Abstract
A facile and ecofriendly synthesis of new chromonyl chalcones 3a-b from 3-formylchromone 1 and active methyl compounds 2a-b is reported under thermal solvent-free heating condition in good yields. The chromonyl chalcones 3a-b were used as intermediates under green condition for the synthesis of new bioactive pyrazoline derivatives 4a-f. The compounds were tested for antimicrobial activity by disk diffusion assay with slight modifications against Gram-positive, Gram-negative strains of bacteria as well as fungal strains. The investigation of antimicrobial screening revealed that compounds 3a-b and 4a-f showed antibacterial and antifungal activities.
Introduction
Human infections particularly those involving microorganisms like bacteria and fungi cause serious damages in tropical and subtropical countries of the world and these infections are a major threat to public health despite tremendous growth in human chemotherapeutic medicine. These infections can occur in invasive form, and are an increasing problem due to the increase of their incidence in hospitals, especially in patients who are undergoing cancer treatment, transplantation or are immune suppressed for other reasonsCitation1. Methicillin-resistant Staphylococcus aureus (MRSA) is common Gram-positive pathogen of nosocomial infections which account for outbreaks and are increasing in frequencyCitation2,Citation3. Escherichia coli is responsible for the world’s most common and serious infectious diseases like invasive dysentery and diarrheaCitation4,Citation5. The different parasitic bacteria such as S. aureus, S. pyogenes, S. typhimurium, E. coli have important effect on the human’s mucosal health. The infection with these microorganisms may have significant impact on huge demolition of host tissues and severe diseasesCitation6,Citation7. Thus, antibiotics provide the main basis for the therapy of microbial (bacterial and fungal) infections. However, overuse of antibiotics has become the major factor for the emergence and dissemination of multi-drug resistant strains of several groups of microorganismsCitation8. Furthermore, the drugs available are either too expensive or have undesirable side effects or contraindicationsCitation9. The aim of this work is the synthesis of new molecules able to inhibit the growth of Gram-positive, Gram-negative bacteria and fungi.
Chromones comprise a vast array of oxygen containing compounds ubiquitous in plantsCitation10. They form basic nucleus of flavones and have been recognized as the essential component of pharmacophores of a large number of bioactive moleculesCitation11. Interest in chromone containing structures stems from their widespread occurrence in molecules that exhibit significant antimicrobialCitation12, antioxidantCitation13, neuroprotectiveCitation14, HIV-inhibitoryCitation15 and anticancerCitation16 activities. In addition, chromone derivatives are also active at benzodiazepine receptors, lipoxygenases and cyclooxygenasesCitation17. Chalcones and pyrazolines are another important class of compounds with wide spread biological applicationsCitation18,Citation19.
Growing concern about environmental damage leads to an urgent requirement for the development of efficient and environmentally benign chemical processes for the synthesis of new molecules. Solvent-free reactions are gaining importance in this context as the procedure demonstrates obvious and significant advantagesCitation20. However, most of the reported solvent-free accomplishments focus on reactions promoted by microwave irradiation, hand grinding and mechanical millingCitation21–23. In contrast, reports on direct heating of the reagents under thermal heating conditions without any solvent are relatively less explored. To the best of our knowledge, no reports have been so far made in the synthesis of chromonyl chalcones and derivatives in solvent-free thermal heating condition. Thus, in the present study, we wish to report the novel synthesis of series of chromonyl chalcones and pyrazolines in excellent yields, under thermal solvent-free heating condition and their evaluation for antimicrobial activities.
Materials and methods
Chemistry
Melting points were taken in Riechert Thermover (Austria)instrument and are uncorrected. The IR spectra were recorded on Perkin Elmer (Spectro Lab, United Kingdom) RXI spectrometer in KBr, 1H NMR on Bruker DRX 300 MHz spectrometer and Bruker Avance (Switzerland) II 400 MHz spectrometer using tetramethyl silane as the internal standard and DMSO-d6/CDCl3 as solvent. Mass spectra were obtained on Jeol-SX-102 (FAB) spectrometer. The microanalytical data were collected on Carlo Erba (Carlo Erba Instruments, Germany) analyzer model 1108. 3-Formylchromone 1Citation24, hydrazinobenzothiazoleCitation25 and 5-acetylbarbituric acid 2bCitation26 were synthesized by reported methods. Dehydroacetic acid 2a was purchased from E. Merck (Merck, Darmstadt, Germany). Other chemicals were of commercial grade and used without further purification. The purity of the compounds was checked by thin layer chromatography (TLC) on glass plates coated with silica gel G254 (E. Merck) using chloroform-methanol (3:1 v/v) mixture as mobile phase and visualized by iodine vapours.
General procedure for the synthesis of chalcones (3a-b) under conventional method
To a solution of 3-formylchromone 1 (1.00 g, 5.7 mmol) and dehydroacetic acid 2a (0.965 g, 5.7 mmol)/5-acetyl barbituric acid 2b (0.970 g, 5.7 mmol) in methanol (12 mL), pyridine (0.5 mL) was added. The reaction mixture was stirred at room temperature/refluxed for a specified time. After completion of the reaction (checked by TLC), the reaction mixture was concentrated and cooled at room temperature. The yellow solid, which precipitated out, was filtered, washed with methanol and dried. The compounds were purified by recrystallization from chloroform-methanol (1:3 v/v) mixture as shining yellow crystals.
General procedure for the synthesis of chalcones (3a-b) under solvent-free condition
A mixture of 3-formylchromone 1 (1.00 g, 5.7 mmol), dehydroacetic acid 2a (0.965 g, 5.7 mmol)/5-acetyl barbituric acid 2b (0.970 g, 5.7 mmol) and pyridine (0.3 mL) were mixed well and air dried. The reaction mixture was then heated at 80 ˚C in a 100 mL beaker. After completion of the reaction (checked by TLC), the reaction mixture was slurred in water (25 mL). The yellow solid obtained was filtered, washed with methanol and dried. The compounds were purified by recrystallization from chloroform-methanol (1:3 v/v) mixture as shining yellow crystals.
(2E)-1-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-3-(4-oxo-4H-1-benzopyran-3-yl)-2-propen-1-one (3a)
Pale yellow crystals; mp 235–237°C. 1H NMR (CDCl3, 300 MHz) δ: 2.28 (3H, s, CH3), 5.96 (1H, s, C-5′), 7.36-7.73 (3H, m, Ar-H), 7.87 (1H, d, J = 15.9 Hz, Ha), 8.32 (1H, s, C-2), 8.35 (1H, dd, J = 7.8 Hz, 1.8 Hz, C-5), 8.77 (1H, d, J = 15.9 Hz, Hb). IR (KBr) υ: 3437 (OH), 1721 (C=O), 1659 (C=O). MS-FAB m/z (%): 324 (M+, 100), 323 (25), 309 (5), 308 (10), 279 (10), 198 (80), 171 (70), 120 (10), 92 (5). Anal. Cald for C18H12O6: C, 66.67; H, 3.73. Found: C, 66.53; H, 3.69.
(2E)-3-(4-Oxo-4H-1-benzopyran-3-yl)-1-(2,4,6-pyrimidinetrione-5-yl)-2-propen-1-one (3b)
Light yellow crystals; mp >300°C. 1H NMR (DMSO-d6, 300 MHz) δ: 7.40 (1H, d, J = 15.2 Hz, Ha), 7.55-7.91 (3H, m, Ar-H), 8.17 (1H, dd, J = 8.0 Hz, 1.6 Hz, C-5), 8.95 (1H, s, C-2), 9.09 (1H, d, J = 15.9 Hz, Hb), 11.06 (1H, s, NH), 11.76 (1H, s, NH). IR (KBr) υ: 3078 (NH), 1740 (C=O), 1643 (C=O). MS-FAB m/z (%): 326 (M+, 60), 206 (10), 198 (70), 171 (65), 145 (50), 120 (40). Anal. Cald for C16H10N2O6: C, 58.83; H, 3.06; N, 8.58. Found: C, 58.72; H, 3.14; N, 8.44.
General procedure for the synthesis of pyrazolines (4a-e) and bipyrazole (4f) under conventional method
The compound 3a-b (1.00 g, 3 mmol) was dissolved in acetic acid (8 mL) and hydrazines (hydrazine hydrate (0.15 g, 3 mmol)/hydrazinobenzothiazole (0.49 g, 3 mmol)/phenylhydrazine (0.51 g, 3 mmol) added to it. The reaction mixture was stirred at room temperature or refluxed in a heating mantle for specified period of time. After that, the reaction mixture was cooled and poured into ice-cold water (20 mL). The solid, which precipitated out, was filtered, washed with water and dried. Purification of 4a-f was achieved by recrystallization from chloroform-methanol (1:4 v/v) mixture.
General procedure for the synthesis of pyrazolines (4a-e) and bipyrazole (4f) under solvent-free heating method
The compound 3a-b (1.00 g, 3 mmol) and hydrazines (hydrazine hydrate (0.15 g, 3 mmol)/hydrazinobenzothiazole (0.49 g, 3 mmol)/phenylhydrazine (0.51 g, 3 mmol) were mixed well and heated at 80°C in a 100 mL beaker. After completion of the reaction (checked by TLC), the reaction mixture was slurred in water (20 mL). The solid, obtained was filtered, washed with methanol and dried. The compounds were purified by recrystallization from chloroform-methanol (1:4 v/v) mixture to get 4a-f.
3-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-5-(4-oxo-4H-1-benzopyran-3-yl)pyrazoline (4a)
White solid; mp 260–262°C. 1H NMR (CDCl3, 300 MHz) δ: 2.27 (3H, s, CH3), 3.69-3.92 (2H, m, He, Hf), 5.20-5.26 (1H, m, Hd), 6.04 (1H, s, C-5′), 6.68 (1H, s, NH), 7.35-7.68 (3H, m, Ar-H), 8.02 (1H, s, C-2), 8.16 (1H, d, J = 7.9 Hz, C-5), 12.78 (1H, s, OH, D2O exchangeable). IR (KBr) υ: 1700 (C=O), 1665 (C=O). MS-FAB m/z (%): 338 (M+, 60), 337 (70), 309 (5), 281 (5), 217 (5), 193 (15), 189 (5), 185 (5), 163 (10), 120 (15), 93 (45), 92 (10). Anal. Cald for C18H14N2O5: C, 63.90; H, 4.17; N, 8.28. Found: C, 63.83; H, 4.24; N, 8.22.
1-Benzothiazolyl-3-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-5-(4-oxo-4H-1-benzopyran-3-yl)pyrazoline (4b)
Pale yellow solid; mp >300°C. 1H NMR (DMSO-d6, 300 MHz) δ: 2.48 (3H, s, CH3), 3.07–4.14 (2H, m, He, Hf), 5.44–5.50 (1H, m, Hd), 6.29 (1H, s, C-5′), 8.02 (1H, d, J = 7.1 Hz, C-5), 7.05-7.81 (7H, m, Ar-H), 8.41 (1H, s, C-2) 12.23 (1H, s, OH, D2O exchangeable). IR (KBr) υ: 1727 (C=O), 1649 (C=O). MS-FAB m/z (%): 471 (M+, 100), 443 (5), 415 (5), 428 (5), 322 (5), 307 (50), 177 (5), 120 (20). Anal. Cald for C25H17N3O5S: C, 63.68; H, 3.63; N, 8.91. Found: C, 63.61; H, 3.67; N, 8.87.
5-(4-Oxo-4H-1-benzopyran-3-yl)-3-(2,4,6-pyrimidinetrione-5-yl)pyrazoline (4c)
White solid; mp >300°C. 1H NMR (DMSO-d6, 300 MHz) δ: 3.43-3.49 (1H, m, He), 3.82–3.89 (1H, m, Hf), 4.69–4.75 (1H, m, Hd), 7.49–7.84 (3H, m, Ar-H), 6.69 (1H, s, NH), 8.09 (1H, dd, J = 8.0 Hz, 1.2 Hz, C-5), 8.29 (1H, s, C-2), 10.31 (1H, s, NH), 10.45 (1H, s, NH). IR (KBr) υ: 3214 (NH), 3069 (NH), 1721(C=O), 1628 (C=O). MS-FAB m/z (%): 340 (M+, 75), 326 (25), 322 (30), 206 (5), 198 (5), 171 (30), 145 (5), 120 (30). Anal. Cald for C16H12 N4 O5: C, 56.47; H, 3.52; N, 16.47. Found: C, 56.31; H, 3.46; N, 16.54.
1-Phenyl-5-(4-oxo-4H-1-benzopyran-3-yl)-3-(2,4,6-pyrimidinetrione-5-yl)pyrazoline (4d)
Pale yellow solid; mp >300°C. 1H NMR (DMSO-d6, 400 MHz) δ: 3.51–3.56 (1H, m, He), 3.73–3.78 (1H, m, Hf), 4.42–4.44 (1H, m, Hd), 7.25–7.45 (8H, m, Ar-H), 7.55 (1H, s, C-2), 7.79 (1H, dd, J = 7.6 Hz, 1.8 Hz, C-5), 10.60 (1H, s, NH), 10.76 (1H, s, NH). IR (KBr) υ: 3068 (NH), 1737 (C=O), 1649 (C=O). MS-FAB m/z (%): 416 (M+, 70), 415(5), 326 (45), 198 (40) 171 (30), 120 (20). Anal. Cald for C22H16N4O5: C, 63.46; H, 3.84; N, 13.46. Found: C, 63.50; H, 3.71; N, 13.38.
1-Benzothiazolyl-5-(4-oxo-4H-1-benzopyran-3-yl)-3-(2,4,6-pyrimidinetrione-5-yl)pyrazoline (4e)
Brown crystals; mp >300°C. 1H NMR (DMSO-d6, 400 MHz) δ: 3.81–3.87 (1H, m, He), 4.10–4.17 (1H, m, Hf), 5.49–5.53 (1H, m, Hd), 7.12–7.76 (7H, m, Ar-H), 8.15 (1H, dd, J = 8.0 Hz, 1.6 Hz, C-5), 8.27 (1H, s, C-2), 11.17 (1H, s, NH), 11.56 (1H, s, NH). IR (KBr) υ: 3069 (NH), 1701(C=O), 1644 (C=O). MS-FAB m/z (%): 473 (M+, 50), 326 (20), 198 (10), 145 (20), 120 (30). Anal. Cald for C23H15N5O5S: C, 58.35; H, 3.17; N, 14.79. Found: C, 58.27; H, 3.25; N, 14.71.
1-Phenyl-3-[4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl]-5-[5-(2-hydroxyphenyl)-1-phenyl pyrazol-4-yl]pyrazoline (4f)
Red crystals; mp 250–253°C. 1H NMR (CDCl3, 300 MHz) δ: 2.35 (3H, s, CH3), 3.38–3.50 (1H, m, He), 3.66–4.11 (1H, m, Hf), 4.99–5.34 (1H, m, Hd), 6.01 (1H, s, C-5′), 6.90-7.13 (4H, m, Ar-H), 7.18–7.42 (14H, m, Ar-H), 7.68 (1H, s, Hc), 13.27 (1H, s, OH, D2O exchangeable). IR (KBr) υ: 3432 (OH), 1701 (C=O). MS-FAB m/z (%): 504 (M+, 70), 476 (5), 460 (15), 449 (8), 445 (10), 379 (5), 352 (20), 279 (15). Anal. Cald for C30H24N4O4: C, 71.41; H, 4.79; N, 11.10. Found: C, 71.26; H, 4.84; N, 11.06.
Antibacterial studies
The newly synthesized compounds were screened for their antibacterial activity against Streptococcus pyogenes (clinical isolate), MRSA (+ve), Pseudomonas aeruginosa (ATCC-27853), Klebsiella pneumoniae (clinical isolate) and E. coli (ATCC-25922) bacterial strains by disk diffusion methodCitation27,Citation28. A standard inoculums (1–2 × 107 c.f.u./mL 0.5 McFarland standards) was introduced on to the surface of sterile agar plates, and a sterile glass spreader was used for even distribution of the inoculums. The disks measuring 6 mm in diameter were prepared from Whatman no. 1 filter paper and sterilized by dry heat at 140°C for 1 h. The sterile disks previously soaked in a known concentration of the test compounds were placed in nutrient agar medium. Solvent and growth controls were kept. Ciprofloxacin (30 µg) was used as positive control while the disk poured in DMSO was used as negative control. The plates were inverted and incubated for 24 h at 37°C. The susceptibility was assessed on the basis of diameter of zone of inhibition against Gram-positive and Gram-negative strains of bacteria. Inhibition zones were measured and compared with the controls. The bacterial zones of inhibition values are given in .
Table 1. Antibacterial activity of compounds 3a-4f.
Minimum inhibitory concentrations (MICs) were determined by broth dilution technique. The nutrient broth, which contained logarithmic serially two fold diluted amount of test compound and controls were inoculated with approximately 5 × 105 c.f.u/mL of actively dividing bacteria cells. The cultures were incubated for 24 h at 37°C and the growth was monitored visually and spectrophotometrically. The lowest concentration (highest dilution) required to arrest the growth of bacteria was regarded as MIC. To obtain the minimum bactericidal concentration (MBC), 0.1 mL volume was taken from each tube and spread on agar plates. The number of c.f.u was counted after 18–24 h of incubation at 35°C. MBC was defined as the lowest drug concentration at which 99.9% of the inoculums were killed. The minimum inhibitory concentration and minimum bactericidal concentration are given in .
Antifungal studies
Antifungal activity was also done by disk diffusion method. For assaying antifungal activity Candida albicans, Aspergillus fumigatus, Trichophyton mentagrophytes and Penicillium marneffei were recultured in DMSO by agar diffusion methodCitation29,Citation30. Sabourauds agar media was prepared by dissolving peptone (1 g), D-glucose (4 g) and agar (2 g) in distilled water (100 mL) and adjusting pH to 5.7. Normal saline was used to make a suspension of spore of fungal strain for lawning. A loopful of particular fungal strain was transferred to 3 mL saline to get a suspension of corresponding species. 20 mL of agar media was poured into each Petri dish. Excess of suspension was decanted and the plates were dried by placing in an incubator at 37°C for 1 h. Using an agar punch, wells were made and each well was labeled. A control was also prepared in triplicate and maintained at 37°C for 3–4 days. The fungal activity of each compound was compared with griseofulvin as standard drug. Inhibition zones were measured and compared with the controls. The fungal zones of inhibition values are given in . The nutrient broth, which contained logarithmic serially two fold diluted amount of test compound and controls was inoculated with approximately 1.6 × 104–6 × 104 c.f.u/mL. The cultures were incubated for 48 h at 35°C and the growth was monitored. The lowest concentration (highest dilution) required to arrest the growth of fungi was regarded as MIC. To obtain the minimum fungicidal concentration (MFC), 0.1 mL volume was taken from each tube and spread on agar plates. The number of c.f.u. was counted after 48 h of incubation at 35°C. MFC was defined as the lowest drug concentration at which 99.9% of the inoculums were killed. The MIC and MFC are given in .
Table 2. Antifungal activity of compounds 3a-4f.
Results and discussion
Chemistry
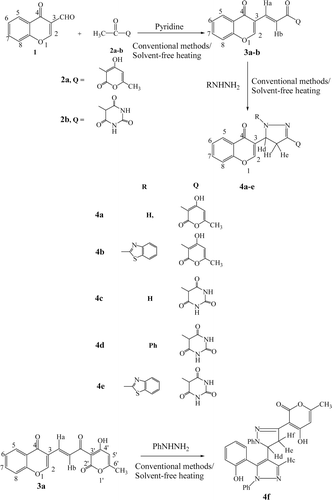
3-Formylchromone has emerged as an important starting material for incorporation of chromone moiety, ever since its convenient synthesis was reported in 1970sCitation24. It has been used as an important synthon due to the presence of three electron deficient centers viz C-2, C-4 carbonyl and C-3 formyl groups. In the present study, we carried out the reaction of 3-formylchromone 1 and active methyl compounds viz dehydroacetic acid 2a and 5-acetyl barbituric acid 2b under different reaction conditions. Initially reactions were conducted at room temperature in the presence of basic catalyst pyridine. The reactions afforded the expected chromonyl chalcones 3a-b in 18–25 hours with 62–70% yields (). The reactions carried using methanol as a solvent under conventional heating condition were found to be completed in 10–16 h, but there was no substantial increase in yields of products. This prompted us to carry out reactions under solvent-free heating technique by simply heating the starting materials with the catalyst. To our pleasant surprise, the reactions were completed in short span of time (12–15 min) with considerable increase in yields (88–92%) and were devoid of any toxic waste products. Thus, the present protocol explains the superiority of thermal solvent-free reactions over the conventional methods. The products obtained were characterized by elemental and spectral (IR, 1H NMR and mass spectrometry) analysis.
Scheme 1. Formation of chalcones 3a-b and their reaction with different hydrazines to give compounds 4a-f.
The 1H NMR spectrum of the compound 3b showed trans olefinic protons Ha and Hb as ortho coupled doublets at δ 7.40 (J = 15.2 Hz) and 9.09 (J = 15.9 Hz), respectively. The value of spin-spin coupling constant Jab, in the range of 15–16 Hz, is indicative of the E-configuration of chalcone. The three aromatic protons of chromone moiety were discernible in the form of multiplet at δ 7.55–7.91 whereas C-2 and C-5 protons appeared as a singlet and doublet of doublet at δ 8.95 and 8.17 respectively. The NH protons of barbituric acid moiety appeared as two broad singlets at δ 11.06 and 11.76.
Nitrogen bases such as hydrazines react with α,β-unsaturated carbonyl compounds to give pyrazolinesCitation31. With the objective of synthesizing pyrazolines containing chromone moiety, chromonyl chalcones 3a-b were allowed to react with different hydrazines in conventional method using acetic acid as solvent and under solvent-free heating conditions. As expected, pyrazolines 4a-e were obtained from hydrazine hydrate, hydrazinobenzothiazole and phenylhydrazine (Scheme 1) and the results were comparable with reactions performed under conventional procedures. However, in case of reaction of 3a with phenylhydrazine, the mixture did not afford the desired pyrazoline, instead a bipyrazole 4f was obtained due to the ring opening reaction of pyrone ring both under conventional and thermal solvent-free methods. All the newly synthesized compounds were characterized by elemental and spectral (IR, 1H NMR and mass spectrometry) analysis.
The 1H NMR spectrum of compound 4f indicated the absence of resonances attributable to C-2 olefinic and C-5 protons of chromone moiety, whereas compound 4a/4b showed these diagnostic signals of C-2 proton as singlet at δ 8.02, 8.41 and C-5 proton as doublet at δ 8.16, 8.02 respectively. These observations suggest the opening of pyrone ring under the action of phenylhydrazine to lead the formation of bipyrazole 4f. The other prominent peaks as obtained are explained in the experimental part.
Pharmacology
The investigation of antibacterial screening data revealed that all the tested compounds 3a-4f showed moderate to good bacterial inhibition. Among the tested bacterial strains, good inhibitory results were obtained against S. pyogenes and E. coli. The structural activity study showed that chalcones 3a, 3b and their derivatives 4a-f have varying degrees of microbial inhibition. The antimicrobial activity seemed to be dependent on the nature of heterocyclic moieties. A comparative study () also revealed that chromonyl chalcones 3a-b, are more potent antibacterial agents than chromonyl pyrazolines 4a-f. Compounds 3a, 3b, 4a, 4b and 4c showed good inhibition (MIC = 25 µg/mL) against S. pyogenes. The maximum inhibition was observed in 3a and 4c against S. aureus (MRSA +ve). Except 3a and 3b all other compounds showed moderate to less activity results against P. aeruginosa, K. pneumoniae and E. coli bacterial strains. The MICs i.e., the lowest concentration of the compounds to inhibit the growth of bacteria completely were in the range of 25–100 µg/mL. The minimum bactericidal concentrations (MBCs) i.e., the lowest concentration of the compounds for killing the bacteria completely were found to be two, three or four folds higher than the corresponding MIC results.
The antifungal screening data of the compounds also revealed good to moderate activity. Among the tested fungal strains, good inhibitory results were obtained against C. albicans, and A. fumigatus. Again the comparative study () revealed that chromonyl chalcones 3a-b, were found to have better inhibitory action than pyrazolines 4a-f. The compounds 3a, 3b, 4a, 4b and 4d showed effective inhibition results (MIC = 12.5–25 µg/mL) against C. albicans. The maximum inhibition was observed in 3a, 3b and 4a against A. fumigatus, and T. mentagrophytes fungal cultures. The inhibitory activity against P. marneffei was significantly higher for the compounds 3a, 3b and 4c than the other tested compounds. Compounds 4c-f were moderately active against most of the fungal strains. The MICs, i.e., the lowest concentration of the compounds to inhibit the growth of fungi completely were in the range of 12.5–100 µg/mL. The MFCs, i.e., the lowest concentration of the compounds for killing the fungi completely were found to be two, three or four folds higher than the corresponding MIC results.
Considering the results obtained from antifungal and antibacterial tests together, it is noteworthy to mention that tested compounds are more active towards fungi than bacteria. Thus, the nature of heterocycles and basic skeleton of molecule have significant influence on the extent of antibacterial and antifungal activities. A comparative study of the activity results ( and ) with standard drugs (ciprofloxacin, griseofulvin) revealed that none of the compound exceeds the activity of commercial drugs. However, compounds have produced the marked enhancement in the potency of these analogues as antibacterial and antifungal agents.
Molecular targets of the synthesized compounds on the bacteria and fungi were identified using BioSpec Module of Raasi Suite. Every molecule has multiple targets on bacteria and fungi. The synthesized molecules have the following molecular targets: ATP-binding cassette, sub-family C, member 1 isoform 1 inhibitor, Pyruvate kinase, muscle isoform M2 activator, Eukaryotic translation initiation factor 4γ, 1 isoform 4 inhibitor.
Conclusions
In summary, a clean and convenient synthesis of novel chromone derivatives has been developed. The procedure offers several advantages including mild reaction conditions as well as simple experimental and product isolation procedures, thus, making the current “green protocol” as a useful and attractive methodology for the synthesis of series of novel heterocycles in excellent yields from cheap and readily available starting materials. The antibacterial, antifungal screening data revealed that newly generated compounds are good antimicrobial agents. The newly generated compounds can be used as template for future development through modification and derivatization to design more potent and selective antimicrobial agents.
Acknowledgments
The authors are thankful to University Grants Commission, New Delhi for providing financial assistance in the form of major research project [F.No. 37-15/2009 (SR)], SAIF, CDRI, Lucknow, India for providing analytical, spectral data and Mr Sameer Chaudhary, MD RASA Life Science Informatics, Pune, India for providing drug target identification using BioSpec Module of Raasi Suite.
Declaration of Interest
The authors have declared no conflicts of Interest.
References
- Oliveira RDR, Maffei CML, Martinez R. Infecção urinária hospitalar por leveduras do gênero Candida. Rev Assoc Med Bras 2001;47:231–235.
- Diekema DJ, BootsMiller BJ, Vaughn TE, Woolson RF, Yankey JW, Ernst EJ et al. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin Infect Dis 2004;38:78–85.
- Georgopapadakou NH. Infectious disease 2001: drug resistance, new drugs. Drug Resist Updat 2002;5:181–191.
- Zhang W, Berberov EM, Freeling J, He D, Moxley RA, Francis DH. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect Immun 2006;74:3107–3114.
- Shaheen HI, Khalil SB, Rao MR, Abu Elyazeed R, Wierzba TF, Peruski LF Jr et al. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J Clin Microbiol 2004;42:5588–5595.
- Puerto AS, Fernández JG, del Castillo Jde D, Pino MJ, Angulo GP. In vitro activity of beta-lactam and non-beta-lactam antibiotics in extended-spectrum beta-lactamase-producing clinical isolates of Escherichia coli. Diagn Microbiol Infect Dis 2006;54:135–139.
- Nolan CM, Chalhub EG, Nash DG, Yamauchi T. Treatment of bacterial meningitis with intravenous amoxicillin. Antimicrob Agents Chemother 1979;16:171–175.
- Harbottle H, Thakur S, Zhao S, White DG. Genetics of antimicrobial resistance. Anim Biotechnol 2006;17:111–124.
- Berger S. Incidence of severe side effects during therapy with sulfonylurea and biguanides. Horm Metab Res 1985;17:111–115.
- Barton D, Ollis WD. (1979). Comprehensive Organic Chemistry,vol 1–6. Oxford: Pergamon.
- Chemler YY, Leonard E, Koffas MAG. Combinatorial mutasynthesis of flavonoid analogues from acrylic acids in microorganisms. Org Lett 2007;9:1855–1858.
- Prakash O, Kumar R, Parkash V. Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl) chromones. Eur J Med Chem 2008;43:435–440.
- Kuroda M, Uchida S, Watanabe K, Mimaki Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry 2009;70:288–293.
- Larget R, Lockhart B, Renard P, Largeron M. A convenient extension of the Wessely-Moser rearrangement for the synthesis of substituted alkylaminoflavones as neuroprotective agents in vitro. Bioorg Med Chem Lett 2000;10:835–838.
- Yu D, Chen CH, Brossi A, Lee KH. Anti-AIDS agents. 60. Substituted 3′R,4′R-di-O-(-)-camphanoyl-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DCP) analogues as potent anti-HIV agents. j Med Chem 2004;47:4072–4082.
- Valenti P, Bisi A, Rampa A, Belluti F, Gobbi S, Zampiron A et al. Synthesis and biological activity of some rigid analogues of flavone-8-acetic acid. Bioorg Med Chem 2000;8:239–246.
- Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem Rev 2003;103:893–930.
- Nowaskowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 2007;42:125–137.
- Acharya BN, Saraswat D, Tiwari M, Shrivastava AK, Ghorpade R, Bapna S et al. Synthesis and antimalarial evaluation of 1, 3, 5-trisubstituted pyrazolines. Eur J Med Chem 2010;45:430–438.
- Tanaka K, Toda F. Solvent-free organic synthesis. Chem Rev 2000;100:1025–1074.
- Cave GW, Raston CL, Scott JL. Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem Commun (Camb) 2001;2159:2169.
- Toda F. (2005). Solid State Reaction topics in Current Organic Chemistry. Berlin, Heidelberg, New York: Springer.
- Wang GW. (2004). Fullerene Mechanochemistry. In: Nalwa HS, ed. Encyclopedia of Nanoscience and Nanotechnology, vol. 3. Stevenson Ranch: American Scientific Publishers, 557.
- Nohara A, Umetani T, Sanno Y. A facile synthesis of 4-oxo-4H-1-benzopyran-3- carboxaldehydes by vilsmeier reagents. Tetrahedron 1974;30:3353–3561.
- Singh SP, Sehgal S, Tarar LS, Dhawan SN. Synthesis of 2-[3-methyl or trifluromethyl-5-(2-thienyl)-pyrazol-1-yl]thiazol and benzothiazoles. Indian J Chem 1990;29B:310–314.
- Jursic BS, Neumann DM. Preparation of 5-formyl and 5-acetyl barbituric acids, including the corresponding Schiff bases and phenyl hydrazones. Tetrahedron Lett 2001;42:8435–8439.
- Cruickshank R, Duguid JP, Marmion BP, Swain RHA. (1975). Medicinal Microbiology. 12th edn, vol. II, London: Churchill Livingstone, 196–202.
- Collins AH. (1976). In: Microbiological Methods, second edn. London: Butterworth.
- Khan ZK. In vitro and vivo screening techniques for bioactivity screening and evaluation, Proc Int Workshop UNIDO-CDRI 1997:210–211.
- Varma RS.(1998). Antifungal Agents: Past, Present and Future prospects. Lucknow, India: National Academy of Chemistry & Biology.
- Wiley RH, Jarboe CH. The Chemistry of Heterocyclic compounds. Interscience, NY: 1967;22:183.
