Abstract
The inhibitory effects of oxyresveratrol, the aglycone of mulberroside A, on mushroom and cellular tyrosinase activities and melanin synthesis were evaluated. Mulberroside A and oxyresveratrol showed inhibitory activity against mushroom tyrosinase, with oxyresveratrol demonstrating a greater inhibitory effect than that of mulberroside A. Oxyresveratrol and mulberroside A strongly inhibited melanin production in Streptomyces bikiniensis and exhibited dose-dependent inhibition of tyrosinase activity and inhibition of melanin synthesis in B16F10 melanoma cells. However, the compounds exhibited nearly similar inhibitory effects on the activity of cellular tyrosinase and melanin synthesis in murine melanocytes. The inhibition of melanin synthesis by mulberroside A and oxyresveratrol was involved in suppressing the expression level of melanogenic enzymes, tyrosinase, tyrosinase-related protein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2). These results indicate that the inhibition rate of mushroom tyrosinase might not provide an accurate estimate of the inhibition rate of melanin synthesis in melanocytes.
Introduction
Biotransformation of glucosides into aglycones improves their biological activitiesCitation1–3. Flavonoids generally occur as glycosides; removal of the glycone results in the enhancement of antioxidant, anticancer, and antimicrobial activity, and bone-loss prevention functionsCitation4–6. Many anticancer agents are activated by enzyme catalysisCitation7. Mulberroside A is a glycosylated stilbene, and oxyresveratrol is an aglycone derivative of mulberroside A. Mulberroside A purified from the methanol extract of Morus alba root has previously demonstrated hypocholesteromic and antioxidant effectsCitation8. Mulberroside A administered orally is converted into oxyresveratrol, which is transported to tissues at high ratesCitation9. Oxyresveratrol obtained from the Morus alba cortex displays a much stronger anti-inflammatory effect than that of mulberroside A, but the edema-suppressing activities of oxyresveratrol and mulberroside A in rats are almost the same after a 5-h treatment, suggesting biotransformation of mulberroside A to oxyresveratrolCitation10.
Melanin synthesis inhibition is mainly achieved via the inhibition of tyrosinase, a key enzyme of the synthetic pathway. Tyrosinase exhibits two enzymatic activities: it functions as a tyrosine hydroxylase that converts tyrosine to 3,4-dihydroxyphenylalanine (DOPA), and as a DOPA oxidase, which oxidizes DOPA to dopaquinoneCitation11. Melanin is synthesized in the melanosomes of melanocytes through a complex process. Melanogenesis is controlled by an enzyme cascade and two tyrosinase-related proteins (TRP-1 and TRP-2) are also involved in the pathwayCitation12. One is DOPAchrome tautomerase (TRP-2) which functions to tautomerize DOPAchrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) and the other is DHICA oxidase (TRP-1) which promotes further oxidation of DHICA. Many inhibitors of melanin synthesis have been isolated from natural sources, including flavonoids, kojic acids, arbutin, hydroquinone, morin, epicatechin gallate, epigallocatechin gallate and quercetinCitation13–16. Although some of them were investigated on the inhibition of cellular tyrosinase, the tyrosinase inhibitory activity of the compounds was mainly evaluated via the inhibition rate of mushroom tyrosinase activity. Mulberroside A was enzymatically transformed into oxyresveratrol using Pectinex® and reported that oxyresveratrol showed more mushroom tyrosinase inhibitory activity than mulberroside ACitation17. In the present study, the inhibition effects of oxyresveratrol on mushroom dihydroxyphenylalanine (DOPA) oxidase activity, cellular DOPA oxidase activity and melanin biosynthesis in melanoma cells were evaluated and were compared to those of mulberroside A.
Materials and methods
Materials and strains
Streptomyces bikiniensis NRRL B-1049 was purchased from the Korean Collection for Type Culture (Daejon, Korea). B16F10 murine melanoma cells (KCLB 80008) were purchased from the Korean Cell Line Bank (Seoul, Korea). Cell culture reagents such as Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Rockville, MD, USA). Antibiotic-antimycotic solution (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 µg/mL amphotericin B) was purchased from WelGENE (Seoul, Korea). Synthetic melanin, mushroom tyrosinase, arbutin, resveratrol, α-melanocyte stimulating hormone (MSH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS) and 3,4-dihydroxyphenylalanine (l-DOPA) were purchased from Sigma (St. Louis, MO, USA).
Mushroom tyrosinase activity assay
DOPA oxidase activity of mushroom tyrosinase was measured according to the previously described method with minor modificationsCitation18. The reaction mixture consisted of 40 µL of 25 mM l-DOPA, 80 µL of 0.1 M phosphate buffer (pH 6.8), 10 µL of a test sample dissolved in DMSO, 30 µL of distilled water and 40 µL of mushroom tyrosinase (125 U/mL). After incubation at 37°C for 20 min, the amount of dopachrome in the reaction mixture was determined by measurement of optical density at 475 nm using a Spectra max 340pc microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Determination of melanin production inhibition in S. bikiniensis
A preserved culture of S. bikiniensis NRRL B-1049 was inoculated onto a Papavizas’ VDYA agar slant containing 200 mL of V-8 juice (Campbell Soup Co., Camden, NJ, USA), 2 g of glucose, 2 g of yeast extract (Difco, Sparks, MD, USA), 1 g of CaCO3, 20 g of agar and 800 mL of distilled water (pH 7.2). After incubation at 28°C for 2 weeks, 2 mL of sterile water was added to the slant culture, and the spore mass that formed on the aerial mycelium was removed with an inoculating loop. Spore suspensions of S. bikiniensis were then inoculated onto agar medium ISP No. 7 supplemented with 0.2% Bacto-yeast extract, uniformly spread over the agar surface. After the agar surfaces were dried, paper discs (8 mm in diameter) soaked with sample solution, were placed on the agar plates, which were then incubated at 28°C for 48 h. The resulting melanin formation inhibitory zones were measured.
Cell culture and chemical treatment
Mouse B16F10 melanoma cells were routinely cultured in DMEM medium containing 10% FBS, 50 μg/mL streptomycin, 50 U/mL penicillin and 0.125 µg/mL amphotericin B in a humidified 5% CO2 atmosphere at 37°C. The medium was changed every 2 days. All experiments were performed using the cells at the same passage. The B16F10 melanoma cells (1 × 105 cells) were seeded into each well of 24-well plates. The cells were allowed to attach to the plates for 12 h, each test compound at the designated concentrations was added, and continuous culture proceeded for 2 days. Another 24-well plate was treated under the same conditions, with DMSO added only as a control. Cell viability, tyrosinase inhibition activity or inhibition of melanin synthesis was determined from these plates. All assays were performed in three independent experiments.
Cell viability
Cell viability was quantified using a colorimetric MTT assay to measure the mitochondrial activity in viable cells, using the method of MosmannCitation19 with minor modifications. At the end of the treatment time, all wells were aspirated, refilled with MTT (1 mg/mL) solution, and incubated for 3 h at 37°C. The formazan crystals that formed in the actively metabolizing cells were extracted with 10% SDS (in 1 N HCl). The absorbance at 540 nm was measured using a microplate reader, with results expressed as a percentage of the untreated control (% of control).
Cellular tyrosinase activity assay
Tyrosinase activity was determined based on a modification of a previously described methodCitation20. Total cell counts were determined using a hemocytometer and 2 × 104 cells/cm2 were treated with various concentrations of the test compounds, followed by addition of α-MSH to give a final concentration of 100 nM. After 2 days, the incubated cells were harvested by trypsinization and washed twice with ice-cold phosphate-buffered saline (PBS) by centrifugation at 1,000 × g for 5 min. The harvested cells were lysed in 1% Triton X-100 and 0.1 mM phenylmethanesulfonyl fluoride (PMSF) in PBS. After lysis for 30 min, tyrosinase from the melanosome membrane was collected by centrifugation at 10,000 × g for 25 min at 4°C. The reaction mixture consisted of 0.05% l-DOPA solution and cell-extracted protein (30 μg) in 0.2 mL of PBS. The protein content in the supernatant was analyzed using a Bio-Rad protein assay with bovine serum albumin (BSA) standardsCitation21. Dopachrome formation at 37°C was monitored every 10 min for 1 h by measuring the absorbance at a wavelength of 475 nm using a microplate reader.
Determination of melanin content
Melanin content of the cells was measured using a modification of a previously described methodCitation22. The cell density and treatment used for determination of melanin content were the same as those for the determination of tyrosinase inhibition activity. After 2 days, the collected medium and trypsinized cells were centrifuged at 3,000 × g for 10 min. Each precipitate was combined, washed twice with PBS, and dried. Dried cells were dissolved in 250 μL of 1 N NaOH containing 10% DMSO at 80°C for 1 h and then centrifuged for 30 min at 16,000 × g. Supernatant optical densities were measured at 405 nm and compared with standard curves of synthetic melanin. Melanin levels were expressed versus cellular protein concentration using a Bio-Rad protein assay with BSA standard.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) and data analysis
Total cellular RNA was prepared using a Pure Link RNA mini kit (Invitrogen, USA) according to the manufacturer’s protocols and then quantified the concentration of RNA using NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, USA). After the preparation of cDNA using Revert Aid First Strand cDNA kit (Fermantas, Germany), quantitative PCR was performed using a DyNAmo™ HS SYBR® Green qPCR kit (FINNZYMES, Finland) in a CFX96 Real Time PCR System (Bio-Rad)Citation23. The oligonucleotide primers used for the PCR are as follows: tyrosinase forward 5′-GGC CAG CTT TCA GGC AGA GGT-3′ and reverse 5′-TGG TGC TTC ATG GGC AAA ATC-3′; TRP-1 forward 5′-GCT GCA GGA GCC TTC TTT CTC-3′ and reverse 5′-AAG ACG CTG CAC TGC TGG TCT-3′; TRP-2 forward 5′-GGA TGA CCG TGA GCA ATG GCC-3′ and reverse 5′-CGG TTG TGA CCA ATG GGT GCC-3′; actin forward 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ and reverse 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. The reaction was preheated for 15 min at 94°C followed by 40 cycles at 94°C for 20 s, 56°C for 20 s, and 72°C for 40 s. Actin primers were used to standardize the amount of RNA in each sample. The reaction without cDNA was used as a negative control. Generation of quantitative data by real-time PCR is based on the number of cycles needed for amplification generated fluorescence to reach a specific threshold of detection (the Ct value). For relative quantification of gene expression on the basis of the same amounts of RNA (1 µg), the average Ct value of each gene was obtained and delta Ct (ΔCt = Cttarget gene – Ctreference gene) was calculated using the Ct values of the genes in the same sample. Actin gene was used as the internal control reference gene. The ΔΔCt value was obtained by calculating using the equation ΔΔCt = (ΔCttreated - ΔCtuntreated). The normalized expression fold was expressed as the value of 2−ΔΔCt (actin control = 1)Citation24.
Western blot
To determine the amount of tyrosinase and tyrosinase-related proteins expression, western blotting analysis was performed. Soluble protein fractions were extracted from cultured cells using protein extraction solution (PRO-PREP™, Intron, Korea) containing 1 mM EDTA, 1 mM PMSF, 1 µM Pepstatin A, 1 µM Leupeptin and 0.1 µM Aprotinin. After centrifugation at 10,000 × g for 30 min at 4°C, the supernatant was collected. Soluble proteins (20 µg) were electrophoresed through 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred onto polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% non-fat skim milk in phosphate-buffered saline/Tween 20 buffer (PBS-T) for overnight at 4°C. The membrane was washed three times with PBS-T and incubated with the primary antibodies for 5 h at room temperature. Tyrosinase, TRP-1, TRP-2, and actin were detected with rabbit polyclonal anti-tyrosinase antibody (dilution 1:1,000), rabbit polyclonal anti-TRP-1 antibody (dilution 1:1,000) and rabbit polyclonal anti TRP-2 antibody (dilution 1:500), respectively, which were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membrane was washed three times with PSB-T and then further incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit IgG antibody at a 1:5,000 dilution from Santa Cruz Biotechnology) for 1 h at room temperature. All bound antibodies were then detected with enhanced chemiluminescence (ECL) reagent (Perkin Elmer, USA).
Statistical analysis
Results are expressed as the mean ± standard deviation (SD). All statistical analyses were performed using SigmaStat 3.5 (Jandel Scientific, San Rafael, CA, USA). The differences among groups were evaluated by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests. A level of p < 0.05 was used as the threshold for statistical significance.
Results
Mushroom tyrosinase inhibition
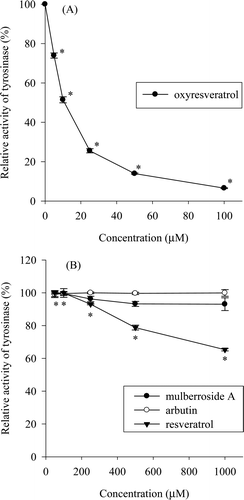
The DOPA oxidase activity of mushroom tyrosinase was assayed with oxyresveratrol and mulberroside A, as well as arbutin and resveratrol. Oxyresveratrol showed strong inhibition of mushroom tyrosinase DOPA oxidase activity (. Oxyresveratrol showed a 50% inhibition of DOPA oxidase at a concentration of 10.3 µM and almost complete inhibition of DOPA oxidase activity at a concentration of 100 µM. On the other hand, mulberroside A and arbutin did not display noteworthy inhibitory activity at concentrations up to 1,000 µM, although mulberroside A exhibited minor inhibitory activity and resveratrol inhibited DOPA oxidase activity to a 34.7% level at 1,000 µM (. This means that oxyresveratrol, which can be enzymatically transformed from mulberroside A, greatly increases the inhibition of mushroom tyrosinase DOPA oxidase activity.
Figure 1. Inhibition of mushroom tyrosinase by oxyresveratrol (A) and mulberroside A, arbutin, and resveratrol (B). The tyrosinase DOPA oxidase activity of the untreated sample was taken as 100%; data are shown as percentage relative activity. Results are expressed as mean ± SD (n = 3). A p-value < 0.05 was considered the threshold for statistical significance.
Melanin synthesis inhibition in S. bikiniensis
S. bikiniensis, a melanin producer, is used to measure the inhibitory activity of oxyresveratrol and mulberroside A on melanin biosynthesis. A strong, dose-dependent inhibition of melanin synthesis by oxyresveratrol and mulberroside A is observed. These inhibitory activities were stronger than that of arbutin, a well-known inhibitor of melanin synthesis (). Calculation of the molarity from the molecular weight of oxyresveratrol (MW 244) and mulberroside A (MW 568) showed that the melanin synthesis inhibition by oxyresveratrol was in fact similar to that of mulberroside A in S. bikiniensis. Growth inhibition by oxyresveratrol and resveratrol was exhibited by amounts over 500 µg.
Table 1. Inhibition of melanin synthesis in Streptomyces bikiniensis by compound of interest.
Cell viability
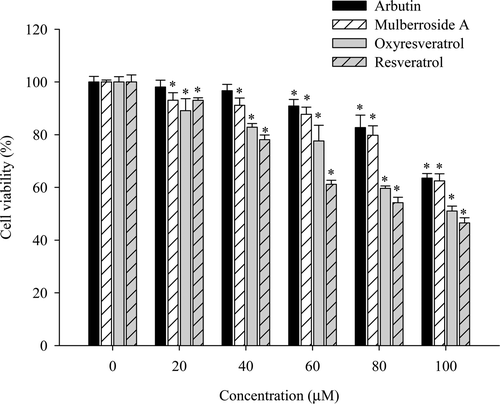
The MTT assay is used to investigate the cell survival rate of B16F10 cells after exposure to oxyresveratrol and mulberroside A, with resveratrol and arbutin as control compounds. Cells exhibited a survival rate of over 90% up to concentrations of 20 µM oxyresveratrol, 40 µM mulberroside A, 20 µM resveratrol and 60 µM arbutin (). Treatment with oxyresveratrol, mulberroside A and resveratrol all resulted in decreased cell viabilities to survival rates of 89.1%, 93.1% and 93.0%, respectively, at 20 µM concentrations; 82.8%, 91.1% and 78.1%, respectively, at 40 µM concentrations; increasing the concentration to 100 µM led to further decreased viability levels of 51.0%, 62.5% and 46.5%, respectively. The cell toxicity was not much different between mulberroside A and oxyresveratrol below 20 µM. However, oxyresveratrol showed more toxicity than mulberroside A at high concentrations.
Figure 2. Effect of oxyresveratrol, mulberroside A, resveratrol, and arbutin on B16F10 melanoma cell viability, as assayed by MTT. The data were normalized by setting 100% equal to the viability of the untreated control group. The results are presented as mean ± SD (n = 3). A p-value < 0.05 was considered the threshold for statistical significance.
Inhibitory effect on cellular tyrosinase and melanin biosynthesis in B16F10 cells
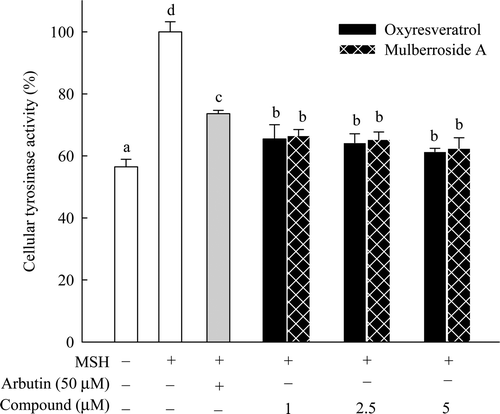
Melanin synthesis occurs through several steps in the cell and is affected by various factorsCitation16. Accordingly, MSH was used to investigate the inhibitory effects of oxyresveratrol and mulberroside A on cellular tyrosinase and melanin synthesis in B16F10 cells. To avoid cellular toxicity from the compounds, 0−5 µM concentrations of oxyresveratrol and mulberroside A and 50 µM arbutin, commonly used in Korea to prevent melanin synthesis in the skin, were used as a control compound. The cellular tyrosinase activity of the sample treated only with MSH was assigned as 100%. The residual activities were 56.5%, 61.4%, 62.5% and 73.6% in the samples treated without MSH, 5 µM oxyresveratrol, 5 µM mulberroside A and 50 µM arbutin, respectively (). Although oxyresveratrol and mulberroside A displayed greater inhibitory activity than arbutin, the two compounds did not differ much from each other in terms of their tyrosinase inhibitory activities.
Figure 3. Effect of oxyresveratrol and mulberroside A on cellular tyrosinase inhibition in B16F10 melanoma cells. Results are depicted relative to the control (100 nM α-MSH treatment only) and expressed as mean ± SD (n = 3). Means with the different letters are significantly different (p < 0.05).
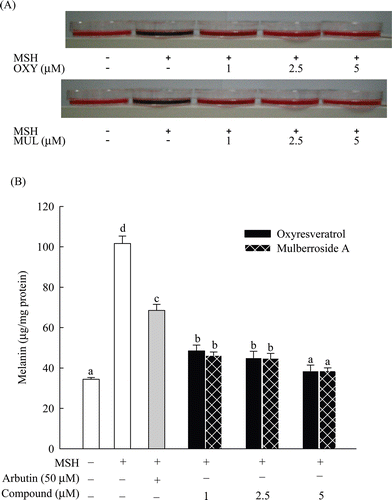
A standard curve was plotted using synthetic melanin, and used this curve to measure melanin concentrations in melanoma cells, expressing the concentrations as µg/mg protein. Melanin concentrations were 34.4 µg/mg, 101.6 µg/mg, 38.5 µg/mg, 38.5 µg/mg and 68.5 µg/mg protein in the samples without MSH, treated with MSH alone, 5 µM oxyresveratrol, 5 µM mulberroside A and 50 µM arbutin, respectively (). The cell pellets treated with mulberroside A or oxyresveratrol showed a visible reduction in melanogenesis compared to control treated with MSH alone. Oxyresveratrol and mulberroside A both displayed greater inhibition of melanin synthesis than that of arbutin, showing similarity to the results for tyrosinase inhibition (). However, there was no difference in melanin synthesis inhibition between the cells treated with either oxyresveratrol or mulberroside A.
Figure 4. Inhibitory effect of oxyresveratrol and mulberroside A on melanogenesis in B16F10 melanoma cells exposed to 100 nM MSH. Cell pellets and culture broth after 2 days of incubation (A) and cellular melanin content (B) are shown; OXY and MUL are abbreviations of oxyresveratrol and mulberroside A, respectively. Results are expressed in µg/mg protein, mean ± SD (n = 3). Means with the different letters are significantly different (p < 0.05).
Expression of melanogenic enzymes
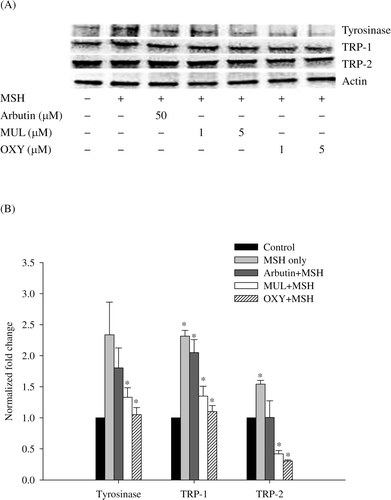
To elucidate the inhibition of melanin synthesis by mulberroside A and oxyresveratrol, we investigated the expression of melanogenic enzymes, tyrosinase, TRP-1, and TRP-2 using western blot ( and analyzed levels of tyrosinase, TRP-1, and TRP-2 mRNA by qRT-PCR (. All compounds suppressed the expression level of the melanogenic enzymes in B16F10 cells compared to cells treated only with MSH and mulberroside A and oxyresveratrol showed dose-dependent inhibition (. The protein content of the sample treated with MSH alone was assigned as 100%. The levels of tyrosinase, TRP-1 and TRP-2 were reduced to 46.0%, 84.5% and 78.3%, respectively, after treatment with 5 µM mulberroside A and MSH; and 26.3%, 73.8%, and 78.7%, respectively, after treatment of 5 µM oxyresveratrol and MSH. The Ct values of each mRNA were evaluated to investigate the effect of mulberroside A and oxyresveratrol on the genes expression. The genes expression of melanogenic enzymes was also suppressed. Compared with the normalized fold expression of tyrosinase and TRP-1 in sample treated with α-MSH only, tyrosinase mRNA decreased to 77.3%, 56.9% and 45.0%, TRP-1 mRNA decreased to 88.6%, 58.3% and 47.6%, and TRP-2 mRNA decreased to 65.2%, 27.3% and 19.8% in samples treated with α-MSH plus arbutin, α-MSH plus mulberroside A and α-MSH plus oxyresveratrol, respectively. Although the expression of TRP-2 gene was more reduced than that of other genes, the suppression of genes expression showed consistent with the inhibition of protein expression (. The results explain that mulberroside A and oxyresveratrol inhibited melanin synthesis by reducing the expression of tyrosinase and TRP-1 in melanocytes. Oxyresveratrol showed approximately 10% more inhibition of the gene expression in every enzyme than mulberroside A.
Figure 5. Western blotting (A) and quantitative real-time RT-PCR (B) of melanogenic enzymes. (A): The cells were treated with α-MSH (100 nM) and each chemical of designated concentration. (B): The cells were treated with α-MSH (100 nM) and arbutin (50 μM), mulberroside A (MUL, 5 μM), or oxyresveratrol (OXY, 5 μM). Data were normalized by using actin as a control. The values of normalized fold expression were determined from three independent experiments and expressed as mean values ± SD. The asterisks denote the statistic significance of p < 0.05, in comparison with the control which was normalized to 1.
Discussion
Biotransformation is an effective technique to produce bioavailable compounds. Flavonoid aglycones exhibit greater bio-availability than their glycosidesCitation2,Citation3. Oxyresveratrol and mulberroside A have displayed antioxidant, anti-inflammatory, and anti-browning activities, those of oxyresveratrol being much greater than mulberroside ACitation10,Citation25. We previously reported that oxyresveratrol inhibits mushroom tyrosinase via a mixed inhibition with l-tyrosine as a substrate, resulting in approximately 110-fold more inhibition than mulberroside ACitation17. Following oral administration, mulberroside A is metabolized in the liver or digestive tract, resulting in an almost complete conversion to oxyresveratrol, which is easily transported in tissueCitation9. It can be concluded that oxyresveratrol is more suitable for the study of bioavailability in tissue than mulberroside A.
Oxyresveratrol and mulberroside A in non-cytotoxic concentrations (1−5 µM) significantly (p < 0.05) inhibited cellular tyrosinase activity and melanin synthesis in murine melanocytes treated with MSH. Although oxyresveratrol and mulberroside A more strongly inhibited cellular tyrosinase and melanin synthesis in melanoma cells than arbutin, they exhibited very similar inhibition levels in both assays, and their cellular inhibitory activities showed a large difference from the inhibitory effects on mushroom tyrosinase. Oxyresveratrol also did not show stronger inhibitory activity than mulberroside A on melanin synthesis in S. bikiniensis. The 50% inhibition concentration (IC50) values of mulberroside A and oxyresveratrol were 53.6 µM and 0.49 µM, respectively, with L-tyrosine as the substrateCitation17. The relative in vitro activities of mushroom tyrosinase using L-DOPA as a substrate were 73.9% and almost 100% with treatment of 5 µM oxyresveratrol or 5 µM mulberroside A, respectively (). Mushroom tyrosinase treated with 1,000 µM mulberroside A still maintained 93.1% activity; in contrast, the enzyme lost almost all activity when treated with 100 µM oxyresveratrol. On the other hand, the relative activities of cellular tyrosinase using l-DOPA as a substrate were 61.4% and 62.5% with treatment of 5 µM oxyresveratrol or 5 µM mulberroside A, respectively (). Notably, mulberroside A exhibited a large difference in inhibitory activities between mushroom tyrosinase and cellular tyrosinase. From these results, it can be inferred that mulberroside A may be converted into oxyresveratrol by enzymatic degradation during the culture of melanocyte cells, consistent with the previous reportsCitation9,Citation10. This suggests that mulberroside A acts like a pro-drug in melanocytes, expressing strong inhibitory activity after conversion to oxyresveratrol in melanocytes. Interestingly, the bioavailability or bioactivity of flavonoids may depend not on the properties of the native compound but rather on the properties of the metabolites produced by biotransformation in vivoCitation26,Citation27. Thus, the bioactivity of a natural compound analyzed in vitro may not represent its bioavailability or bioactivity in vivo. Tyrosinase is a key enzyme of melanin synthesis; thus mushroom tyrosinase is usually used in screens for inhibitors of melanin synthesis. However, it is important to point out that in vitro inhibition rates of mushroom tyrosinase might not represent melanin synthesis inhibition rates in melanocytes. The previous study indicated that oxyresveratrol strongly inhibited mushroom tyrosinase activity and acted through reversible inhibition of tyrosinase activity rather than suppression of the expression and synthesis of the enzymeCitation28. Thus, the inhibition effect is derived not from the level of regulation of the gene expression of the enzyme but from the level of the inhibition of the enzyme activity.
The MSH regulates melanogenic enzymes activities directly in the melanosome. Mulberroside A and oxyresveratrol inhibited the α-MSH-stimulated expression of tyrosinase, TRP-1 and TRP-2 and the inhibition showed a dose-dependent pattern, which is correlated with the down-regulation of their genes. Therefore, the results suggest that the effect of mulberroside A and oxyresveratrol on the inhibition of melanin synthesis is mainly due to the reduction of the melanogenic enzymes expression. Although the inhibitory effects of both compounds on the inhibition of cellular tyrosinase and melanin synthesis are almost same, the down-regulation rates of the genes expression by mulberroside A and oxyresveratrol exhibit approximately 10% difference. Further studies are needed to explain the different results between the down-regulation rates of the genes expression and the inhibition of cellular tyrosinase and melanin synthesis.
In conclusion, oxyresveratrol and mulberroside A showed strong inhibitory activities against mushroom and cellular tyrosinase resulting in the inhibition of melanin synthesis. Oxyresveratrol and mulberroside A showed almost the same effect on the inhibition of cellular tyrosinase and melanin biosynthesis in melanocytes, although oxyresveratrol showed stronger inhibition of mushroom tyrosinase activity than mulberroside A. Thus, the in vitro inhibition rate of mushroom tyrosinase might not indicate the inhibition rate of melanin synthesis in in vivo systems.
Declaration of interest
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (20080563).
References
- Marotti I, Bonetti A, Biavati B, Catizone P, Dinelli G. Biotransformation of common bean (Phaseolus vulgaris L.) flavonoid glycosides by bifidobacterium species from human intestinal origin. J Agric Food Chem 2007;55:3913–3919.
- Nielsen IL, Chee WS, Poulsen L, Offord-Cavin E, Rasmussen SE, Frederiksen H et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr 2006;136:404–408.
- Otieno DO, Shah NP. A comparison of changes in the transformation of isoflavones in soymilk using varying concentrations of exogenous and probiotic-derived endogenous beta-glucosidases. J Appl Microbiol 2007;103:601–612.
- Feng X, Zhang L, Zhu H. Comparative anticancer and antioxidant activities of different ingredients of Ginkgo biloba extract (EGb 761). Planta Med 2009;75:792–796.
- Habauzit V, Nielsen IL, Gil-Izquierdo A, Trzeciakiewicz A, Morand C, Chee W et al. Increased bioavailability of hesperetin-7-glucoside compared with hesperidin results in more efficient prevention of bone loss in adult ovariectomised rats. Br J Nutr 2009;102:976–984.
- Pereira JA, Pereira AP, Ferreira IC, Valentão P, Andrade PB, Seabra R et al. Table olives from Portugal: phenolic compounds, antioxidant potential, and antimicrobial activity. J Agric Food Chem 2006;54:8425–8431.
- Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev 2004;56:53–102.
- El-Beshbishy HA, Singab AN, Sinkkonen J, Pihlaja K. Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci 2006;78:2724–2733.
- Qiu F, Komatsu K, Saito K, Kawasaki K, Yao X, Kano Y. Pharmacological properties of traditional medicines. XXII. Pharmacokinetic study of mulberroside A and its metabolites in rat. Biol Pharm Bull 1996;19:1463–1467.
- Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH et al. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol 2003;55:1695–1700.
- García-Molina F, Muñoz JL, Varón R, Rodríguez-López JN, García-Cánovas F, Tudela J. A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J Agric Food Chem 2007;55:9739–9749.
- Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc 1999;4:24–28.
- Chen JS, Wei CI, Marshall MR. Inhibition mechanism of kojic acid on polyphenol oxidase. J Agric Food Chem 1991;39:1897–1901.
- Kubo I, Kinst-Hori I, Chaudhuri SK, Kubo Y, Sánchez Y, Ogura T. Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem 2000;8:1749–1755.
- No JK, Soung DY, Kim YJ, Shim KH, Jun YS, Rhee SH et al. Inhibition of tyrosinase by green tea components. Life Sci 1999;65:PL241–PL246.
- Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK et al. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res 2006;20:921–934.
- Kim JK, Kim M, Cho SG, Kim MK, Kim SW, Lim YH. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J Ind Microbiol Biotechnol 2010;37:631–637.
- Matsuda H, Nakamura S, Kubo M. Studies of cuticle drugs from natural sources. II. Inhibitory effects of Prunus plants on melanin biosynthesis. Biol Pharm Bull 1994;17:1417–1420.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Wu L, Chang L, Chen S, Fan N, Ho JA. Antioxidant activity and melanogenesis inhibitory effect of the acetonic extract of Osmanthus fragrans: A potential natural and functional food flavor additive. LWT – Food Sci Technol 2009;42:1513–1519.
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76–85.
- Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res 1998;11:275–282.
- Yoshida Y, Hachiya A, Sriwiriyanont P, Ohuchi A, Kitahara T, Takema Y et al. Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB J 2007;21:2829–2839.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408.
- Li H, Cheng KW, Cho CH, He Z, Wang M. Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples. J Agric Food Chem 2007;55:2604–2610.
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132:3577–3584.
- Spencer JP, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal 2001;3:1023–1039.
- Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 2002;277:16340–16344.
