Abstract
Seven novel 6-aryl-2-(p-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-ones (2a-g) were synthesized by the condensation of appropriate aroylpropionic acid and 4-hydrazinobenzenesulfonamide hydrochloride in ethanol. Structure of all compounds have been elucidated by elemental analysis, IR, 1H NMR, 13C NMR, DEPT and MS spectrscopy. These compounds were tested for their anti-inflammatory activity in carrageenan-induced rat paw edema model. Compound 2b exhibited anti-inflammatory activity comparable to that of celecoxib (at 5 h). Two other compounds 2d and 2g showed promising anti-inflammatory activity (edema reduction more than 80% at 5 h). These compounds (2b, 2d and 2g) did not produce any ulceration in gastric region.
Introduction
Pyridazin-3(2H)-one derivatives represent one of the most active class of compounds possessing a wide spectrum of pharmacological activities ranging from cardiovascular properties, anti-inflammatory, antidiabetic, analgesic, anti-AIDs, anticancer and anticonvulsant activitiesCitation1,Citation2. Pyridazinone derivatives also possess affinity for benzodiazepine receptorsCitation3 and the ability to inhibit the human matrix metalloproteinaseCitation4 and aldose reductaseCitation5 enzymes. Recently, these derivatives have been reported as potent hepatoprotective agentsCitation6, antibacterial and antifungal agentsCitation7, COX-2 inhibitorsCitation8, platelet aggregation inhibitorCitation9–12, phosphodiesteraseCitation13,Citation14. They also act as cytotoxic (cell-killing) agents and anti-hormonal drugs, which reduce the proliferation of the tumorsCitation15–16.
The sulfonamides constitute an important class of drugs, with several types of pharmacological agents possessing antibacterialCitation17, anti-carbonic anhydraseCitation18,Citation19, diureticCitation18,Citation20, hypoglycemicCitation21, antithyroidCitation22, and antiprotons activitiesCitation23–25. A large number of structurally novel sulfonamide derivatives have recently been reported to show substantial antitumor activity, both in vitro and/or in vivo. Although they have a common chemical motif of aromatic/heterocyclic sulfonamide, there are a variety of mechanisms of their antitumor action, most of them poorly understood at this moment. Some of these derivatives are currently being evaluated in clinical trials, and there is much optimism that they may lead to novel alternative anticancer drugs, devoid of the side effects of the presently available pharmacological agentsCitation26. Anti-inflammatory activity of celecoxib (celebrex) which was used clinically in the 1990s is attributed to the presence of SO2NH2 substituted at the para position of one aryl groupsCitation27,Citation28.
Therefore, it was thought worthwhile to synthesize novel pyridazinone derivatives bearing sulphonamide moiety and screen them for the anti-inflammatory activity.
Results and discussion
Synthesis of compounds
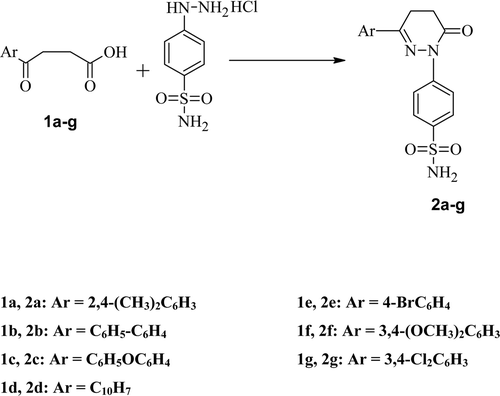
The synthetic route used to synthesize title compounds (2a-j) is outlined in . Various intermediates 1,4-ketoacids (β-aroylpropionic acids) required for the synthesis of pyridazinones were prepared by Friedel-Crafts succinoylation of substituted aromatic compoundsCitation29. The cyclization to pyridazinone derivatives bearing a benzene sulfonamide moiety was afforded by the condensation of appropriate aroylpropionic acid and 4-hydrazinobenzenesulfonamide hydrochloride in ethanol in 60–75% yield. The structures of pyridazinone derivatives (2a-g) were determined by elemental analysis, IR, 1H NMR, 13C NMR, DEPT and MS.
The structure was supported by the evidence of presence of prominent bands in IR spectra for NH2 (3284–3449, 3019–3298 and 2916–2954), cyclic carbonyl (1637–1657), C=N (1462–1595) and SO2N< (1310–1377 and 1151–1189) groups. The structure was further established by 1HNMR spectral data. The signal for SO2NH2 was observed as two-proton singlet at δ 7.10–7.98. Two triplets each integrating for two protons observed at δ 2.74–2.87 and δ 3.03–3.21 indicate the presence of−CH2–CH2– system of dihydropyridazinone ring. DEPT spectra indicate the presence of CH3, CH2 and CH carbons. Elemental analysis (C, H, N and S) data were within ±0.4 of the theoretical values.
Biological activity
All compounds (2a-g) where tested for anti-inflammatory activity by using carrageenan-induced rat hind paw edema methodCitation30. All the pyridazinone derivatives (2a-g) exhibited varying degree of anti-inflammatory activity (23.3–78.9% at 3 h and 52.4–88.1% at 5 h) (). Compound 2b has shown maximum activity at 5h (comparable to that of celecoxib at 5 h). Compound 2d and 2g showed promising activity.
Table 1. Effect of pyridazinone derivatives on carrageenan induced hind paw edema in rats.
No specific structural activity relationship was established from the data shown in .
Acute gastric ulcerogenic effect of the compound 2b, 2d and 2g at 60 mg/kg (three times) was evaluated in Wistar ratsCitation31. No ulceration in gastric region was observed.
Experimental
Melting points were determined by open capillary tubes and are uncorrected. All the Fourier Transform Infra Red spectra were recorded on a Brukers Vector 22 spectrophotometer in film; υmax values are given in cm−1. 1H NMR spectra were recorded on a Bruker Spectrospin DPX 300-MHz spectrometer using deuterated DMSO as a solvent and tetramethyl silane (TMS) as an internal standard. Chemical shifts are given in δ (ppm) scale and coupling constants (J values) are expressed in Hz. Mass spectra were scanned by affecting FAB ionization JEOL-JMS-DX 303 system, equipped with direct inlet probe system and ESI Bruker Esquire 3000. The m/z values of the more intense peaks are mentioned. 13C NMR and DEPT spectra were recorded on Bruker spectrospin DPX 400 MHz and 500 MHz using deuterated DMSO as a solvent and TMS as internal standard. Purity of the compounds was checked on TLC plates (silica gel G) which were visualized by exposing to iodine vapors. Elemental analysis was carried out on CHNS Elementar (Vario EL III).
Procedure for the synthesis of Aroylpropionic acids (1a-g)
To liquid aromatic hydrocarbon (30 mL), anhydrous aluminium chloride (16.6 g, 0.125 mole) was added. It was stirred on magnetic stirrer at room temperature for 30 min. To it succinic anhydride (5 g, 0.05 mol) was added in five portions with continuous stirring. Vigorous reaction started with evolution of HCl gas. Stirring was continued for another 6 h at room temperature. It was left at room temperature for 48 h and then decomposed by adding ice-cold hydrochloric acid (50%, 100 mL). The excess solvent was removed by steam distillation. The solid precipitated out was treated with aqueous saturated sodium bicarbonate solution and filtered. Filtrate was acidified with dilute HCl (4% v/v) to give a precipitate. It was filtered and residue was washed with cold water, dried and crystallized from the appropriate solvent to give (1a-g)Citation29.
General Procedure for the synthesis of pyridazinones (2a-g)
A mixture of appropriate aroylpropionic acid (1a-g) (0.001 mol) and 4-hydrazinobenzenesulfonamide hydrochloride (0.001 mol) in absolute ethanol (20–30 mL) was refluxed for 18–24 h. The reaction mixture was concentrated to one-third of its volume and left at room temperature when a solid separated out. Crude product was filtered off, washed with little volume of alcohol. The residue thus obtained was stirred with 5% sodium bicarbonate solution (25 mL) for 1h. It was filtered, washed with 2% acetic acid and then with water. It was dried and crystallized from methanol to give (2a-g).
6-(2′,4′-Dimethylphenyl)-2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2a)
Yield = 61%, m.p. 156–1580C, Rf = 0.61 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3304, 3232 and 2920 (NH2), 1649 (C=O), 1310 and 1185 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 2.30 (3H, s, CH3), 2.41 (3H, s, CH3), 2.78 & 3.03 (each t, 2x-CH2-), 7.11 (2H, s, SO2NH2), 7.38 (3H, m, H-2′, H-5′, H-6′), 7.72 (2H, d, J = 8.59 Hz, H-3′′, H-5′′), 7.85 (2H, d, J = 8.59 Hz, H-2′′, H-6′′). ESI+ (m/z): 356 (M+-1), 380 (M++Na). 13C NMR (DMSO, δ): 20.92 (-CH3, C-3′), 22.19 (-CH3, C-4′), 27.42 (-CH2 of pyridazinone), 46.16 (-CH2 of pyridazinone), 165.73 (C=N), 177.95 (C=O). DEPT (DMSO, δ): 19.036 (2x-CH3, C-3′, C-4′), 27.875 (-CH2 of pyridazinone), 46.680 (-CH2 of pyridazinone). Anal. Calcd. For C18H19N3O3S, Calculated: C = 60.49, H = 5.36, N = 11.76, S = 8.97, Found: C = 60.87, H = 4.98, N = 12.02, S = 9.13.
6-(4′-Phenylphenyl)- 2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2b)
Yield = 55%, m.p. >260°C, Rf = 0.70 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3317, 1657 (C=O), 1595 (C=N), 1326 and 1151 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 2.80 (2H, t, CH2 of pyridazinone), 3.21 (2H, t, CH2 of pyridazinone), 7.40-7.98 (15H, m, N-phenyl protons, biphenyl protons, SO2NH2). ESI+ (m/z): 405 (M+), 406 (M+ +1), 407 (M+ +2). 13C NMR (DMSO, δ): 24.4 (-CH3), 32.4 (-CH2 of pyridazinone), δ 32.4 (-CH2 of pyridazinone), 152.50 (C=N), 165.70 (C=O). DEPT (DMSO, δ): 22.20 (-CH2 of pyridazinone), 27.44 (-CH2 of pyridazinone). Anal. Calcd. For C22H19N3O3S, Calculated: C = 65.17, H = 4.72, N = 10.36, S = 7.91, Found: C = 65.12, H = 4.69, N = 10.36, S = 7.89.
6-(4′-phenoxyphenyl)- 2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2c)
Yield = 65%, m.p. 235–2360C, Rf = 0.57 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3449 (NH2), 1637 (C=O), 1329 and 1153 cm−1 (SO2N). 1H NMR (300 MHz, DMSO,d): 2.80 (2H, t, -CH2 of pyridazinone), 3.18 (2H, t, -CH2 of pyridazinone), 7.10- 7.94 (15H, m, SO2NH2, phenyl phenoxy & N-phenyl). ESI+ (m/z): 421 (M+), 422 (M+ +1), 423 (M+ +2), 299. 13C NMR (DMSO, δ): 22.47 (-CH2 of pyridazinone), 27.59 (-CH2 of pyridazinone), 152.33 (C=N), 165.56 (C=O). DEPT (DMSO, δ): 22.23 (-CH2 of pyridazinone), 27.46 (-CH2 of pyridazinone). Anal. Calcd. for C22H19N3O4S, Calculated: C = 62.69, H = 4.54, N = 9.97, S = 7.61, Found: C = 62.63, H = 4.51, N = 9.96, S = 7.60.
6-(β-napthyl)-2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one(2d)
Yield = 65%, m.p. 252–2540C, Rf = 0.57 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3298 (NH2), 1657 (C=O), 1590 (C=N), 1377 and 1189 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 2.87 (2H, t, -CH2 of pyridazinone), 7.60-8.21 (10H, m, N-phenyl and napthelene protons except H-α), 7.43 (2H, s, SO2NH2), 8.44 (1H, s, H-α). ESI+ (m/z): 379 (M+), 337, 300. 13C NMR (DMSO, δ): 22.82 (-CH2 of pyridazinone), 28.07 (-CH2 of pyridazinone), 153.19 (C=N), 166.20 (C=O). DEPT (DMSO, δ): 20.76 (-CH2 of pyridazinone), 27.47 (-CH2 of pyridazinone). Anal. Calcd. For C20H17N3O3S, Calculated: C = 63.31, H = 4.52, N = 11.07, S = 8.45, Found: C = 63.25, H = 4.48, N = 11.07, S = 8.43.
6-(4′-bromophenyl)-2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2e)
Yield = 55%, m.p. 241–2420C, Rf = 0.59 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3312, 3019 and 2954 (NH2), 1651 (C=O), 1462 (C=N), 1338 and 1154 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 2.76 (2H, t, -CH2 of pyridazinone), 3.14 (2H, t, -CH2 of pyridazinone), 7.37 (2H, s, SO2NH2), 7.64− 7.87 (8H, m, N-phenyl protons & bromophenyl protons). ESI+ (m/z): 409 (M++1), 431 (M++Na), 407 (M+-1), 405, 378, 338. 13C NMR (DMSO, δ): 22.11 (-CH2 of pyridazinone), 27.28 (-CH2 of pyridazinone), 151.79 (C=N), 165.57 (C=O). DEPT (DMSO, δ): 22.18 (-CH2 of pyridazinone), 27.35 (-CH2 of pyridazinone). Anal. Calcd. For C16H14BrN3O3S, Calculated: C = 47.07, H = 3.46, N = 10.29, S = 7.85, Found: C = 47.03, H = 3.43, N = 10.29, S = 7.84.
6-(3′,4′-dimethoxyphenyl)-2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2f)
Yield = 65%, m.p. 203–2040C, Rf = 0.5 (toluene: ethyl formate: formic acid, 7.5: 2: 0.5). 1H NMR (300 MHz, DMSO, δ): 2.74 (2H, t, -CH2 of pyridazinone), 3.13 (2H, t, -CH2 of pyridazinone), 3.79 (6H, s, 2 × OCH3), 7.02 (1H, d, J= 8.4 Hz, H-5′), 7.39 (4H, m, H-2′, H-6′, SO2NH2), 7.82 (4H, m, N-phenyl protons). ESI+ (m/z): 389 (M+), 388 (M+-1), 412 (M++Na), 325, 311, 255. 13C NMR (DMSO, δ): 22.16 (-CH2 of pyridazinone), 27.6 (-CH2 of pyridazinone), 55.55 (OCH3,C-3′ and C-4′), 148.68 (C=N), 165.74 (C=O). DEPT (DMSO, δ): 22.23 (-CH2 of pyridazinone), 27.63 (-CH2 of pyridazinone), δ 55.615 (2x OCH3, C-3′, C-4′). Anal. Calcd. for C18H19N3O5S, Calculated: C = 55.52, H = 4.92, N = 10.79, S = 8.23, Found: C = 55.47, H = 4.88, N = 10.78, S = 8.22.
6-(3′,4′-dichlorophenyl)-2-(4-sulfamoylphenyl)-4,5-dihydropyridazin-3(2H)-one (2g)
Yield = 70%, m.p. 260–2610C, Rf = 0.70 (toluene: ethyl acetate: formic acid, 5: 4: 1). IR υmax (Solvent, in cm−1): 3284, 3218 and 2916 (NH2), 1643 (C=O), 1468 (C=N), 1336 and 1151 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 2.74 (2H, t, -CH2 of pyridazinone), 3.15 (2H, t, -CH2 of pyridazinone), 7.37 (2H, s, SO2NH2), 7.71-7.87 (6H, m, N-phenyl protons, H-5′, H-6′), 8.05 (1H, s, H-2′). ES+-MS (m/z): 397 (M+), 399 (M+2), 365, 348. 13C NMR (DMSO, δ): 22.78 (-CH2 of pyridazinone), 28.07 (-CH2 of pyridazinone), 153.19 (C=N), 166.20 (C=O). DEPT (DMSO, δ): 26.394 (-CH2 of pyridazinone), 28.225 (-CH2 of pyridazinone). Anal. Calcd. for C16H13Cl2N3O3S, Calculated: C = 48.25, H = 3.29, N = 10.55, S = 8.05, Found: C = 48.21, H = 3.26, N = 10.55, S = 8.04.
Anti-inflammatory activity
Carrageenan-induced hind paw edema method was used for evaluating anti-inflammatory activityCitation30. Wistar rats (either sex) weighing 140–180 g were procured from Central Animal House facility of Jamia Hamdard, New Delhi (Registration no. 173/CPCSEA). The experiments were performed in accordance with the guidelines for the care and use of laboratory animals, laid down by the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA), Ministry of Social Justice and Empowerment, Govternment of India, January 2000. Overnight fasted rats (16 h) were divided into groups of six animals each. One group of rats, which served as control was given vehicle (1% Carboxymethyl cellulose (CMC) in water in a volume of 10 mL/kg p.o.) only. Test compounds (20 mg/kg body weight.) and celecoxib (20 mg/kg b.w.) suspended in vehicle (10 mL/kg) were administered orally to respective groups. After 30 min, all animals were injected with 0.1 mL of 1% carrageenan solution (prepared in normal saline) in the subplantar aponeurosis of left hind paw to induce inflammation and the volume of injected paw was measured by using plethysmometer immediately (at 0 h). The paw volume was again measured after 3 h and 5 h. The average paw volume in a group of treated rats was compared with vehicle (control group) and the percentage inhibition of edema was calculated by using the formula:
Where Vt is the mean paw volume of the treated rats and Vc is the mean paw volume of the control.
Ulcerogenic activity
Acute gastric ulcerogenic effect of the compound 2b, 2d and 2g was evaluated in Wistar ratsCitation31. Albino rats of Wistar strain (150–200 g) fasted over 24 h were randomly allotted into three groups of six animals each. The animals of one group were given vehicle 10 mL/kg (CMC 1% w/v in distilled water) orally. The compound 2b, 2d and 2g standard drug (60 mg/kg) suspended in vehicle was administered orally in a volume of 10 mL/kg to the animals. They were scarified under deep ether anesthesia after 6 h of the oral treatment at a dose of 60 mg/kg. Their stomach were removed and opened through greater curvature for examining lesions or bleedings.
Conclusions
The structures proposed to the synthesized compounds (2a-g) are well supported by spectroscopic data and elemental analysis. Among the synthesized compounds 2b exhibited potent anti-inflammatory activity which is comparable to that of celecoxib (at 5 h). Other two compounds (2d and 2g) were found to have promising anti-inflammatory activity (edema reduction more than 80% at 5 h). These compounds (2b, 2d and 2g) did not produce any ulceration in gastric region.
Acknowledgments
This work was supported by Grant No. 32–228/2006 (SR) from the University Grants Commission, New Delhi, India. One of the authors, Rafia Bashir is thankful to UGC for fellowship. Thanks are due to SAIF, CDRI, Lucknow, for providing Mass spectra.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Siddiqui AA, Kushnoor A, and Wani SM. Synthesis and hypotensive activity of some 6-(substituted aryl)-4-methyl-2,3-dihydropyridazin-3-ones. Indian J Chem-B 2004;43B:1574–1579
- Banerjee PS, Sharma PK, and Nema RK. Synthesis and anticonvulsant activity of pyridazinone derivatives. Int J Chem Tech Research 2009;1:522–525
- Tanaka H, Kirihara S, Yasumatsu H, Yakushiji T, and Nakao T. Synthesis and evaluation of novel 2-aryl-2,5,6,7-tetrahydro-3H-thieno[2′,3′:6,7]cyclohepta[1,2-c]pyridazin-3-ones and 2-aryl-5,6-dihydrothieno[2,3-h]cinnolin-3(2H)-ones as anxiolytics. Eur J Med Chem 1997;32:607–615.
- Pinna GA, Curzu MM, Murineddu G, Chelucci G, Cignarella G, Menta E et al. Preparation of thieno[3,2-h]cinnolinones as matrix metalloproteinase inhibitors. Arch Pharm (Weinheim) 2000;333:37–47.
- Pau A, Asproni B, Boatto G, Grella GE, De Caprariis P, Costantino L et al. Synthesis and aldose reductase inhibitory activities of novel thienocinnolinone derivatives. Eur J Pharm Sci 2004;21:545–552.
- Kwon SK, Moon A. Synthesis of 3-alkylthio-6-allylthiopyridazine derivatives and their antihepatocarcinoma activity. Arch Pharm Res 2005;28:391–394.
- Sönmez M, Berber I, Akbas E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. Eur J Med Chem 2006;41:101–105.
- Harris RR, Black L, Surapaneni S, Kolasa T, Majest S, Namovic MT et al. ABT-963 [2-(3,4-difluoro-phenyl)-4-(3-hydroxy-3-methyl-butoxy)-5-(4-methanesulfonyl-phenyl)-2H-pyridazin-3-one], a highly potent and selective disubstituted pyridazinone cyclooxgenase-2 inhibitor. J Pharmacol Exp Ther 2004;311:904–912.
- Estevez I, Ravina E, Sotelo E. Synthesis of 6-aryl-5-amino-3(2H)-pyridazinones as potential platelet aggregation inhibitors. J Heterocyclic Chem 1998;35:1421–1428.
- Haider N, Hartmann RW, Steinwender A. Synthesis of 2- [2-(1-imidazolyl)ethyl]-4-phenylcycloalka [ g ] phthalazin-1(2H)-ones as thromboxane A 2 synthase inhibitors, Arch Pharm Pharm Med Chem 1999;332:408–409.
- Amgad G, Habeeb PN, Parveen R, Edward EK. Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: Replacement of sulphonamide and methylsulfonyl pharmacophores by an azido bioisosters. J Med Chem 2001;44:3039–3042.
- Sotelo E, Fraiz N, Yáñez M, Laguna R, Cano E, Brea J et al. Pyridazines. Part 28: 5-alkylidene-6-phenyl-3(2H)-pyridazinones, a new family of platelet aggregation inhibitors. Bioorg Med Chem Lett 2002;12:1575–1577.
- Coelho A, Sotelo E, Fraiz N, Yáñez M, Laguna R, Cano E et al. Pyridazines. Part 36: Synthesis and antiplatelet activity of 5-substituted-6-phenyl-3(2H)-pyridazinones. Bioorg Med Chem Lett 2004;14:321–324.
- Van der Mey M, Boss H, Couwenberg D, Hatzelmann A, Sterk GJ, Goubitz K et al. Novel selective phosphodiesterase (PDE4) inhibitors. 4. Resolution, absolute configuration, and PDE4 inhibitory activity of cis-tetra- and cis-hexahydrophthalazinones. J Med Chem 2002;45:2526–2533.
- Van der Mey M, Bommelé KM, Boss H, Hatzelmann A, Van Slingerland M, Sterk GJ et al. Synthesis and structure-activity relationships of cis-tetrahydrophthalazinone/pyridazinone hybrids: a novel series of potent dual PDE3/PDE4 inhibitory agents. J Med Chem 2003;46:2008–2016.
- Gilchrest BA, Eller MS. Cancer therapeutics: smart and smarter. Drugs of the Future 2009;34:205–216.
- Pau A, Murineddu G, Asproni B, Murruzzu C, Grella GE, Pinna GA et al. Synthesis and cytotoxicity of novel hexahydrothienocycloheptapyridazinone derivatives. Molecules 2009;14:3494–3508.
- Drew J. Drug discovery: A historical perspective. Science 2000;287:1960–1964.
- Supuran CT and Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Exp Opin Ther Patents 2000;10:575–600.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem Immunol Endoc Metab Agents 2001;1:61–97.
- Maren TH. Relations between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 1976;16:309–327.
- Boyd AE 3rd. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 1988;37:847–850.
- Thornber CW. Isosterism and molecular modification in drug design. Chem Soc Rev 1979;8:563–580.
- Ogden RC, Flexner CW. (2001). Protease Inhibitors in AIDS Therapy. New York, Basel: Marcel Dekker.
- Supuran C, Scozzafava A, Mastrolorenzo A. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Expert Opin Ther Patents 2001;11:221–259.
- Scozzafava A, Supuran CT. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 2000;43:3677–3687.
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002;2:55–75.
- Khanna IK, Weier RM, Yu Y, Collins PW, Miyashiro JM, Koboldt CM et al. 1,2-Diarylpyrroles as potent and selective inhibitors of cyclooxygenase-2. J Med Chem 1997;40:1619–1633.
- Bashir R. (2010). Thesis, Synthesis of novel heterocyclic compounds of pharmaceutical interest. New Delhi, India: Jamia Hamdard.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547.
- Rathish IG, Javed K, Ahmad S, Bano S, Alam MS, Pillai KK et al. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 2009;19:255–258.