Cleavage of N-arylmethyleneaminophthalimides by nucleophilic reagent. Ind J Chem 1982;21B:462.
- Kandile NG, Zaky HT, Soliman M. Novel pyrimidine derivatives for drug design. Egypt J Chem 2004;47:283–292.
- Kandile NG, Mohamed MI, Zaky H, Mohamed HM. Novel pyridazine derivatives: synthesis and antimicrobial activity evaluation. Eur J Med Chem 2009;44:1989–1996.
- Mohamed MI. Synthesis and antibacterial activity of some novel heterocycles. Bulg Chem Commun 2004;36:241.
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Novel heterocyclic systems of pyridazine. Afinidad 2005;62:48–56.
- Mohamed MI. Synthesis and antimicrobial activity of new pyridazinyl sulfon amide derivatives. Bulg Chem Commun 2007;39:152–158.
- Zaky HT. Action of amines and Grignard reagents on some new N-arylidene aminophthalimides. Heterocyclic Commun 2002;8:355–360.
- Zaky HT, Mohamed MI, Nail AM, Kandile NG. Synthesis of biologically active of some novel sulphonamides. Egypt J Chem 2004;47:321–331.
- Popp FD. Potential anticonvulsants. IX. Some isatin hydrazones and related compounds. J Heterocyclic Chem 1984;21:1641–1645.
- Molloy BB. Pharmaceutical compositions containing substituted 2-oxo-indolines and the use thereof to treat anxiety and tension. US Patent 1975; 3882236.
- Parquet E, Lin Q. Microwave-assisted Wolff–Kishner reduction reaction. J Chem Edu 1997;74:1225.
- Hlavac J, Buchtik R, Slouka J, Hradil P, Wiedermannova I. Synthesis of oxo analogs of lamotrigine and some related compounds Arkivoc 2003;(i):22–38.