Abstract
Several 9H-carbazole derivatives are used for various pharmacological applications. Many of these compounds demonstrated cytotoxic and anticancer activities. In this work, we have investigated the cytotoxic activity of some substituted carbazoles against cancer cell lines (MCF-7, and ISK). The derivative 2a showed the highest inhibitory activity against both cell lines.
Introduction
The indole nucleus is often found embedded in compounds with a wide range of biological activitiesCitation1. Carbazoles, whether synthetic or naturally occurring, are important members of indole-containing heterocyclesCitation1,Citation2. Several 9H-carbazoles possess various pharmacological applications and also they are used in dyes-, insecticide-, and plastic industries. In addition, several derivatives of 1,2,3,4-tetrahydro-9H-carbazole and 9-methyl-9H-carbazole have been known by their medicinal use as antitubercular, antifungal, antibacterial, β3-adrenoceptor agonists and anticancer activitiesCitation2–4.
The precise mechanisms of their anti-neoplastic activity have not yet been explained. Many of these carbazoles are cytostatic and their activity results from alternative cytotoxic effects. It was suggested that the prevalent mechanisms of the antitumor, mutagenic and cytotoxic activities are their intercalation into DNA and inhibition of DNA-topoisomerase II activityCitation5,Citation6.
Based on this, the aim of this work is to investigate the cytotoxic activity of the prepared carbazoles () against a cancer cell line. We have used MCF-7 cell line derived from a pleural effusion of a postmenopausal 69-year-old patient with metastatic breast cancer in 1970Citation7. This cell line was grown in Dulbecco’s modified Eagle’s medium (DMEM), without phenol red, containing 10% foetal calf serum (Invitrogen, Milan, Italy) and 1 mg/mL penicillin-streptomycin. The MCF-7 cell line is the most widely used and best characterized of all the human breast cancer cell linesCitation8. MCF-7 cells respond to estrogens and anti-estrogens, have differential expression of estrogen receptors (ER), progesterone receptor, and have high proliferation ratesCitation9. MCF-7 cells are also a perfect model to study the pathway of malignant progression as they can be subjected to appropriate endocrinologic and physiologic selective pressures for the derivation of variants with more progressed phenotypesCitation8.
Further in this work, some tests were performed on Ishikawa cell line (ISK) derived from an endometrial adenocarcinoma of a 39-year-old Asian womanCitation10. This cell line was maintained in Dulbecco’s modified Eagle’s medium-F12 (DMEM/F12), without phenol red, supplemented with 10% foetal bovine serum (Invitrogen, Milan, Italy) and 1 mg/mL penicillin-streptomycin.
Ishikawa cells express estrogenCitation11, progesteroneCitation12, androgenCitation13 and aryl hydrocarbons receptorsCitation14. There is a big evidence for the hormonal responsiveness of Ishikawa cells. This evidence is based mainly on hormonal modification of proliferation, cellular functions or gene expressionCitation15. In particular, these cells are used in vitro for the elucidation of molecular mechanisms of hormone action (e.g., in drug and discovery processes), testing of potential agonistic functions of anti-estrogens or selective estrogen receptor modulators in an endometrial-derived modelCitation16, in studies of ligand independent activation of the estrogen receptor and anchorage independent tumour growthCitation17. In addition, they are used for studies on factors controlling hormonal receptivityCitation18 and on environmental toxicology on the function of phytoestrogensCitation19 and endocrine-disrupting chemicalsCitation20 in an endometrial model; paracrine cell/cell-interactionCitation21, signalling cross-talkCitation14 and othersCitation22. Because of their hormonal responsiveness in vitro, Ishikawa endometrial adenocarcinoma cells have also been developed as an estrogen sensitive in vivo tumour model, which is particularly suitable for the study of hormonal growth controlCitation23.
Results and discussion
Carbazoles 1a–g were synthesizedCitation24–27 and characterizedCitation28–34 using earlier reported methods including ours.
In particular, the 9H-carbazoles 1a–d were prepared by the reaction of appropriate indoles with hexane-2,4-dione in the presence of p-toluenesulphonic acid. The 9H-carbazole 1e was instead prepared by reaction of 1a with ethyl chloroformate under standard conditionsCitation35. Furthermore, we have used 1a as starting compound for the preparation of 1f–g. In fact, 1a was N-methylated by iodomethane and in the presence of sodium hydride to give compound 1f. The carbazole NH could be N-protected by (Boc)2O to give 1g ().
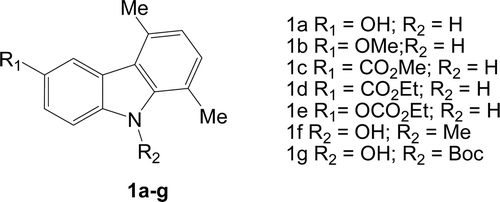
In order to evaluate the cytotoxic activity of prepared compounds (1a–g) against MCF-7 and ISK cell lines, an MTT assay was performedCitation36 using increasing concentrations (0.1, 1, 10 μM) of the compounds under testing. Compound 1a showed a significant inhibition at 1 and 10 μM, against both MCF-7 and ISK cell lines (, and ). Concerning the MCF-7 cells, a 30% inhibition was observed by the lower concentrations of 0.1 and 1 μM of 1b and 1c, respectively (. However, with ISK cells, compound 1b displayed a significant inhibitory effect on cell proliferation only at 10 μM concentration, while the inhibitory effect of 1c was observed at a concentration of 0.1 and 10 μM (. On the other hand, 1d showed an inhibitory effect against MCF-7 cells at 1 μM concentration (, and the same compound, however, displayed more inhibition against ISK cells at a concentration of 1 and 10 μM (. Compound 1e significantly reduced cell proliferation of both MCF-7 and ISK cells at a concentration of 0.1 μM and 1 μM (, and ).
Figure 2. Effect of carbazole derivatives (1a–e) against MCF-7 and ISK cell proliferation. MCF-7 (A) and ISK (B) cells were treated for 96 or 24 h, respectively, after 24-h starvation, with the indicated concentrations of substances 1a, 1b, 1c, 1d and 1e. Cells proliferation was evaluated by MTT assay. Statistically significant differences are indicated. Histograms; mean of three independent experiments each performed with triplicate samples expressed as percent of basal; bars, SE (*P < 0.01 compared with basal).
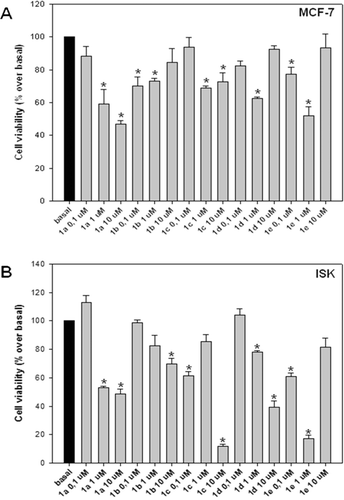
Based on the above results indicating the ability of compound 1a to inhibit proliferation of both cell lines (, and ), structural modifications of 1a were made in order to find out whether the introduction of a substituent such as a methyl- (1f) or a Boc substituent (1g), at the 9 position of the carbazole nucleus could affect the antiproliferative activity.
showed that the introduction of a methyl group (an electron-donating small substituent), improved the inhibitory activity, whereas the introduction of Boc group did not show any improvement of activity. The derivative 1f showed the highest inhibitory activity against both cell lines (at low concentration of 0.1 µM) (, and ), the matter that encourages its use as a possible antitumor agent. However, the observation that higher concentrations (1, 10 µM) of 1f do not induce dose-response inhibitory effect on both MCF-7 and ISK cell viability suggests a possible role of 1f in blocking a survival pathway binding to receptor desensitized at high concentration. This hypothesis is currently under investigation in our laboratories.
Figure 3. Effect of carbazole derivatives (1f–g) against MCF-7 and ISK cell proliferation. MCF-7 (A) and ISK (B) cells were treated for 96 or 24 h, respectively, after 24-h starvation, with the indicated concentrations of substances 1f and 1g. Cells proliferation was evaluated by MTT assay. Statistically significant differences are indicated. Histograms; mean of three independent experiments each performed with triplicate samples expressed as percent of basal; bars, SE (*P < 0.01 compared with basal).
Acknowledgements
We are thankful to Dr. Ewa Surmacz (Sbarro Institute for Cancer Research and Molecular Medicine, Philadelphia, PA, USA) and to Dr. D. Picard (University of Geneva, Geneva, Switzerland) for their kind offer of MCF-7 and Ishikawa cell lines, respectively.
Declaration of interest
The authors report no conflicts of interest.
References
- De Koning CB, Michael JP, Rousseau AL. A versatile and convenient method for the synthesis of substituted benzo[a]carbazoles and pyrido[2,3-a]carbazoles. J Chem Soc Perkin Trans 2000;1:1705–1713.
- Caruso A, Lancelot JC, El-Kashef H, Sinicropi MS, Legay R, Lesnard A, Rault S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009;65:10400–10405.
- Caruso A, Voisin-Chiret AS, Lancelot JC, Sinicropi MS, Garofalo A, Rault S. Efficient and simple synthesis of 6-aryl-1,4-dimethyl-9H-carbazoles. Molecules 2008;13:1312–1320.
- Waldau D, Mikolasch A, Lalk M, Schauer F. Derivatization of bioactive carbazoles by the biphenyl-degrading bacterium Ralstonia sp. strain SBUG 290. Appl Microbiol Biotechnol 2009;83:67–75.
- Auclair C. Multimodal action of antitumor agents on DNA: The ellipticine series. Arch Biochem Biophys 1987;259:1–14.
- Stiborova M, Rupertova M, Schmeiser HH, Frei E. Molecular mechanisms of antineoplastic action of an anticancer drug ellipticine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2006;150:13–23.
- Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 1973;51:1409–1416.
- Bullinger D, Neubauer H, Fehm T, Laufer S, Gleiter CH, Kammerer B. Metabolic signature of breast cancer cell line MCF-7: Profiling of modified nucleosides via LC-IT MS coupling. BMC Biochem 2007;8–25.
- Matthews J, Gustafsson JA. Estrogen signaling: A subtle balance between ER α and ER β. Mol Interv 2003;3:281–292.
- Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nippon Sanka Fujinka Gakkai Zasshi 1985;37:1103–1111.
- Holinka CF, Hata H, Kuramoto H, Gurpide E. Responses to estradiol in a human endometrial adenocarcinoma cell line (Ishikawa). J Steroid Biochem 1986;24:85–89.
- Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K et al. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): Progesterone-induced expression of the α1 integrin. J Steroid Biochem Mol Biol 1996;59:31–39.
- Lovely LP, Appa Rao KB, Gui Y, Lessey BA. Characterization of androgen receptors in a well-differentiated endometrial adenocarcinoma cell line (Ishikawa). J Steroid Biochem Mol Biol 2000;74:235–241.
- Wormke M, Castro-Rivera E, Chen I, Safe S. Estrogen and aryl hydrocarbon receptor expression and crosstalk in human Ishikawa endometrial cancer cells. J Steroid Biochem Mol Biol 2000;72:197–207.
- Vollmer G. Endometrial cancer: Experimental models useful for studies on molecular aspects of endometrial cancer and carcinogenesis. Endocr Relat Cancer 2003;10:23–42.
- Labrie F, Labrie C, Bélanger A, Simard J, Giguère V, Tremblay A et al. EM-652 (SCH57068), a pure SERM having complete antiestrogenic activity in the mammary gland and endometrium. J Steroid Biochem Mol Biol 2001;79:213–225.
- Holinka CF, Anzai Y, Hata H, Kimmel N, Kuramoto H, Gurpide E. Proliferation and responsiveness to estrogen of human endometrial cancer cells under serum-free culture conditions. Cancer Res 1989;49:3297–3301.
- Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR et al. Osteopontin and its receptor αvβ(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab 2001;86:4991–5000.
- Frigo DE, Duong BN, Melnik LI, Schief LS, Collins-Burow BM, Pace DK et al. Flavonoid phytochemicals regulate activator protein-1 signal transduction pathways in endometrial and kidney stable cell lines. J Nutr 2002;132:1848–1853.
- Bergeron RM, Thompson TB, Leonard LS, Pluta L, Gaido KW. Estrogenicity of bisphenol A in a human endometrial carcinoma cell line. Mol Cell Endocrinol 1999;150:179–187.
- Arnold JT, Lessey BA, Seppälä M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res 2002;62:79–88.
- Kanishi Y, Kobayashi Y, Noda S, Ishizuka B, Saito K. Differential growth inhibitory effect of melatonin on two endometrial cancer cell lines. J Pineal Res 2000;28:227–233.
- Nishida M, Kasahara K, Tsuji T, Kaneko M, Iwasaki H. Mechanisms of the antitumor action of gestagens on endometrial cancer. Nippon Sanka Fujinka Gakkai Zasshi 1986;38:2214–2222.
- (a) Cranwell A, Saxton JE. A synthesis of ellipticine. J Chem Soc 1962;3482–3487; (b) Lancelot JC, Rault S, Robba M, Nguyen HD. Efficient synthesis of 6H-pyrido[3,2-b]carbazole derivatives from 3-amino-1,4-dimethylcarbazole. Chem Pharm Bull 1987;35:425–428.
- (a) Tabka T, Letois B, Lancelot JC, Gauduchon P, Heron JF, Le Talaer JY, Rault S, Robba M. Etude de la cytotoxicité in vitro de dérivés du carbazole II. Nitro et amino halogéno-6 diméthyl-1,4 9H-carbazoles. Eur J Med Chem, 1989;24:605–610; (b) Testard A, Picot L, Fruitier-Arnaudin I, Piot JM, Chabane H, Domon L, Thiery V, Besson T. Microwave-assisted synthesis of novel thiazolocarbazoles and evaluation as potential anticancer agents. Part III. J Enzym Inhib Med Chem, 2004;19:467–473.
- Moinet-Hedin V, Tabka T, Sichel F, Gauduchon P, Le Talaer JY, Saturnino C, Letois B, Lancelot JC, Robba M. In vitro cytotoxicity of carbazole derivatives. V. 9-Halogeno-substituted 5,11-dimethyl-6H-pyrido[3,2-b]carbazoles. Eur J Med Chem 1997;32:113–122.
- Caruso A, Voisin-Chiret AS, Lancelot JC, Sinicropi MS, Garofalo A, Rault S. Novel and efficient synthesis of 5,8-dimethyl-9H-carbazol-3-ol via a hydroxydeboronation reaction. Heterocycles 2007;71(10):2203–2210.
- (1a) White solid, mp 174°C. IR (KBr) (cm-1): 3517, 3415, 1461, 1165, 847, 809, 543. 1HNMR (DMSO-d6): δ 2.50 (s, 3H, CH3), 2.61 (s, 3H, CH3); 6.83 (d, J = 7.36 Hz, 1H, Ar); 6.95 (d, J = 8.56 Hz, 1H, Ar); 7.08 (d, J = 7.36 Hz, 1H, Ar); 7.40 (d, J = 8.56 Hz, 1H, Ar); 7.53 (s, 1H, Ar); 9.12 (br, 1H, OH); 10.88 (s, 1H, NH). MS (ESI+): 212 (M+ +1).
- (1b) White solid, mp 150°C. IR (KBr) (cm-1): 3406, 2959, 1481, 1210, 1045, 812, 545. 1H NMR (DMSO-d6): δ 2.62 (s, 3H, CH3), 2.69 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 6.85 (d, J = 7.08 Hz, 1H, Ar); 7.09 (d, J = 8.28 Hz, 2H, Ar), 7.48 (d, J = 8.56 Hz, 1H, Ar), 7.64 (s, 1H, Ar), 11.01 (br, 1H, NH). MS (ESI+): 226 (M+ +1), 224 (M+ -1).
- (1c) White solid, mp 266°C. IR (KBr) (cm-1): 3337, 1702, 1615, 1430, 1290, 1122, 989, 803, 764, 735, 557. 1H NMR (DMSO-d6): δ 2.52 (s, 3H, CH3), 2.71 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 6.89 (d, J = 7.84 Hz, 1H, Ar); 7.11 (d, J = 7.32 Hz, 1H, Ar), 7.55 (d, J = 8.08 Hz, 1H, Ar), 7.98 (dd, J1 = 1.48 Hz, J2 = 8.56 Hz, 1H, Ar), 8.67 (s, 1H, Ar), 11.62 (s, 1H, NH). MS (ESI+): 254 (M+ +1).
- (1d) White solid, mp 253°C. IR (KBr) (cm-1): 3339, 1705, 1618, 1430, 1295, 1129, 990, 806, 765, 738, 560. 1H NMR (DMSO-d6): δ 1.67 (s, 3H, CH3), 2.53 (s, 3H, CH3), 2.80 (s, 3H, CH2CH3), 4.30-4.60 (m, 2H, OCH2), 6.95 (d, J = 7.50 Hz, 1H, Ar); 7.16 (d, J = 7.20 Hz, 1H, Ar), 7.55 (d, J = 8.70 Hz, 1H, Ar), 8.04 (d, J = 8.70 Hz, 1H, Ar), 8.72 (s, 1H, Ar), 11.59 (br, 1H, NH). MS (ESI+): 268 (M+ +1).
- (1e) White solid, mp 130°C. IR (KBr) (cm-1): 3396, 1744, 1462, 1253, 1181, 1002, 811, 780, 552. 1H NMR (DMSO-d6): δ 1.32-1.40 (t, 3H, CH2CH3), 2.57 (s, 3H, CH3), 2.78 (s, 3H, CH3), 4.28–4.35 (q, 2H, CH2CH3), 6.91 (d, 1H, J = 7.08 Hz, Ar), 7.16 (d, 1H, J = 7.32 Hz, Ar), 7.29 (dd, 1H, J1 = 1.72 Hz, J2 = 8.56 Hz, Ar), 7.58 (d, 1H, J = 8.56 Hz, Ar), 7.94 (s, 1H, Ar), 11.36 (s, 1H, NH). MS (ESI+): 284 (M+ +1).
- (1f) White solid, mp 202°C. IR (KBr) (cm-1): 3415, 1587, 1460, 1165, 809, 543. 1H NMR (CDCl3): δ 8.23 (d, J = 1.96 Hz, 1H, Ar); 7.52 (dd, J = 8.80 Hz, 1H, Ar); 7.23 (d, J = 8.80 Hz, 1H, Ar); 7.07 (d, J = 7.32 Hz, 1H, Ar); 6.88 (d, J = 7.32 Hz, 1H, Ar); 4.07 (s, 3H, NCH3); 2.80 (s, 3H, CH3); 2.78 (s, 3H, CH3). MS (ESI+): 226 (M+ +1).
- (1g) White solid, mp 205°C. IR (KBr) (cm-1): 3410, 1701, 1449, 1329, 1157, 807. 1HNMR (CDCl3): δ 1.64 (s, 9H, CH3); 2.43 (s, 3H, CH3); 2.74 (s, 3H, CH3); 6.91 (d, J = 8.56 Hz, 1H, Ar); 6.98 (d, J = 7.32 Hz, 1H Ar,); 7.09 (d, J = 7.32 Hz, 1H, Ar); 7.46 (s, 1H, OH); 7.63 (d, J = 8.56 Hz, 1H, Ar); 7.85 (s, 1H, Ar). MS (EI) m/z (%): 311 (M+, 15), 255 (48) (M+, -tBu), 211 (100) (M+, -CO2tBu).
- Caruso A. Ph.D. Thesis. Synthesis of novel dimethylcarbazole, dimethylpyrimidocarbazole, benzofuro quinazolinone, benzothienoquinazolinone derivatives with potential anticancer activity. University of Calabria-Italy, 17.12. 2008.
- Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 2011;716:157–168.