Abstract
Novel chemotypes with carbonic anhydrase (CA; EC 4.2.1.1) inhibitory action, in addition to the sulphonamide and sulphamate were discovered, many of which are based on natural products. Caffeine and piperine were extracted and tested for inhibition of the human (h) cytosolic isoforms hCA I and II. The IC50 values of caffeine against hCA I was of 55 mM, whereas that of piperine of 60 mM. The IC50 values of caffeine and piperine against hCA II were of 2 mM. Although these are quite weak inhibitors they may constitute leads for developing tighter binding compounds.
Introduction
Carbonic anhydrases (CAs; also known as carbonate dehydratases EC 4.2.1.1) are ubiquitous metalloenzymes present in prokaryotes and eukaryotes that are encoded by five evolutionarily unrelated gene families. These are the α-CAs (present in vertebrates, bacteria, algae and cytoplasm of green plants); the β-CAs (predominantly in bacteria, algae and chloroplasts of monodicotyledons and dicotyledons); the γ-CAs (mainly in archaea and some bacteria); and the δ-CAs and ζ-CAs (present in some marine diatoms)Citation1–7. In mammals, 16 α-CA isozymes or CA-related proteins with different catalytic activity, subcellular localization and tissue distribution are thereCitation8–25.
CAs catalyze a simple physiological reaction the conversion of CO2 to the bicarbonate ion and protons. The active site of most CAs contains a zinc ion (Zn2+), which is essential for catalysis. The CA reaction is involved in many physiological and pathological processes, including respiration and transport of CO2 and bicarbonate between metabolizing tissues and lungs; pH and CO2 homeostasis; electrolyte secretion in various tissues and organs; biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis); bone resorption, calcification, and tumorigenicityCitation8–18.
Several classes of CA inhibitors (CAIs) are known: the metal-complexing anions and the unsubstituted sulphonamides and their bioisosteres, for example, sulphamates and sulphamides compounds, the coumarins and the polyaminesCitation1–25. In addition to the sulphonamide and sulphamate, natural products such as phenols/polyphenols, phenolic acids, and coumarins were recently investigated in detail as CAIsCitation1–25. Their detailed mechanism of inhibition has been explained by means of kinetic and x-ray crystallographic studies and can be used for the rational drug design of other agentsCitation26–33.
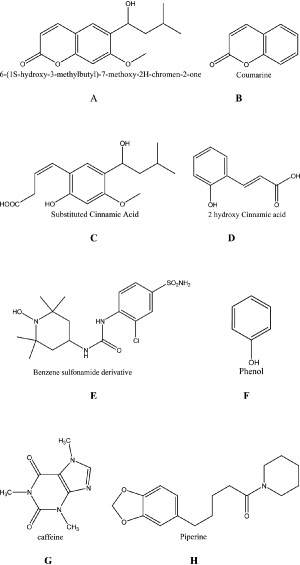
Structure A-H
It was also be found that coumarins A and B and hydrolyzed coumarins C and D were potent inhibitors against some investigated human CA isoforms, which makes this entire class of derivatives of paramount interest for designing novel applications for the CAIs. The binding of the hydrolyzed coumarins C and D to hCA II where the structures of a benzene sulphonamide CAI E and of simple phenol F are also presented, stressing the novelty of the binding mode of this chemotype to the enzyme, in comparison to the classical inhibitors (sulphonamides, which interact with the zinc ion) or phenols F (which interact with the zinc-coordinated water molecule)Citation27,Citation28.
Natural products were less investigated as CAIsCitation26 and we decided to undergo such a work. Caffeine G and piperine H have the C=O functional group as coumarins, which creates a novel interest for the hCA inhibition study.
Materials and methods
Caffeine G and piperine H were isolated from leaves of Camellia sinensis family Theaceae and fruits of Piper nigrum family Piperaceae, respectivelyCitation34,Citation35. The purity of the compounds has determined by finding out the melting point and thin-layer chromatography (TLC) by comparing the melting point and Rf factor with pure compoundsCitation36,Citation37. The melting point of caffeine and piperine were found to be 225–230°C and 129–132°C, respectively which almost matches the theoretical melting point of it. The TLC of caffeine determined by taking 1.5 × 8 cm TLC plate, TLC tank, lid, ultraviolet (UV) lamp and TLC solvent (5% acetic acid in ethyl acetate). The TLC analysis of a known sample of pure caffeine resulted in a spot with a Rf value of 0.23, then it is reasonably conclude that the compounds present in the unknown sample is caffeine which have the Rf value of 0.24. The TLC of piperine determined by taking 1.5 × 8 cm TLC plate, TLC tank, lid, UV lamp and TLC solvent (toluene:ethyl acetate in 70:3 ratios). The standard Rf value of pure piperine was 0.25. The Rf value of purified piperine from TLC was found to be 0.24. So it was confirmed that the product obtained from the black pepper powder contains piperine.
Both the isolated caffeine G and piperine H were tested against isozymes (of human origin) hCA I, hCA II (University of Florence, Dipartimento di Chimica Bioinorganica, Florence, Italy)Citation38.
Enzyme inhibition of caffeine and piperine on hCA I and hCA II
An SX.18MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic/inhibition of various CA isozymes as reported by KhalifahCitation38. Phenol Red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.4) as buffer, 0.1 M Na2SO4 or NaClO4 (for maintaining constant the ionic strength; these anions are not inhibitory in the used concentration), following the CA-catalyzed CO2 hydration reaction for a period of 5–10 s. Saturated CO2 solutions in water at 25°C were used as substrate. Stock solutions of inhibitors were prepared at a concentration of 10 mM (in dimethyl sulphoxide-water 1:1, v/v) and dilutions up to 1 mM done with the assay buffer mentioned above. At least four different inhibitor concentrations have been used for measuring the inhibition constant. Inhibitor and enzyme solutions were preincubated together for 10 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. Triplicate experiments were done for each inhibitor concentration, and the values reported throughout the paper are the mean of such results. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlier, and represent the mean from at least three different determinations. All CA isozymes used here were recombinant proteins obtained as reported earlier by our groupCitation38–40. As seen in , several millimolar CAIs against the cytosolic isoforms hCA I and hCA II, have been determined.
Table 1. Enzyme inhibition of caffeine and piperine on hCA I and hCA II by a stopped-flow CO2 hydrase assayCitation38.
Results and discussion
The IC50 values of caffeine against hCA I was found to be 55 mM which whereas that of piperine was of 60 mM. The IC50 values of caffeine against hCA II was found to be 2 mM which the same as that of piperine (IC50 values of 2 mM; ).
Acetazolamide which taken as the reference compound have the IC 50 values against hCA I and hCA II of 350 nM and 24 nM, respectively, being a highly potent inhibitor against both the isoforms. Although caffeine and piperine are much less potent, CAIs compared to acetazolamide these compounds may be used as leads for developing novel inhibitors.
The caffeine and piperine may create a novel interesting chemotypes, in addition to the sulphonamide and sulphamate and other natural products such as phenols/polyphenols, phenolic acids, and coumarins were discovered. The new applications of CAIs range from anti-glaucoma agents with topical activity, to anti-convulsants, anti-pain, anti-obesity, and anti-tumour agents/diagnostic tools for cancer. This idea is not widely accepted, there is potential to develop anti-infectives (anti-malarials, anti-fungal, and anti-bacterial agents) belonging to the CAIs, targeting enzymes from various pathogens. The pharmacological effects of caffeine and piperine still to develop clinically as hCAIs. It is thus, that the novel therapeutic applications will emerge for this natural product-based enzyme inhibitors in the near future.
Acknowledgements
This work is acknowledged to our Principal and Dean Dr. P. Suresh GITAM Institute of Pharmacy, GITAM University, Visakhapatnam who has given all the opportunity of laboratory and instruments. The authors are greatly indebted to Dr. C. T. Supuran for the enzyme assay.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Pat 2006;16:1627–1664.
- Supuran CT, Scozzafava A, Conway J. Carbonic Anhydrase—its inhibitors and Activators. Boca Raton, FL: CRC, 2004:1–363.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 2000;24:335–366.
- Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–573.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Nishimori I, Minakuchi T, Onishi S, Vullo D, Cecchi A, Scozzafava A et al. Carbonic anhydrase inhibitors: cloning, characterization, and inhibition studies of the cytosolic isozyme III with sulfonamides. Bioorg Med Chem 2007;15:7229–7236.
- Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mitochondrial isozyme V with aromatic and heterocyclic sulfonamides. J Med Chem 2004;47:1272–1279.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2005;48:7860–7866.
- Nishimori I, Minakuchi T, Onishi S, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. DNA cloning, characterization, and inhibition studies of the human secretory isoform VI, a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2007;50:381–388.
- Vullo D, Voipio J, Innocenti A, Rivera C, Ranki H, Scozzafava A et al. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2005;15:971–976.
- Nishimori I, Supuran CT, Scozzafava A, Conway J. Carbonic Anhydrase—its Inhibitors and Activators. Boca Raton, FL: CRC, 2004:25–43.
- Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S et al. Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2003;13:1005–1009.
- Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastoreková S et al. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides-a new target for the design of antitumor and antiglaucoma drugs? Bioorg Med Chem Lett 2005;15:963–969.
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem 2004;279:2719–2727.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg Med Chem Lett 2005;15:3828–3833.
- Alterio V, Vitale RM, Monti SM, Pedone C, Scozzafava A, Cecchi A et al. Carbonic anhydrase inhibitors: X-ray and molecular modeling study for the interaction of a fluorescent antitumor sulfonamide with isozyme II and IX. J Am Chem Soc 2006;128:8329–8335.
- Köhler K, Hillebrecht A, Schulze Wischeler J, Innocenti A, Heine A, Supuran CT et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–7699.
- Supuran CT, Ilies MA, Scozzafava A. Carbonic anhydrase inhibitors. Part 29. Interaction of isozymes I, II and IV with benzolamide-like derivatives. Eur J Med Chem 1998;33:739–752.
- Supuran CT, Scozzafava A, Ilies MA, Briganti F. Carbonic anhydrase inhibitors: synthesis of sulfonamides incorporating 2,4,6-trisubstituted-pyridinium-ethylcarboxamido moieties possessing membrane-impermeability and in vivo selectivity for the membrane-bound (CA IV) versus the cytosolic (CA I and CA II) isozymes. J Enzym Inhib 2000;15:381–401.
- Scozzafava A, Briganti F, Ilies MA, Supuran CT. Carbonic anhydrase inhibitors: synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus cytosolic isozymes. J Med Chem 2000;43:292–300.
- Winum JY, Temperini C, El Cheikh K, Innocenti A, Vullo D, Ciattini S et al. Carbonic anhydrase inhibitors: clash with Ala65 as a means for designing inhibitors with low affinity for the ubiquitous isozyme II, exemplified by the crystal structure of the topiramate sulfamide analogue. J Med Chem 2006;49:7024–7031.
- Saczewski F, Slawinski J, Kornicka A, Brzozowski Z, Pomarnacka E, Innocenti A et al. Carbonic anhydrase inhibitors. Inhibition of the cytosolic human isozymes I and II, and the transmembrane, tumor-associated isozymes IX and XII with substituted aromatic sulfonamides activatable in hypoxic tumors. Bioorg Med Chem Lett 2006;16:4846–4851.
- De Simone G, Vitale RM, Di Fiore A, Pedone C, Scozzafava A, Montero JL et al. Carbonic anhydrase inhibitors: Hypoxia-activatable sulfonamides incorporating disulfide bonds that target the tumor-associated isoform IX. J Med Chem 2006;49:5544–5551.
- Supuran CT. Carbonic anhydrase inhibition with natural products: novel chemotypes and inhibition mechanisms. Mol Divers 2010.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of mammalian isoforms I-XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–7428.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Innocenti A, Beyza Oztürk Sarikaya S, Gülçin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–2164.
- Innocenti A, Gülçin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–5053.
- Davis RA, Innocenti A, Poulsen SA, Supuran CT. Carbonic anhydrase inhibitors. Identification of selective inhibitors of the human mitochondrial isozymes VA and VB over the cytosolic isozymes I and II from a natural product-based phenolic library. Bioorg Med Chem 2010;18:14–18.
- Murray DS, Hansen PJ. Extraction of caffeine from coffee or tea. J Chem Educ 1995;72:851.
- Epstein WW, Netz DF, Seidel JL. Isolation of Piperine from Black Pepper. J Chem Ed 1993; 70;598–599.
- Pavlik AW. TLC detection of caffeine in commercial products. J Chem Educ 1973;50: 134–137.
- Madhavi BB, Nath AR, Banji D, Madhu NM, Ramalingam R, Swetha D. Extraction, identification, formulation and evaluation of piperine in alginate beads. International Journal of Pharmacy and Pharmaceutical Sciences 2009;1:156–161.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–2573.
- Thiry A, Rolin S, Vullo D, Frankart A, Scozzafava A, Dogné JM et al. Indanesulfonamides as carbonic anhydrase inhibitors and anticonvulsant agents: structure-activity relationship and pharmacological evaluation. Eur J Med Chem 2008;43:2853–2860.
- Innocenti A, Lehtonen JM, Parkkila S, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of the newly isolated murine isozyme XIII with anions. Bioorg Med Chem Lett 2004;14:5435–5439.
