Abstract
A series of 6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-substituted phenylmethanone/ethanone derivatives were synthesized and in vitro activity against mycobacterium tuberculosis (MTB) and INHR-MTB were carried out. Among the synthesized compounds, compound (4h) 6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-pyridyl methanone was found to be the most active agent against MTB and INHR-MTB with a minimum inhibitory concentration of 0.22 μM.
Introduction
Tuberculosis (TB) is a major disease in the world estimated to kill over 2 million people annually. The majority of cases occur in developing countries among which the sub-Sahara Africa has the highest incidence rate per capita and the Southeast Asian regions have the largest number of cases. It is estimated that there were 8.3 million new cases of TB worldwide in 2000 and that TB was the cause of 11% of all adult acquired immune deficiency syndrome (AIDS) deaths in 2000. In South Africa alone, there were 2 million people co-infected with mycobacterium tuberculosis (MTB) and HIVCitation1.
One-third of the world’s population is infected with MTB or has active TB, making it the second leading cause of infectious death globally. In 2006, 9.2 million incident cases were reported and 1.2 million people died as a result of TBCitation2,Citation3. Although the global incidence rate appears to be slowing, the absolute number of new cases is increasing because of the worldwide population growth. Countries in Southeast Asia, Africa, and the former Soviet Union are regions of particular concern as the surge of cases in these regions threatens global TB control. The breakdown of the health care infrastructure after the dissolution of the Soviet Union has resulted in poorly functioning TB control programs, and there is evidence of increasing HIV infection contributing to the TB epidemicCitation4. In Asia and Africa, the increase in TB directly corresponds to the worsening of the HIV epidemic over the past 2 decadesCitation5.
Modern chemotherapy has played a major role in the control of TB. Yet TB still remains a leading infectious disease worldwide, largely owing to persistence of tubercle bacillus and inadequacy of the current chemotherapy. The increasing emergence of drug-resistant TB along with the HIV pandemic threatens the disease control and highlights the necessity for the understanding of the mechanism of the current drugs and the importance to develop more effective drugs. The next threat for TB is the emergence of drug-resistant strains of MTBCitation5,Citation6. The anti-TB drugs that are currently utilized in the treatment are associated with severe toxicity and adverse effects. In spite of toxicity on repeated dosing of isoniazid (INH), it still considered to be a first line drug in the chemotherapy of TBCitation7. The statistics of the disease rate, drug-resistant strains of the tubercle bacilli and the adverse effects of the current chemotherapeutic agents clearly indicate the necessity of new drugs with effective mechanism of actions. However, highly effective new anti-TB drugs have not been developed in the last 40 years. Literature survey of novel chemotherapeutic agents reveals that the substituted pyrazoline derivatives are proved to be active against many mycobacterial speciesCitation8–14. Based on these literatures, the current work was designed to synthesize novel indane substituted pyrazoline derivatives. The present investigation of the new compounds shows encouraging antimycobacterial activity against MTB and INHR-MTB.
Results and discussion
Chemistry
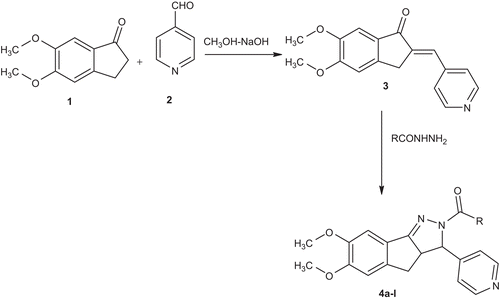
6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-substituted phenyl methanone/ethanone derivatives 4a–l described in this study are shown in and a reaction sequence for the preparation is outlined in . In the initial step, 5, 6-dimethoxy-2-[(E)-1-(4-pyridyl)methylidene]-1-indanone analogs were synthesized by condensing 5,6-dimethoxy-1-indanone with isonicotinaldehyde in diluted methanolic sodium hydroxide (30%) solution at room temperature. The product 5,6-dimethoxy-2-[(E)-1-phenylmethylidene]-1-indanone was then treated with appropriate acid hydrazide in the presence of glacial acetic acid to get titled compounds in 62–84% yield after recrystallization with ethanol. The purity of the compounds was checked by thin layer chromatography (TLC) and elemental analyses. Both analytical and spectral data (1H-NMR, IR) of all the synthesized compounds were in full agreement with the proposed structures. The elemental analysis results were within ±0.4% of the theoretical values.
Table 1. Physical constant and antimycobacterial activity of the synthesized compounds.
Antimycobacterial activity
Among the 12 compounds synthesized, most of the compounds were found to be most active with minimum inhibitory concentration of less than 7 μΜ. In addition, the in vitro test results indicated that the new compounds were active against MTB and INH resistant MTB. Compounds with electron rich groups substituted on the phenyl ring were shown to have higher activity potentials. Among the 12 newly synthesized compounds, compound 1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-pyridylmethanone (4h) was found to have maximum activity against MTB and INHR-MTB. The minimum inhibitory concentration of this compound (4h) was found to be <0.30 μM. When compared to INH, the compound (4h) was found to be 3.12-fold and 15.7-fold more active against MTB and INHR-MTB, respectively. Following this compound, 4-chlorophenyl (4f), 2,-chlorophenyl (4e) substituents show good activity with a MIC of 0.96, 2.56 and 1.28, 2.48 μM for MTB and INHR-MTB, respectively. However, the compounds substituted with electron rich groups such as, 4-chlorophenyl, 2-chlorophenyl, 4-flurophenyl, 4-bromophenyl and 4-nitrophenyl produced good to excellent inhibitory activity against both the species of MTB and INHR-MTB. On the other hand, the compounds like 4-methoxy phenyl (4l), 4-methyl phenyl (4f), 2-methyl phenyl (4i), phenyl methyl (4j) and phenoxy methyl (4k) showed relatively moderate to good antitubercular activity. Out of all the substitutions of the phenyl ring, the results indicate that the pyridyl substitution causes remarkable improvement in antimycobacterial activity. Among the newly synthesized compounds, two compounds were found to be most active compounds with minimum inhibitory concentration of less than 1 μM. Among them, compound (4h) pyridyl substituent was found to be the most active against INH and INHR-MTB. Among the newer derivatives, it is conceivable that derivatives showing antimycobacterial activity can be further modified to exhibit better potency than the standard drugs. Further studies to acquire more information about target and quantitative structure-activity relationships (QSAR) are in progress in our laboratory.
All the compounds were tested for cytotoxicity (1C50) in VERO cells at concentrations 62.5 μg/mL or 10 times. After 72 h exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega CellTiter 96 Non-radioactive cell proliferation method. Most of the active compounds were found to be nontoxic below 62.5 μg/mL.
Materials and methods
The entire chemicals were supplied by E. Merck (Germany) and S.D fine chemicals (India). Melting points were determined by open tube capillary method and are uncorrected. Purity of the compounds was checked on TLC plates (silica gel G) with the solvent system toluene-ethyl formate-formic acid (5:4:1) and benzene-methanol (8:2), the spots were located under iodine vapors or UV light. IR spectrums were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr Pellets). 1H-NMR spectra were recorded on a Bruker AC 300 MHz spectrometer using TMS as internal standard in DMSO-d6/CDCl3.
General procedure
Synthesis of 5,6-dimethoxy-2-[(E)-1-(4-pyridyl)methylidene]-1-indanone (3)
5,6-dimethoxy-1-indanone (1.92 g, 0.01 mol) were dissolved in methanol. Then, a solution of sodium hydroxide (30%, 5 mL) and isonicotinaldehyde (1.02 g, 0.01 mol) in 10 mL of pet. ether was added to the resulting solution with continuous stirring. After 4 h of stirring, the resulting solution was allowed to stand overnight. It was poured into ice-cold water and then neutralized with hydrochloric acid. The solid separate was filtered off, dried and purified from ethanol.
IR: (KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C). 1HNMR (DMSO-d6, ppm): δ3.1–3.23 (2H, m, CH2), 3.6 (6H, s, OCH3), 7.20 (1H, s, CH), 6.70–6.96 (2H, m, Aromatic); 7.5–8.80 (4H, m, pyridine); Mass (m/z) 282 M+1); Cal/Ana [C(72.58) 72.56, H(5.37) 5.35, N(4.98) 4.96].
General procedure
Synthesis of compound (4a–l)
To the compound 5,6-dimethoxy-2-[(E)-1-(4-pyridyl)methylindene]-1-indanone (3) (0.001 mol) in 15 mL of glacial acetic acid, 0.001 mol of appropriate acid hydrazide was added and the reaction mixture was refluxed for 12 h and cooled. The progress of the reaction was monitored by TLC and after completion of the reaction, the solvent was evaporated by vacuo and the reaction mixture was poured on to crushed ice (20 gm). The product obtained was filtered and washed with water and then recrystallized from methanol.
6, 7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-phenyl methanone (4a) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (7H, m, Aromatic);7.4–8.50 (4H, m, pyridine); Mass (m/z) 400 (M+1); Cal/Ana [C(72.17) 72.10, H(5.30) 5.29, N(10.52) 10.50].
4-bromophenyl-6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl methanone(4b) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 479 (M+1); Cal/Ana [C(60.26) 60.24, H(4.21) 4.20, N(8.78) 8.76].
4-fluorophenyl-6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-methanone(4c) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 418 (M+1); Cal/Ana [C(60.06) 69.04, H(4.83) 4.81, N(10.07) 10.09].
6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-nitrophenyl methanone(4d) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 445 (M+1); Cal/Ana [C(68.86) 68.88, H(4.54) 4.52, N(12.61) 12.63].
2-chlorophenyl-6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl methanone(4e) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 434 (M+1); Cal/Ana [C(66.44) 66.42, H(4.65) 4.63, N (9.688) 9.66].
4-chlorophenyl-6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl methanone(4f) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 434 (M+1); Cal/Ana [C(66.44) 66.42, H(4.65) 4.63, N(9.688) 9.66].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-methylphenyl methanone(4g) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ1.72 (3H, s, CH3) 2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 414 (M+1); Cal/Ana [C(72.62) 72.60, H(5.61) 5.60, N(10.16) 10.14].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-pyridyl methanone(4h) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ1.72 (3H, s, CH3), 2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (2H, m, Aromatic); 7.4–8.50 (8H, m, pyridine); Mass (m/z) 401 (M+1); Cal/Ana [C(68.99) 68.97, H(5.03) 5.01, N(13.99) 13.97].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-2-methylphenyl methanone(4i) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ1.72 (3H, s, CH3) 2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 414 (M+1); Cal/Ana [C(72.62) 72.60, H(5.61) 5.60, N(10.16) 10.14].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl]-2-phenyl-1-ethanone(4j) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1H-NMR (DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.2 (2H, m, benzyl-CH2), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 414 (M+1); Cal/Ana [C(72.62) 72.60, H(5.61) 5.60, N(10.16) 10.14].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl]-2-phenoxy-1-ethanone(4k) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4 (2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), 3.2 (2H, m, pheoxy-CH2), 3.6 (6H, s, OCH3), 5.5 (1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 430M+1); Cal/Ana [C(69.92) 69.90, H(5.40) 5.38, N(9.78) 9.76].
1-[6,7-dimethoxy-3-(4-pyridyl)-2,3,3a,4-tetrahydroindeno[1,2-c]pyrazol-2-yl-4-methoxyphenyl methanone(4l) IR:(KBr, cm−1): 3042 (CH), 1642 (C=O), 1439 (C=C), 766 (C-F). 1HNMR(DMSO-d6, ppm): δ2.1–2.4(2H, m, CH2), 2.5–2.6 (1H, m, CH-Indane), CH2), 3.6 (9H, s, OCH3), 5.5(1H, d, pyrazoline-CH), 6.60–7.10 (6H, m, Aromatic); 7.4–8.50 (4H, m, pyridine); Mass (m/z) 430M+1); Cal/Ana [C(69.92) 69.90, H(5.40) 5.38, N(9.78) 9.76].
Biology
The primary screening was conducted at a concentration of 6.25 µg/mL (or molar equivalent of highest molecular weight compound in a series of congeners) against MTB H37Rv (ATCC27294) and INH resistant MTB in BACTEC 460 radiometric systemCitation15. Compound demonstrating at least 90% inhibition in the primary screen was re-examined at lower concentration (MIC) in broth microdilution assay with alamarBlue. The MIC was defined as the lowest concentration inhibiting 99% of the innoculum. Concurrent with the determination of MICs, compounds were tested for cytotoxicity (IC50) in VERO at concentration equal to and greater than the MIC for MTB H37Rv and INH resistant MTB after 72 h exposure. Viability was assessed on the basis of cellular conversion of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) into a formazan product using the Promega CellTiter 96 Non-radioactive cell proliferation assayCitation16.
Acknowledgments
The authors wish to express their thanks to Pharmacogenetic and Pharmacogenomic Research, Institute for Research in Molecular Medicine, Universiti of Sains Malaysia, Pennag, Malaysia and Alwar Pharmacy College, Alwar, Rajasthan, India, for providing research facilities.
Declaration of interest
The authors report no conflicts of interest.
References
- Robert JN, Yu-Jin J. Immunity to tuberculosis. Annu Rev Immunol 2004;22:599–623.
- Kazionny B, Wells CD, Kluge H, Gusseynova N, Molotilov V. Implications of the growing HIV-1 epidemic for tuberculosis control in Russia. Lancet 2001;358:1513–1514.
- Morozova I, Riekstina V, Sture G, Wells C, Leimane V. Impact of the growing HIV-1 epidemic on multidrug-resistant tuberculosis control in Latvia. Int J Tuberc Lung Dis 2003;7:903–906.
- Sbarbaro JA. ‘Multidrug’-resistant tuberculosis. It is time to focus on the private sector of medicine. Chest 1997;111:1149–1151.
- Fujiwara PI, Cook SV, Rutherford CM, Crawford JT, Glickman SE, Kreiswirth BN et al. A continuing survey of drug-resistant tuberculosis, New York City, April 1994. Arch Intern Med 1997;157:531–536.
- Schaberg T, Gloger G, Reichert B, Mauch H, Lode H. Resistant lung tuberculosis in Berlin 1987–1993. Pneumologie 1996;50:21–27.
- Blair IA, Mansilla Tinoco R, Brodie MJ, Clare RA, Dollery CT, Timbrell JA et al. Plasma hydrazine concentrations in man after isoniazid and hydralazine administration. Hum Toxicol 1985;4:195–202.
- Ali MA, Shaharyar M, Siddiqui AA. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 2007;42:268–275.
- Ali MA, Yar MS, Hasan MZ, Ahsan MJ, Pandian S. Design, synthesis and evaluation of novel 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenyl-3,4-substituted phenyl methanone analogues. Bioorg Med Chem Lett 2009;19:5075–5077.
- Ali MA, Samy JG, Manogaran E, Sellappan V, Hasan MZ, Ahsan MJ et al. Synthesis and antimycobacterial evaluation of novel 5,6-dimethoxy-1-oxo-2,5-dihydro-1H-2-indenyl-5,4-substituted phenyl methanone analogues. Bioorg Med Chem Lett 2009;19:7000–7002.
- Küçükgüzel SG, Rollas S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Farmaco 2002;57:583–587.
- Ali MA, Shaharyar M. Oxadiazole mannich bases: Synthesis and antimycobacterial activity. Bioorg Med Chem Lett 2007;17:3314–3316.
- Shaharyar M, Ali MA, Bakht MA, Murugan V. Synthesis and antimycobacterial activity of 4-[5-(substituted phenyl)-4, 5-dihydro-3-isoxazolyl]-2-methylphenols. J Enzyme Inhib Med Chem 2008;23:432–436.
- Ragavan RV, Vijayakumar V, Kumari NS. Synthesis and antimicrobial activities of novel 1,5-diaryl pyrazoles. Eur J Med Chem 2010;45:1173–1180.
- Heifets LB, Flory MA, Lindholm-Levy PJ. Does pyrazinoic acid as an active moiety of pyrazinamide have specific activity against mycobacterium tuberculosis? Antimicrob Agents Chemother 1989;33:1252–1254.
- Gundersen LL, Nissen-Meyer J, Spilsberg B. Synthesis and antimycobacterial activity of 6-arylpurines: The requirements for the N-9 substituent in active antimycobacterial purines. J Med Chem 2002;45:1383–1386.