Abstract
In the present study, apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and Kit were studied. For testing the possible apoptotic effects of majdine and isomajdine from V. herbacea, DNA fragmentation assay was conducted on the rat brain cortical tissue homogenates, in vitro. Also their possible effects on mitochondrial activity were tested by using the same tissue samples of rats. In addition, the antioxidant activity of isomajdine and majdine was determined using various in vitro antioxidant assays, including 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+) radical scavenging and N,N-dimethyl-p-phenylenediamine (DMPD•+) radical scavenging, ferric ions (Fe3+) and cupric ions (Cu2+) reducing abilities and ferrous ions (Fe2+) chelating activity. On the other hand, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol and trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were used as reference antioxidants.
Introduction
Antioxidants are substances that delay the oxidation process, inhibit the polymerisation chain, initiated by free radicals and other subsequent oxidising reactions. Thereby, they help prevent cancer, heart disease, diabetes mellitus, neurodegenerative and inflammatory diseasesCitation1. Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate and tert-butylhydroquinone are the most commonly used synthetic antioxidantsCitation2. Although, many synthetic antioxidants are promising for various human ailments, pro-oxidant or cytotoxic natures at higher concentration limit their long term use. In addition, these are suspected to be responsible for liver damage and carcinogenesis. Therefore, new interest has been developed to search natural and safe antioxidative agentsCitation3.
Many antioxidant compounds, naturally occurring in plant sources, have been identified as free radical or active oxygen scavengers. Recently, interest has considerably increased in finding naturally occurring antioxidants for use in foods or medical materials to replace synthetic antioxidants, which are being restricted due to their side effects such as carcinogenicity. Natural antioxidants can protect the human body from free radicals and retard the progress of many chronic diseases as well as lipid oxidative rancidity in foodsCitation3.
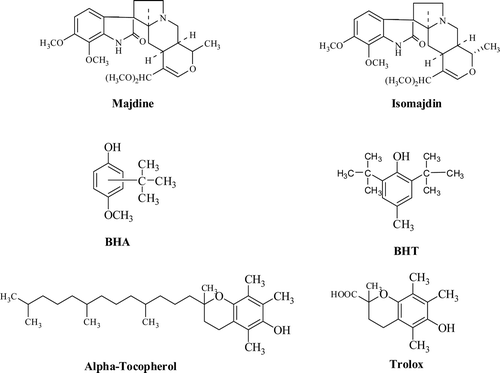
Vinca herbacea Waldst. and Kit. is in the Apocynaceae family, which is composed of dicots, usually herbs that have two either opposite or whorled entire leavesCitation4. This plant has many alkaloid kinds with high amountCitation12. Vincoline an alkaloid previously isolated from V. herbacea has been isolated and its structure elucidatedCitation5. The chemical structures of majdine and isomajdine isolated from V. herbacea Waldst. and Kit were given in .
Figure 1. Chemical structures of standard antioxidants (BHA, BHT, α-tocopherol and trolox), majdine and isomajdine from Vinca herbacea Waldst. and Kit.
Apoptosis is a form of cell death in which a cell undergoes an internally controlled transition from an intact metabolically active state into a number of shrunken remnants retaining their membrane bound integrity. Moreover, direct exposure of cells to various oxidants causes multiple intracellular alterations, particularly inflammation. Therefore, the presence of apoptosis directly linked with antioxidants in living organismsCitation6.
The aim of the present study was to investigate apoptotic and antioxidant activities of isomajdine and majdine isolated from V. herbacea by using different antioxidant tests including ABTS•+ scavenging, DMPD•+ scavenging, ferric ions (Fe3+) reducing ability by Fe3+–Fe2+ transformation, cupric ions (Cu2+) reducing ability by CUPRAC method and ferrous ions (Fe2+) chelating activity. An important goal of this research was to compare these compounds to most commonly used synthetic antioxidants such as BHA, BHT, α-tocopherol, and trolox.
Materials and methods
Chemicals
N,N-dimethyl-p-phenylenediamine (DMPD), neocuproine (2,9-dimethyl-1,10-phenanthroline), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), 3-(2-pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), α-tocopherol and trolox were obtained from Sigma (Sigma–Aldrich GmbH, Steinheim, Germany). All other chemicals used, were of analytical grade, and obtained from either Sigma–Aldrich or Merck.
Plant materials
The roots of V. Herbacea Waldst. and Kit (Fam. Apocynaceae) were collected in the East of Georgia in a phase of flowering (2005). The plant was identified in the department of Pharmacobotany I. Kutateladze Institute of Pharmacochemistry [Herbarium #9 (120) Tbilisi, Georgia].
Isolation of majdine and isomajdine
The air-dried plant material (10 kg) was basified with ammoniac (25%) and extracted with chloroform (3 × 50 L) at room temperature. The combined extracts were concentrated in vacuum to a 5 L and fractioned with gradient pH with citrate–phosphate buffer. Crude alkaloids obtained at pH: 3.6 (30 g) from methanol was considered as isomajdine (2.3 g) and at pH: 3.0 (30 g) from methanol was considered as majdine (4.6 g). It was proved that isolated compounds are majdine and isomajdine by thin layer chromatography, than NMR spectra has been performed. Isomajdine and majdine were dissolved in DMSO (20%) and final concentrations of 5, 10, 25, 50, 100 and 200 µM for both compounds were prepared. Cortical homogenates were incubated 24 h with H2O2 (1 mM), DMSO (20%) and majdine or isomajdine solutions.
Determination of antioxidant effects of isomajdine and majdine
Fe3+ reducing power assay
Reducing power of majdine and isomajdine were measured by direct reduction of Fe3+(CN−)6 to Fe2+(CN−)6, and were determined by again, measuring absorbance resulted from the formation of the Perl’s Prussian blue complex following the addition of excess ferric ions (Fe3+). For determination of the ferric reducing antioxidant power of isomajdine and majdine, the method of Oyaizu was usedCitation7. This method is based on the reduction of (Fe3+) ferricyanide in stoichiometric excess, relative to the antioxidants. Different concentrations of isomajdine and majdine (10–30 µg/mL) in 0.75 mL of distilled water were mixed with 1.25 mL of 0.2 M, pH 6.6 sodium phosphate buffer and 1.25 mL of potassium ferricyanide [K3Fe(CN)6] (1%). The mixture was incubated at 50°C for 20 min. After 30 min of incubation, the reaction mixture was acidified with 1.25 mL of trichloroacetic acid (10%). Finally, 0.5 mL of FeCl3 (0.1%) was added to this solution, and the absorbance was measured at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicates grater reduction capabilityCitation8.
Cupric ions (Cu2+) reducing-CUPRAC assay
In order to determine the cupric ions (Cu2+) reducing ability of isomajdine and majdine, the method proposed by Apak et alCitation9. For this reason, 0.25 mL CuCl2 solution (0.01 M), 0.25 mL ethanolic neocuproine solution (7.5 × 10−3 M) and 0.25 mL CH3COONH4 buffer solution (1 M) were added to a test tube, followed by mixing with different concentrations of isomajdine and majdine (10–30 μg/mL). Then, total volumes were adjusted to 2 mL with distilled water, and mixed well. The tubes were stoppered and kept at room temperature. Absorbance was measured at 450 nm against a reagent blank, 30 min later. Increased absorbance of the reaction mixture indicates increased reduction capabilityCitation10.
Chelating activity on ferrous ions (Fe2+)
Ferrous ions (Fe2+) chelating activity of isomajdine and majdine was measured by inhibiting the formation of Fe2+–ferrozine complex after the treatment of test material with Fe2+, following the method of Dinis and co-workersCitation11. Fe2+-chelating ability of isomajdine and majdine was monitored by the absorbance of the ferrous iron–ferrozine complex at 562 nm. Briefly, different concentrations of isomajdine and majdine (10–30 µg/mL) in 0.4 mL methanol were added to a solution of 0.6 mM FeCl2 (0.1 mL). The reactions were initiated by the addition of 5 mM ferrozine (0.1 mL) dissolved in methanol. Then, the mixture was shaken vigorously and left at room temperature for 10 min. Absorbance of the solution was then measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated by using the formula given below:
where λ562-C is the absorbance of control and λ562-S is the absorbance in the presence of amandine, isomajdine or standards. The control contains only FeCl2 and ferrozineCitation12.
ABTS•+ scavenging activity
In this method, an antioxidant is added to a pre-formed ABTS radical solution, and after a fixed time period, the remaining ABTS•+ is quantified spectrophotometrically at 734 nmCitation12. The ABTS•+ was produced by reacting 2 mM ABTS in H2O with 2.45 mM potassium persulfate (K2S2O8), stored in the dark at room temperature for 6 h. The ABTS•+ solution was diluted to give an absorbance of 0.750 ± 0.025 at 734 nm in 0.1 M sodium phosphate buffer (pH 7.4). Then, 1 mL of ABTS•+ solution was added to 3 mL of isomajdine and majdine solution in ethanol at different concentrations (10–30 µg/mL). The absorbance was recorded 30 min after mixing, and the percentage of radical scavenging was calculated for each concentration relative to a blank, containing no scavenger. The extent of decolourisation is calculated as the percentage reduction of absorbance. The ABTS•+ concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (r2: 0.9899):
The scavenging capability of test compounds was calculated using the following equation:
where λ734-C is absorbance of a control lacking any radical scavenger and λ734-S is the absorbance of the remaining ABTS•+ in the presence of scavengerCitation13.
DMPD•+ scavenging activity
The second antiradical capacity of isomajdine and majdine were analysed by DMPD•+ assay. DMPD radical scavenging ability of isomajdine and majdine were performed according to Fogliano and co-workersCitation14. In the presence of Fe3+, a coloured DMPD radical cation is generated; antioxidant compounds able to transfer a hydrogen atom to DMPD•+ cause a decolourisation of the solution measured by the decrease in the absorbance at 505 nm. DMPD (100 mM) was prepared by dissolving 209 mg of DMPD in 10 mL of deionised water, and 1 mL of this solution was added to 100 mL of 0.1 M acetate buffer (pH 5.25), and the coloured radical cation (DMPD•+) was obtained by adding 0.2 mL of a solution of 0.05 M ferric chloride (FeCl3). The absorbance of this solution, which is prepared daily, is constant up to 12 h at room temperature. Different concentrations of standard antioxidants or isomajdine and majdine (10–30 μg/mL) were added in test tubes, and the total volumes were adjusted to 0.5 mL with distilled water. Ten minutes later, the absorbance was measured at 505 nm. One millilitre of DMPD•+ solution was directly added to the reaction mixture, and its absorbance was measured at 505 nm. The buffer solution was used as a blank sample. The DMPD•+ concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (r2: 0.9993):
The scavenging capability of ABTS•+ radical was calculated using the following equation:
where in λ505-C is the initial concentration of the DMPD•+ and λ505-S is absorbance of the remaining concentration of DMPD•+ in the presence of isomajdine and majdine.
Determination of apoptotic effects of isomajdine and majdine
Animals
A total of 6 mature (180–220 g) male Sprague-Dawley rats were used in the present study. The procedure was suitable for the Animal Care and Ethics Committee of Abant Izzet Baysal University, Medical Faculty. Animals were anaesthetised under the ether vapour, decapitated and the temporal cortical tissues were dissected out and homogenised in 5 mL of Dulbecco’s Minimal Essential Medium (DMEM, Sigma) solution, by using ultraturrax. Then, the homogenised cortical tissues were aliquoted equally into 10 eppendorf tubes with glycine (20%) and kept at−80°C until the pharmacological procedure.
DNA fragmentation assay
The DNA fragmentation assay allows determining the amount of DNA that is degraded upon treatment of cells with the following doses of majdine and isomajdine as well as reference compound of this test, hydrogen peroxide (H2O2). The dishes were kept at 37°C in humidified 95% air and 5% CO2, with H2O2, DMSO, isomajdine or majdine for 24 h, in DMEM. After that period, homogenates were centrifuged at 3000 rpm for 3 min; pellets were washed twice with fresh Hank’s Balanced Salt Solution (HBSS). Than the pellets were lysed in a buffer containing 0.1 M NaCl, 10 mM Tris (pH: 8.0), 1 mM EDTA (pH: 8.0), 1% SDS and 2% proteinase K for additional 24 h at 50°C. Lysates were vortexed and cleared by centrifugation at 10000 ×g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of neutral phenol:chloroform:isoamyl alcohol mixture (25:24:1) and analysed electrophoretically on 1.5% agarose gels containing 0.1 µg/mL ethidium bromide at 100 V for 50 min.
WST-1 assay
For testing mitochondrial damage, cell proliferation reagent of WST-1 (Roche) was used. Briefly, the test was based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. After incubating the cortical tissue homogenates with H2O2, DMSO, isomajdine or majdine for 24 h, in Dulbecco’s Modified Eagle Medium (DMEM), at 37°C in humidified 95% air and 5% CO2 in the doses which described above. They were centrifuged at 3000 rpm for 3 min, after that the supernatant was removed. Pellets were washed twice with fresh HBSS solution. HBSS solution of 180 µL and WST-1 reagent of 20 µL were added into the each tube and homogenates were left in same conditions as described above for additionally 4 h. At the end of that period, tubes were centrifuged at 2000 ×g for 4 min, 50 µL of supernatant from each tube was transferred into the 96 well plates. By using a microplate reader, mitochondrial activity was measured at 480 nm wavelength while the reference wavelength set up at 690 nm.
Statistical analysis
The experimental results were performed in triplicate. The data were recorded as mean ± standard deviation and analysed by SPSS (version 11.5 for Windows 2000, SPSS Inc.). One-way analysis of variance ANOVA was performed by procedures. Significant differences between means were determined by Duncan’s Multiple Range tests, and p < 0.05 was regarded as significant, and p < 0.01 was very significant.
Results
Reducing power reflects the electron donating capacity of bioactive compounds and is associated with antioxidant activity. Isomajdine and majdine had effective reducing power determined by using the potassium ferricyanide reduction and cupric ions reducing methods, when compared to the standards. For the measurements of the reductive ability of isomajdine and majdine, Fe3+–Fe2+ transformation was investigated in the presence of isomajdine and majdine using the method of OyaizuCitation7. As can be seen from , isomajdine (r2: 0.921) and majdine (r2: 0.979) demonstrated effective Fe3+ reducing ability with these differences being statistically significant (p < 0.01). The reducing power of isomajdine, majdine, BHA, BHT, α-tocopherol and trolox increased steadily with increasing concentration of samples. Reducing power of isomajdine, majdine and standard compounds was as follows: BHA > trolox ≈ BHT > α-tocopherol > majdine > isomajdine (at the concentration of 30 µg/mL). These results demonstrated that isomajdine and majdine had marked ferric ions (Fe3+) reducing ability and had electron donor properties for neutralising free radicals by forming stable products. The outcome of the reducing reaction is to terminate the radical chain reactions that may otherwise be very damaging.
Figure 2. (A) Fe3+-Fe2+ reductive potential of different concentrations (10–30 g/mL) of majdine (r2:0.933), isomajdine (r2:0.933) and reference antioxidants BHA, BHT, α-tocopherol and trolox using spectrophotometric detection of the Fe+3-Fe+2 transformation. (B) Cupric ions (Cu2+) reducing ability (Cuprac method) of different concentrations (10–30 g/mL) of majdine (r2: 0.9330), isomajdine (r2: 0.9330), BHA, BHT, α-tocopherol and trolox using spectrophotometric detection of the Cu+2-Cu+ transformation. (BHA: Butylated hydroxyanisole, BHT: Butylated hydroxytoluene).
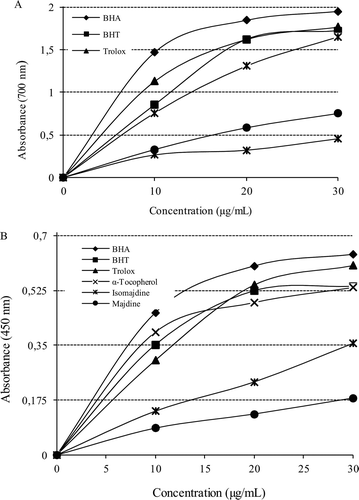
A cupric ion (Cu2+) reducing ability of isomajdine and majdine is shown in . A correlation was observed between the cupric ions reducing ability and isomajdine (r2: 0.994) or majdine concentrations (r2: 0.9767). Cu2+ reducing capability of isomajdine and majdine by CUPRAC method was found to be concentration- dependent (10–30 μg/mL). Cu2+ reducing powers of isomajdine, majdine and standard compounds at the same concentration (30 μg/mL) were as follows: BHA > trolox α BHT > α-tocopherol > isomajdine > majdine.
All the tested compounds exhibited effective ABTS•+ radical cation scavenging activity. As seen in , isomajdine and majdine have an effective ABTS•+ radical scavenging activity. EC50 value for isomajdine and majdine in this assay were 34.11 and 57.69 μg/mL, respectively. On the other hand, EC50 values for BHA, BHT, α-tocopherol and trolox were found to be 4.65, 52.81, 73.15 and 62.32 μg/mL, respectively. The scavenging effect of isomajdine, majdine and standards on ABTS•+ decreased in the following order: BHA > isomajdine > BHT > majdine > trolox > α-tocopherol (98.1, 97.8, 96.9, 86.3, 79.6 and 55.9%, respectively) at the same concentration (30 μg/mL).
Table 1. Comparison of IC50 values of ABTS•+ scavenging, DMPD•+ scavenging and ferrous ion (Fe2+) chelating activity of majdine, isomajdine and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene, ABTS•+:2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radicals, DMPD•+: N,N-dimethyl-p-phenylenediamine radicals).
As shown in , isomajdine and majdine were an effective DMPD•+ radical scavenger in a concentration-dependent manner (10–30 μg/mL). EC50 for isomajdine and majdine were found 32.9 and 71.24 μg/mL. This value was found as 63.25 μg/mL for BHA and 14.89 μg/mL for trolox. There was a significant decrease (p < 0.05) in the concentration of DMPD•+ due to the scavenging capacity at all isomajdine and majdine concentrations.
The data obtained from reveal that isomajdine and majdine possess a marked capacity for iron binding, suggesting that their main action as a peroxidation inhibitor may be related to their iron binding capacity. In this assay, isomajdine and majdine interfered with the formation of the ferrous–ferrozine complex. It suggests that isomajdine and majdine have chelating activity and are able to capture ferrous ion before ferrozine. Isomajdine and majdine had strong chelating effect on ferrous ions (Fe2+). As can be seen in , the ferrous ion chelating effect of isomajdine and majdine were compared to that of BHA, BHT, α-tocopherol and trolox. IC50 values of ferrous ion chelating capacity of BHA, BHT, α-tocopherol and trolox were found to be 34.31, 13.72, 16.31 and 16.17 µg/mL, respectively. On the other hand, IC50 values of ferrous ion chelating capacity of isomajdine and majdine were found to be 10.05 and 14.01, respectively. These results show that the ferrous ion chelating effect of isomajdine and majdine were statistically similar to that of BHT, trolox and α-tocopherol (p > 0.05) but higher than that of BHA (p < 0.01).
According to the results of DNA fragmentation and WST-1 assays, isomajdine and majdine induce apoptosis more potently than H2O2. As can be seen in and , H2O2 in 1 mM concentration, for 24 h, induces DNA fragmentation, in respect to controls. Isomajdine, especially in higher concentrations such as 200 and 100 µM, showed same DNA fragmentation profile with H2O2 and this effect maintained until 10 and 5 µM concentrations. Despite the fact that DNA fragmentation effect of majdine was not as strong as isomajdine, almost all doses of majdine slightly induced DNA fragmentation, in respect to controls. It is obvious that 10 and 5 µM concentrations of isomajdine decreased DNA fragmentation in respect to higher concentrations of it and H2O2.
Figure 3. WST-1 assay of the groups. [H2O2: 1 mM of hydrogen peroxide. Iso: isomajdine and majd: majdine in µM concentrations. *p < 0.05, **p < 0.01 with respect to control group (Con), WST-1: water-soluble tetrazolium salt].
![Figure 3. WST-1 assay of the groups. [H2O2: 1 mM of hydrogen peroxide. Iso: isomajdine and majd: majdine in µM concentrations. *p < 0.05, **p < 0.01 with respect to control group (Con), WST-1: water-soluble tetrazolium salt].](/cms/asset/1b3885b7-9b55-453b-b471-caab639c2fa8/ienz_a_604318_f0003_b.gif)
Controversially to our expectations in the ANOVA and the independent samples t-test, H2O2, isomajdine and majdine were found to induce mitochondrial activity, as a result of WST-1 assay. All of the groups tested, including H2O2, induce formazan formation from tetrazolium salt, more than the control group. During the pre-apoptotic period, the consumption and demand of ATP increase for intracellular chromatin condensation, formation of apoptotic vacuoles, etc. The highest mitochondrial activity was found in the groups of isomajdine’s 5 and 10 µM and majdine’s 25 µM concentrations ().
Discussion
It was suggested that the electron donating capacity, reflecting the reducing power of bioactive compounds, is associated with antioxidant activityCitation15. Antioxidants can be reductants, and inactivation of oxidants by reductants can be described as redox reactions in which one reaction species is reduced at the expense of the oxidation of the other. The presence of reductants, such as antioxidant substances in the samples, causes the reduction of the Fe3+–ferricyanide complex to the ferrous form. Fe2+ formed can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm. An increase in absorbance of the reaction mixture would indicate an increase in the reducing capacity due to an increase in the formation of the complex. Antioxidants can exert their effects via reducing and inactivating the oxidants. The reducing capacity of a compound can be measured by the direct reduction of Fe[(CN)6]3 to Fe[(CN)6]2. There are a number of assays designed to measure overall antioxidant activity, or reducing potential, as an indication of a host’s total capacity to withstand free radical stressCitation16. The ferric ion reducing antioxidant power assay takes advantage of an electron transfer reaction in which a ferric salt is used as an oxidant. In this assay, the yellow colour of the test solution changes to various shades of green and blue depending on the reducing power of antioxidant samples. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activityCitation8.
In the present study, we also used the CUPRAC assay which is based on reduction of Cu2+ to Cu+ by antioxidants. CUPRAC chromogenic redox reaction is carried out at a pH (7.0) close to the physiological pH, and the method is capable of measuring thiol-type antioxidants such as glutathione and non-protein thiols unlike the widely applied FRAP test, which is non-responsive to -SH group antioxidantsCitation8.
Elemental species, such as ferrous iron (Fe2+), can facilitate the production of ROS within living organisms, and the ability of substances to chelate iron can be valuable for antioxidant property. Ferrous ions are the most effective pro-oxidants in food systems; the good chelating effect would be beneficial, and removal of free iron ion from circulation could be a promising approach to prevent oxidative stress-induced diseases. Iron, in nature, can be found as either ferrous (Fe2+) or ferric ion (Fe3+), with the latter form predominant in foods. Ferrous chelation may render important antioxidative effects by retarding metal-catalysed oxidationCitation17.
Ferrous ion chelating activities of isomajdine, majdine, BHA, BHT, α-tocopherol and trolox are shown in . The chelation of ferrous ions by isomajdine and majdine or standards was determined according to the method of Dinis et alCitation11. An effective ferrous ion chelator affords protection against oxidative damage by removing iron that may otherwise participate in HO• generation via the Fenton type reactions. Ferric ions (Fe3+) also produce radicals from peroxides although the rate is 10-fold less than that of ferrous ionCitation18. Minimising ferrous ion may afford protection against oxidative damage by inhibiting production of ROS and molecular damage. Also, ferrozine can quantitatively form complexes with Fe2+ in this method. In the presence of chelating agents, complex formation is disrupted, resulting in a reduction in the red colour of the complex. Measurement of colour reduction therefore allows estimation of the metal chelating activity of the coexisting chelator. Lower absorbance indicates higher metal chelating activityCitation19.
One measurement method of the metal-chelating activity of an antioxidant is based on the absorbance measurement of Fe2+–ferrozine complex after prior treatment of a ferrous ion solution with test material. Ferrozine forms a complex with free Fe2+, but not with Fe2+ bound to other chelators; thus, a decrease in the amount of ferrozine–Fe2+ complex formed after treatment indicates the presence of antioxidant chelatorsCitation19. The ferrozine–Fe2+ complex produces a red chromophore with absorbance that can be measured at λ562 nm. A significant drawback of this complexation reaction in measuring the presence of antioxidant chelator is that the reaction is affected by both the antioxidant-Fe2+ and ferrozine–Fe2+ complex formation constants, and the competition between the two chelators for binding to iron. Thus, a weak antioxidant iron chelator would be seriously underestimated in quantitative determination. Nonetheless, this reaction serves as a convenient assay to access iron chelating activity of antioxidantsCitation19.
The antioxidants are believed to intercept the free radical chain of oxidation and donate hydrogen from the phenolic hydroxyl groups, thereby form a stable end-product which does not initiate or propagate further oxidation of lipids. Assays based upon the use of ABTS•+ and DMPD•+ radicals are among the most popular spectrophotometric methods for determination of the antioxidant capacity of foods, beverages and vegetable extracts. Both chromogens and radical compounds can directly react with antioxidants. ABTS•+ are more reactive radicals, which involve H atom transfer and the reactions with ABTS•+ radicals involve an electron transfer process. Generation of the ABTS radical cation forms the basis of one of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity of pure substances, aqueous mixtures and beveragesCitation20. A more appropriate format for the assay is a decolourisation technique in which the radical is generated directly in a stable form prior to the reaction with putative antioxidants.
The ABTS assay is based on the inhibition of the absorbance of the radical cation ABTS•+, which has a characteristic long-wavelength absorption spectrum showing absorption at 734 nm. Bleaching of a pre-formed solution of the blue-green radical cation ABTS•+ has been extensively used to evaluate the antioxidant capacity of complex mixtures and individual compounds. The reaction of the pre-formed radical with free radical scavengers can be easily monitored by following the decay of the sample absorbance at 734 nmCitation20.
The principle of the DMPD•+ assay is that DMPD can form a stable and coloured radical cation (DMPD•+) at acidic pH and in the presence of a suitable oxidant solution. The UV–visible spectrum of DMPD•+ shows a maximum absorbance at 505 nm. Antioxidant compounds, which are able to transfer a hydrogen atom to DMPD•+, quench the colour and produce a decolourisation of the solution. This reaction is rapid, and the end point, which is stable, is taken as a measure of the antioxidative efficiency. Therefore, this assay reflects the ability of radical hydrogen-donors to scavenge the single electron from DMPD radicalsCitation21.
Preliminary experiments show that the choice of oxidant solution and the ratio between the concentration of DMPD•+ and the concentration of the oxidative compound are crucial for the effectiveness of the method. In fact, the formation of radical cation is very slow and results in a continuous increase of the absorbance. The best results were obtained with FeCl3, which gives a stable coloured solution up to a final concentration of 0.1 mM. Moreover, this method ensures low cost and highly reproducible analysisCitation22.
DMPD assay is particularly suitable for hydrophilic antioxidants, but is less sensitive to hydrophobic bioactive compoundsCitation14, the opposite of the other two tests. In contrast to the ABTS procedure, the DMPD•+ method guarantees a very stable end point. This is particularly important when a large-scale screening is required. It was reported that the main drawback of the DMPD•+ method is that its sensitivity and reproducibility dramatically decreases when hydrophobic antioxidants such as α-tocopherol or BHT were used. Hence, these standard antioxidant compounds were not used in this antiradical assayCitation22.
Phytochemicals have been known to possess a number of physiological benefits, including antioxidant, anti-inflammatory, antiapoptotic and anti-carcinogenic activitiesCitation8,Citation23. Moreover, direct exposure of cells to various oxidants causes multiple intracellular alterations, particularly inflammation. The response of a cell to an increase in oxidants or inflammatory stimuli often involves the activation of numerous intracellular signalling pathways. These cytosolic pathways can, in turn, regulate a host of transcriptional changes that allow the cell to respond appropriately to oxidative stress. The evidence suggests that certain transcription factors can directly or indirectly alter their activities in the activation of numerous intracellular signalling pathways, depending on cellular redox conditionsCitation24.
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include loss of cell membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentationCitation25. Increase in mitochondrial activity, instead of mitochondrial damage and inactivity in WST-1 results gives strong clues that apoptosis works on the non-mitochondrial pathway. It is possible that cytoplasmic proteins such as granzyme B, JNK or NFB activation may be responsible for the non-mitochondrial pathway of apoptosis. Another possibility is that H2O2, majdine and isomajdine induce nuclear p53 directly, which upregulates proapoptotic genes such as Bax, Bad, Fas, TRAIL, Apaf-1 and/or AIP1. Increased mitochondrial activity in respect to control group could be explained as being a compensatory activation of antiapoptotic pathways via mitochondria. In our study, both majdine and isomajdine induced apoptosis via non-mitochondrial pathwaysCitation25.
As a conclusion, according to those results, it can be speculated that majdine and isomajdine had effective antioxidant and antiradical activity in different in vitro antioxidant assays including ABTS•+ and DMPD•+ radical scavenging, ferric ions (Fe3+) and cupric ions (Cu2+) reducing abilities and ferrous ions (Fe2+) chelating activity. The both compounds have phenolic groups. It is well-known that the phenolic compounds were demonstrated antioxidant effect and powerful reducing ability. Also, majdine and isomajdine induce apoptosis via non-mitochondrial pathways.
Declaration of interest
This study was partially supported by a Research Fund of Atatürk University. The authors are grateful to Atatürk University for financial support (Project no: 2008/75). The authors report no conflicts of interest.
References
- Gülçin İ, Topal F, Çakmakçı R, Gören AC, Bilsel M, Erdoğan U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:585–593.
- Gülçin İ, Topal F, Oztürk Sarikaya SB, Bursal E, Gören AC, Bilsel M. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–175.
- Gülçin I, Bursal E, Sehitoglu MH, Bilsel M, Gören AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–2238.
- Hashemloian BD, Azimi AA, Majd A, Ebrahimzadeh H. Abnormal plantlets regeneration through direct somatic embryogenesis on immature seeds of Vinca herbacea Waldst. and Kit. Afr J Biotechnol 2008;7:1679–1683.
- Aynilian GH, Weiss SG, Cordell GA, Abraham DJ, Crane FA, Farnsworth NR. Catharanthus alkaloids. XXIX. Isolation and structure elucidation of vincoline. J Pharm Sci 1974;63:536–538.
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002;108:153–164.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nut 1986;44:307–315.
- Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37.
- Apak R, Güçlü K, Ozyürek M, Esin Karademir S, Erçag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr 2006;57:292–304.
- Bursal E, Gülçin İ,. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–1489.
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–169.
- Gülçin İ,. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–220.
- Gülçin İ,. Antioxidant and antiradical activities of L-Carnitine. Life Sci 2006;78:803–811.
- Fogliano V, Verde V, Randazzo G, Ritieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 1999;47:1035–1040.
- Güllçin I, Dastan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–695.
- Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wissen Technol 2003;36:263–271.
- Gülçin İ. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007;32:431–438.
- Balaydin HT, Gülçin Í, Menzek A, Göksu S, Sahin E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–695.
- Gülçin İ. Antioxidant properties of resveratrol: A structure-activity insight. Innov Food Sci Emerg 2010;11:210–218.
- Talaz O, Gülçin I, Göksu S, Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–6589.
- Gülçin İ. Antioxidant activity of L-Adrenaline: An activity-structure insight. Chem Biol Interact 2009;179:71–80.
- Şerbetçi Tohma H, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–671.
- Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 1999;428:305–327.
- Haddad JJ. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for hypoxia-inducible factor-1alpha. Crit Care 2003;7:47–54.
- Alberts K, Johnson A, Lewis J, Raff M, Roberts WP. Apoptosis: Programmed cell death eliminates unwanted cells. Molecular biology of the cell. Chapter 18, 5th ed. Garland Science. 2008; p.1115.
