Abstract
Xanthene intermediates 4a and 4b were obtained from the reduction of nitro xanthene derivatives 3a and 3b which were synthesized via condensation of dimedone with m-nitrobenzaldehyde and p-nitrobenzaldehyde, respectively. Then xanthene sulfonamide 6a–n, and xanthene carboxamide derivatives 8a–h were synthesized by reaction of amino xanthene 4a, 4b with sulfonyl chlorides 5a–g and acyl chlorides 7a–d. Structures of the novel amino xanthene compounds and xanthene sulfonamide/carboxamide derivatives were established by their spectral data and elemental analyses. Furthermore, all the synthesized compounds were tested in vitro for their antimicrobial activity. The results were compared with reference standard antibiotics, erythromycin and nystatin. 6c, 6f, 6m and 8b Compounds were found to display most effective antimicrobial activity against a series of bacteria and fungi.
Introduction
Drug resistance in diseases treatments created a need for the research and development of novel medicine agents. In the last few decades, the chemistry of xanthene and their fused heterocyclic derivatives have received considerable attention owing to their synthetic and effective biological importance. For example, a large number of xanthene-containing ring systems have been incorporated into a wide variety of therapeutically interesting drug candidates including antibacterial activitiesCitation1–4, photodynamic therapyCitation5, anti-inflammatory activitiesCitation6 and antiviral effectsCitation7, agricultural bactericide effectsCitation8, and antagonist for the paralyzing action of zoxazolamineCitation9. Especially, xanthene-1,8-dione derivatives constitute a structural unit in several natural productsCitation10.
Sulfonamide and carboxamide derivatives are well known for their broad range of pharmaceutically relevant properties. Sulfonamides exhibited a large spectrum of bioactivities, including antimicrobialCitation11, antimalarialCitation12,Citation13, antibacterialCitation14 agents. Amides are known to exhibit antimicrobialCitation15, antifungalCitation16, antibacterialCitation17, and antimalarialCitation18.
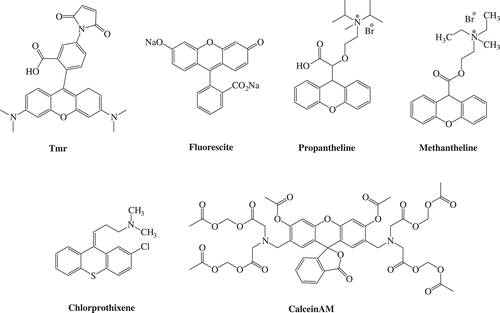
Also, the well-known drugs containing the xanthene group e.g. Tmr (tetramethylrodamine-5-maleimide), Fluorescite, Propantheline, Methantheline, Calcein AM, Chlorprothixene () should be given as example of the large number of xanthene-containing ring systems as therapeutically interesting drug candidates.
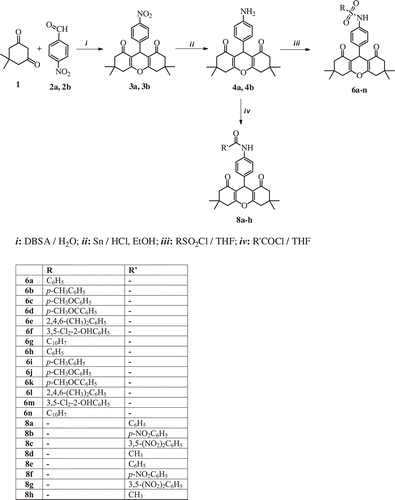
In this study, we report the synthesis of novel sulfonamide and carboxamide derivatives 6a-n and 8a-h containing a xanthene ring system .
Material and methods
Materials
All chemicals and solvents used for the synthesis were spectroscopic reagent grade. Melting points were measured on a Bibby Stuart Scientific apparatus. FT-IR spectra were recorded from a Bruker Optics, Andrtex 70 FT-IR spectrometer with an ATR diamond crystal. 1H-NMR, and 13C-NMR spectra were obtained with a Bruker DPX-300 FT-NMR instrument in CDCl3 and DMSO-d6 as a solvent, at 300 and 400 MHz. Chemical shifts are expressed in δ units (ppm). The elemental analyses (C, H, N and S) were conducted by using the Elemental Analyzer LECO CHNS-932. The microbial subcultures for Esherichia coli ATCC 35218, Bacillus subtilis RSKK 244, Salmonella enteretidis ATCC 13076, Staphylococcus aureus ATCC 29213, Candida albicans ATCC 90028, C. glabrata RSKK 04019 were obtained from Erciyes University, Faculty of Pharmacy, Pharmaceutical Microbiology Department. Bacterial strains were cultured overnight at 37°C in Nutrient Broth and the yeast were cultured overnight at 30°C in YEPD Broth for antibacterial and antifungal activity tests. These stock cultures were stored in the dark at 4°C during the survey.
General procedure for preparation of amino xanthenes 4a, 4b
Sn (3.0 g) and concentrate portion % 37 HCl (10 mL) were added to a solution of the appropriate nitro xanthene compound 3a (1 g, 2.53 mmol) in ethanol (10 mL). The mixture was heated under reflux for 1 h. Then, 15% NaOH solution was added until pH 7–8 was obtained and the white product was separated. The crude product was washed three times with 50 mL distilled H2O and dried over MgSO4. Then, the obtained solid was recrystallized from ethanol.
9-(4-Aminophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8-(2H)-dione (4a) This compound was recrystallized from ethanol as white solid to give 0.48 g (52%), mp 350°C (decompoze); ir: 3445, 3359, 3009, 2960, 1657, 1620, 1282, 1190 cm-1. 1H nmr (CDCl3, 400 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.16 (d, 2H, J = 16.1 Hz, CH2), 2.23 (d, 2H, J = 16.2 Hz, CH2), 2.47 (s, 4H, 2xCH2), 3.50 (br, 2H, NH2), 4.66 (s, 1H, CH), 6.56 (d, 2H, J = 8.1 Hz), 7.08 (d, 2H, J = 8.1 Hz) ppm. 13C nmr (CDCl3, 100 MHz): δ 196.2, 162.1, 144.1, 134.1, 129.1, 115.9, 115.1, 51.5, 41.05, 32.2, 30.03, 28.2, 27.1 ppm. Anal. Calcd. for C23H27NO3: C, 75.59; H, 7.45; N, 3.83. Found: C, 75.47; H, 7.39; N, 3.80.
9-(3-Aminophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8-(2H)-dione (4b) This compound was recrystallized from ethanol as white solid to give 0.57 g (62%), mp 223°C; ir: 3475, 3378, 3029, 2956, 1660, 1617, 1357, 1194 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.11 (s, 6H, 2xCH3), 2.17 (d, 2H, J = 16.2 Hz, CH2), 2.23 (d, 2H, J = 16.2 Hz, CH2), 2.46 (s, 4H, 2xCH2), 3.57 (br, 2H, NH2), 4.66 (s, 1H, CH), 6.44 (dxd, 1H, J = 0.9 Hz and J = 7.8 Hz, Ar-H), 6.59 (dxt, 1H, J = 2.4 Hz and J = 7.8 Hz, Ar-H), 6.72 (t, 1H, J = 1.9 Hz, Ar-H), 6.99 (t, 1H, J = 7.8 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.35, 162.20, 146.05, 145.14, 128.89, 118.29, 116.00, 115.66, 113.43, 50.79, 40.88, 32.21, 31.56, 29.19, 27.49 ppm. Anal. Calcd. for C23H27NO3: C, 75.59; H, 7.45; N, 3.83. Found: C, 75.48; H, 7.36; N, 3.77.
General procedure for preparation of xanthene sulfonamides 6a-n
A mixture of the amino xanthene derivative 4a (0.5 mmol) and the sulphonyl chlorides 5a-g (0.5 mmol) in dry THF (15 mL) was stirred and refluxed for 24 h. After the solvent was removed in vacuo, the crude product was purified by recrystallization from ethanol.
N-(4-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6a) This compound was recrystallized from ethanol as white solid to give 0.21 g (81%), mp 241°C; ir: 3198, 3028, 2969, 1650, 1622, 1342, 1293 cm-1.1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.17 (d, 2H, J = 16.3 Hz, CH2), 2.29 (d, 2H, J = 16.3 Hz, CH2), 2.47 (s, 4H, 2xCH2), 4.66 (s, 1H, CH), 6.86 (d, 2H, J = 8.5 Hz, Ar-H), 7.10 (d, 2H, J = 8.4 Hz, Ar-H), 7.20 (br, 1H, NH), 7.37 (t, 2H, J = 7.4 Hz, Ar-H), 7.50 (t, 1H, J = 7.4 Hz, Ar-H), 7.67 (d, 1H, J = 8.6 Hz, Ar-H), 7.69 (s, 1H, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.69, 162.60, 141.15, 139.26, 134.70, 132.76, 129.10, 128.52, 127.11, 121.57, 115.35, 50.68, 40.83, 32.22, 31.20, 29.26, 27.20 ppm. Anal. Calcd. for C29H31NO5S: C, 68.89; H, 6.18; N, 2.77; S, 6.34. Found: C, 68.81; H, 6.13; N, 2.74; S, 6.29.
4-Methyl-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6b) This compound was recrystallized from ethanol as yellow solid to give 0.18 g (69%), mp 216°C; ir: 3214, 3029, 2959, 1656, 1624, 1359, 1197 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.98 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.17 (d, 2H, J = 16.2 Hz, CH2), 2.25 (d, 2H, J = 16.3 Hz, CH2), 2.38 (s, 3H, ArCH3), 2.47 (s, 4H, 2xCH2), 4.68 (s, 1H, CH), 6.70 (br, 1H, NH), 6.89 (d, 2H, J = 8.4 Hz, Ar-H), 7.13–7.19 (m, 4H, Ar-H), 7.56 (d, 2H, J = 8.3 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.60, 162.55, 143.51, 141.02, 136.38, 134.89, 129.46, 129.07, 127.15, 121,45, 115.38, 50.70, 40.83, 32.19, 31.16, 29.24, 27.21, 21.50 ppm. Anal. Calcd. for C30H33NO5S: C, 69.34; H, 6.40; N, 2.70; S, 6.17. Found: C, 69.29; H, 6.32; N, 2.66; S, 6.11.
4-Methoxy-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6c) This compound was recrystallized from ethanol as yellow solid to give 0.20 g (73%), mp 235°C; ir: 3257, 3016, 2956, 1667, 1618, 1360, 1154 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.11 (s, 6H, 2xCH3), 2.12 (d, 2H, J = 16.2 Hz, CH2), 2.21 (d, 2H, J = 16.2 Hz, CH2), 2.46 (s, 4H, 2xCH2), 2.54 (s, 3H, ArOCH3), 4.72 (s, 1H, CH), 6.52 (br, 1H, NH), 6.63 (d, 2H, J = 7.8 Hz, Ar-H), 6.87 (s, 1H, Ar-H), 6.90 (s, 1H, Ar-H), 7.06 (t, 2H, J = 7.7 Hz, Ar-H), 7.17 (d, 2H, J = 7.7 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.66, 162.89, 162.58, 140.95, 135.03, 131.98, 130.91, 129.08, 121.38, 115.38, 114.01, 55.54, 50.70, 40.82, 32.21, 31.65, 29.24, 27.21 ppm. Anal. Calcd. for C30H33NO6S: C, 67.27; H, 6.21; N, 2.61; S, 5.99. Found: C, 67.19; H, 6.16; N, 2.58; S, 5.90.
4-Acetyl-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6d) This compound was recrystallized from ethanol as yellow solid to give 0.19 g (70%), mp 238°C; ir: 3167, 3056, 2954, 1688, 1652, 1615, 1338, 1136 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.17 (d, 2H, J = 16.3 Hz, CH2), 2.29 (d, 2H, J = 16.3 Hz, CH2), 2.48 (s, 4H, 2xCH2), 2.63 (s, 3H, CH3), 4.69 (s, 1H, CH), 6.90 (d, 2H, J = 8.4 Hz, Ar-H), 6.99 (br, 1H, NH), 7.15 (d, 2H, J = 8.4 Hz, Ar-H), 7.76 (d, 2H, J = 8.3 Hz, Ar-H), 7.96 (d, 2H, J = 8.4 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.91, 196.86, 162.74, 143.17, 141.50, 139.96, 134.35, 129.25, 128.70, 127.44, 121.72, 115.26, 50.68, 40.83, 32.24, 31.30, 29.20, 27.23, 26.87 ppm. Anal. Calcd. for C31H33NO6S: C, 67.99; H, 6.07; N, 2.56; S, 5.85. Found: C, 67.89; H, 6.00; N, 2.52; S, 5.79.
2,4,6-Trimethyl-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6e) This compound was recrystallized from ethanol as yellow solid to give 0.21 g (75%), mp 232°C; ir: 3246, 3026, 2963, 1663, 1617, 1362, 1145 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.15 (d, 2H, J = 16.3 Hz, CH2), 2.23 (d, 2H, J = 16.3 Hz, CH2), 2.28 (s, 3H, ArCH3), 2.45 (s, 4H, 2xCH2), 2.51 (s, 6H, 2xArCH3), 4.62 (s, 1H, CH), 6.43 (br, 1H, NH), 6.89-6.78 (m, 4H, Ar-H), 7.14 (d, 2H, J = 8.4 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.51, 162.44, 142.32, 141.19, 139.28, 134.60, 133.61, 131.94, 129.09, 121.77, 115.38, 50.64, 40.80, 32.18, 31.18, 29.28, 27.17, 22.95, 20.93 ppm. Anal. Calcd. for C32H37NO5S: C, 70.17; H, 6.81; N, 2.56; S, 5.85. Found: C, 70.08; H, 6.73; N, 2.52; S, 5.80.
3,5-Dichloro-2-hydroxy-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6f) This compound was recrystallized from ethanol as pink solid to give 0.24 g (81%), mp 228°C; ir: 3231, 3089, 2957, 1664, 1616,1359, 1156 cm-1.
1H nmr (DMSO-d6, 300 MHz): δ 0.83 (s, 6H, 2xCH3), 1.03 (s, 6H, 2xCH3), 2.04 (d, 2H, J = 16.2 Hz, CH2), 2.22 (d, 2H, J = 16.2 Hz, CH2), 2.50 (s, 4H, 2xCH2), 4.41 (s, 1H, CH), 6.91 (d, 2H, J = 8.4 Hz), 7.01 (d, 2H, J = 8.4 Hz, Ar-H), 7.56 (d, 1H, J = 2.6, Ar-H), 7.77 (d, 1H, J =2.6, Ar-H), 10.12 (br, 1H, NH), 10.90 (br, 1H, OH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.55, 163.40, 150.50, 140.49, 135.64, 134.23, 129.73, 129.04, 128.41, 124.26, 123.20, 119.96, 114.65, 56.50, 50.41, 32.28, 30.85, 29.07, 26.83 ppm. Anal. Calcd. for C29H29Cl2NO6S: C, 58.98; H, 4.95; N, 2.37; S, 5.43. Found: C, 58.86; H, 4.90; N, 2.33; S, 5.39.
N-(4-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)naphthalene-2-sulfonamide (6g) This compound was recrystallized from ethanol as yellow solid to give 0.21 g (75%), mp 278°C; ir: 3216, 3012, 2955, 1658, 1621, 1360, 1160 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.13 (d, 2H, J = 16.2 Hz, CH2), 2.22 (d, 2H, J = 16.3 Hz, CH2), 2.44 (s, 4H, 2xCH2), 4.68 (s, 1H, CH), 6.54 (br, 1H, NH), 6.92 (d, 2H, J = 8.4 Hz, Ar-H), 7.15 (d, 2H, J = 8.4 Hz, Ar-H), 7.55-7.67 (m, 3H, Ar-H), 7.84-7.89 (m, 3H, Ar-H), 8.27 (s, 1H, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.52, 162.47, 141.37, 136.20, 134.85, 134.62, 131.97, 129.28, 129.23, 128.73, 128.67, 127.87, 127.36, 122.30, 121.62, 115.32, 50.66, 40.80, 32.18, 31.21, 29.19, 27.22 ppm. Anal. Calcd. for C33H33NO5S: C, 71.33; H, 5.99; N, 2.52; S, 5.77. Found: C, 71.23; H, 5.94; N, 2.48; S, 5.70.
N-(3-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6h) This compound was recrystallized from ethanol as white solid to give 0.14 g (53%), mp 240°C; ir: 3288, 3046, 2952, 1651, 1608, 1355, 1162 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.15 (s, 6H, 2xCH3), 2.16 (d, 2H, J = 16.2 Hz, CH2), 2.24 (d, 2H, J = 16.2 Hz, CH2), 2.47 (s, 4H, 2xCH2), 4.65 (s, 1H, CH), 6.42 (br, 1H, NH), 6.79 (d, 1H, J = 6.7 Hz, Ar-H), 7.02-7.17 (m, 3H, Ar-H), 7.40-7.53 (m, 3H, Ar-H), 7.68 (d, 2H, J = 7.4 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.51, 162.66, 145.50, 139.25, 136.37, 132.70, 128.92, 128.86, 127.10, 125.70, 121.96, 119.78, 115.13, 50.70, 40.79, 32.14, 31.64, 29.14, 27.42 ppm. Anal. Calcd. for C29H31NO5S: C, 68.89; H, 6.18; N, 2.77; S, 6.34. Found: C, 68.82; H, 6.14; N, 2.74; S, 6.29.
4-Methyl-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6i) This compound was recrystallized from ethanol as orange solid to give 0.12 g (45%), mp 215°C; ir: 3240, 3047, 2956, 1660,1622, 1358, 1159 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.15 (d, 2H, J = 16.2 Hz, CH2), 2.24 (d, 2H, J = 16.2 Hz, CH2), 2.39 (s, 3H, ArCH3), 2.47 (s, 4H, 2xCH2), 4.71 (s, 1H, CH), 6.52 (br, 1H, NH), 6.77 (d, 1H, J = 7.7 Hz, Ar-H), 7.01-7.22 (m, 5H, Ar-H), 7.55 (d, 2H, J = 8.1 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.46, 162.64, 145.47, 143.45, 136.54, 136.36, 129.55, 128.83, 127.15, 125.74, 121.55, 119.54, 115.15, 50.72, 40.80, 32.13, 31.62, 29.13, 27.43, 21.55 ppm. Anal. Calcd. for C30H33NO5S: C, 69.34; H, 6.40; N, 2.70; S, 6.17. Found: C, 69.27; H, 6.35; N, 2.66; S, 6.12.
4-Methoxy-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6j) This compound was recrystallized from ethanol as yellow solid to give 0.21 g (75%), mp 200°C; ir: 3165, 3093, 2951, 1660, 1592, 1357, 1200 cm-1. 1H nmr (CDCl3, 300 MHz): 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.15 (d, 2H, J= 16.2 Hz, CH2), 2.24 (d, 2H, J = 16.2 Hz, CH2), 2.47 (s, 4H, 2xCH2), 3.82 (s, 3H, ArOCH3), 4.73 (s, 1H, CH), 6.41 (br, 1H, NH), 6.78–6.80 (m, 1H, Ar-H), 6.86-6.89 (m, 2H, Ar-H), 6.99 (t, 1H, J = 1.7 Hz, Ar-H), 7.07-7.17 (m, 2H, Ar-H), 7.59 (d, 2H, J = 9.0 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 195.06, 161.46, 161.22, 144.06, 135.14, 129.39, 127.88, 127.44, 124.31, 120.45, 118.39, 113.73, 112.69, 54.13, 49.30, 39.40, 30.74, 30.21, 27.72, 26.02 ppm. Anal. Calcd. for C30H33NO6S: C, 67.27; H, 6.21; N, 2.61; S, 5.99. Found: C, 67.20; H, 6.17; N, 2.58; S, 5.93.
4-Acetyl-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6k) This compound was recrystallized from ethanol as brown solid to give 0.19 g (69%), mp 189°C; ir: 3231, 3087, 2958, 1664, 1642, 1617, 1360, 1132 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.98 (s, 6H, 2xCH3), 1.11 (s, 6H, 2xCH3), 2.14 (d, 2H, J = 16.3 Hz, CH2), 2.23 (d, 2H, J = 16.2 Hz, CH2), 2.47 (s, 4H, 2xCH2), 2.64 (s, 3H, CH3), 4.65 (s, 1H, CH), 6.50 (br, 1H, NH), 6.87 (s, 1H, Ar-H), 7.03-7.13 (m, 3H, Ar-H), 7.75 (d, 2H, J = 8.2 Hz, Ar-H), 7.98 (d, 2H, J = 7.7 Hz, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 197.08, 196.60, 162.64, 145.65, 142.89, 139.89, 135.81, 129.07, 128.81, 127.43, 125.56, 123.04, 120.35, 115.08, 50.61, 40.82, 32.17, 31.72, 29.19, 27.28, 26.92 ppm. Anal. Calcd. for C31H33NO6S: C, 67.99; H, 6.07; N, 2.56; S, 5.85. Found: C, 67.88; H, 6.02; N, 2.52; S, 5.81.
2,4,6-Trimethyl-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6l) This compound was recrystallized from ethanol as white solid to give 0.21 g (76%), mp 218°C; ir: 3283, 3021, 2960, 1657, 1624, 1331, 1194 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.10 (d, 2H, J = 16.2 Hz, CH2), 2.20 (d, 2H, J = 16.3 Hz, CH2), 2.26 (s, 3H, ArCH3), 2.44 (s, 6H, 2xArCH3), 2.52 (s, 4H, 2xCH2), 4.71 (s, 1H, CH), 6.40 (br, 1H, NH), 6.61 (d, 1H, J = 7.6 Hz, Ar-H), 6.85-6.89 (m, 2H, Ar-H), 7.02–7.07 (m, 1H, Ar-H), 7.16-7.18 (m, 1H, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.33, 162.47, 145.42, 142.28, 139.19, 136.32, 133.62, 131.99, 128.80, 126.02, 121.14, 119.20, 115.09, 50.70, 40.81, 32.13, 31.65, 29.04, 27.59, 23.01, 20.98 ppm. Anal. Calcd. for C32H37NO5S: C, 70.17; H, 6.81; N, 2.56; S, 5.85. Found: C, 70.09; H, 6.77; N, 2.52; S, 5.81.
3,5-Dichloro-2-hydroxy-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzenesulfonamide (6m) This compound was recrystallized from ethanol as white solid to give 0.19 g (65%), mp 259°C; ir: 3162, 3064, 2970, 1657, 1619, 1397, 1202 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.82 (s, 6H, 2xCH3), 1.07 (s, 6H, 2xCH3), 2.01 (d, 2H, J = 16.1 Hz, CH2), 2.24 (d, 2H, J = 16.1 Hz, CH2), 2.41 (d, 2H, J = 17.7 Hz, CH2), 2.56 (d, 2H, J = 17.6 Hz, CH2), 4.45 (s, 1H, CH), 6.81-6.90 (m, 3H, Ar-H), 7.07 (t, 1H, J = 7.6 Hz, Ar-H), 7.56 (d, 1H, J = 2.4 Hz, Ar-H), 7.85 (d, 1H, J = 2.4 Hz, Ar-H), 10.21 (br, 1H, NH), 11.05 (br, 1H, OH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.28, 163.40, 150.55, 145.58, 137.58, 134.29, 130.01, 128.82, 128.33, 124.61, 124.27, 123.31, 119.06, 118.52, 114.55, 50.42, 40.81, 32.13, 31.45, 29.25, 26.79 ppm. Anal. Calcd. for C29H29Cl2NO6S: C, 58.98; H, 4.95; N, 2.37; S, 5.43. Found: C, 58.91; H, 4.91; N, 2.33; S, 5.39.
N-(3-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)naphthalene-2-sulfonamide (6n) This compound was recrystallized from ethanol as brown solid to give 0.22 g (75%), mp 218°C; ir: 3148, 3021, 2959, 1658, 1617, 1363, 1155 cm-1. 1H nmr (CDCl3, 300 MHz): δ 0.92 (s, 6H, 2xCH3), 1.09 (s, 6H, 2xCH3), 2.06 (d, 2H, J = 16.3 Hz, CH2), 2.19 (d, 2H, J = 16.2 Hz, CH2), 2.41 (s, 4H, 2xCH2), 4.71 (s, 1H, CH), 6.63 (br, 1H, NH), 6.80 (d, 1H, J = 7.4 Hz, Ar-H), 7.04-7.16 (m, 3H, Ar-H), 7.56-7.68 (m, 3H, Ar-H), 7.87-7.94 (m, 3H, Ar-H), 8.27 (s, 1H, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 196.37, 162.49, 145.40, 136.30, 136.17, 134.75, 131.90, 129.35, 129.22, 128.79, 128.61, 128.50, 127.76, 127.21, 125.65, 122.24, 121.44, 119.50, 114.98, 50.51, 40.62, 31.96, 31.50, 28.98, 27.14 ppm. Anal. Calcd. for C33H33NO5S: C, 71.33; H, 5.99; N, 2.52; S, 5.77. Found: C, 71.22; H, 5.93; N, 2.48; S, 5.71.
General procedure for preparation of xanthene amides 8a–h
The resulting xanthene amines 4a (0.5 mmol) were dissolved in 10 mL THF and added to a solution of 0.5 mmol acyl chlorides 7a–d in THF (5 mL). The mixture was heated under reflux for 24 h. After the solvent was removed in vacuo, the crude products were purified by recrystallization from ethanol.
N-(4-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8a) This compound was recrystallized from ethanol as yellow solid to give 0.19 g (82%), mp 277°C; ir: 3314, 3012, 2956, 1655, 1601, 1360, 1193 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.92 (s, 6H, 2xCH3), 1.09 (s, 6H, 2xCH3), 2.09 (d, 2H, J = 16.2 Hz, CH2), 2.27 (d, 2H, J = 16.1 Hz, CH2), 2.48–2.61 (m, 4H, 2xCH2), 4.51 (s, 1H, CH), 7.14 (d, 2H, J = 8.5 Hz, Ar-H), 7.48–7.61 (m, 5H, Ar-H), 7.92 (d, 2H, J = 6.7 Hz, Ar-H), 11.23 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.53, 165.82, 163.22, 140.22, 137.65, 135.44, 131.94, 128.81, 128.66, 128.03, 120.54, 114.90, 50.54, 40.84, 32.33, 31.22, 29.15, 26.93 ppm. Anal. Calcd. for C30H31NO4: C, 76.73; H, 6.65; N, 2.98. Found: C, 76.62; H, 6.60; N, 2.95.
4-Nitro-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8b) This compound was recrystallized from ethanol as yellow solid to give 0.20 g (83%), mp 300°C; ir: 3307, 3062, 2956, 1660, 1598, 1354, 1195 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.97 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.08 (d, 2H, J = 16.2 Hz, CH2), 2.27 (d, 2H, J = 16.1 Hz, CH2), 2.40-2.51 (m, 4H, 2xCH2), 4.52 (s, 1H, CH), 7.17 (d, 2H, J = 8.4 Hz, Ar-H), 7.60 (d, 2H, J = 8.3 Hz, Ar-H), 8.14 (d, 2H, J = 8.7 Hz, Ar-H), 8.36 (d, 2H, J = 8.7 Hz, Ar-H), 10.45 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.55, 164.14, 163.26, 149.56, 141.10, 140.75, 137.18, 129.59, 128.78, 124.01, 120.64, 114.83, 50.51, 40.82, 32.34, 31.27, 29.15, 26.90 ppm. Anal. Calcd. for C31H33NO4: C, 76.99; H, 6.88; N, 2.90. Found: C, 76.90; H, 6.82; N, 2.86.
3,5-Dinitro-N-(4-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8c) This compound was recrystallized from ethanol as white solid to give 0.24 g (84%), mp 323°C; ir: 3189, 3097, 2961, 1666, 1640, 1600, 1365, 1200 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.99 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.09 (d, 2H, J = 16.1 Hz, CH2), 2.28 (d, 2H, J = 16.2 Hz, CH2), 2.49–2.56 (m, 4H, 2xCH2), 4.52 (s, 1H, CH), 7.18 (d, 2H, J = 8.5 Hz, Ar-H), 7.67 (d, 2H, J = 8.5 Hz, Ar-H), 8.98 (t, 1H, J = 2.0 Hz, Ar-H), 9.18 (d, 2H, J = 2.0 Hz, Ar-H), 11.05 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.56, 163.31, 161.64, 148.53, 141.03, 137.94, 136.90, 128.75, 128.56, 121.45, 120.88, 114.81, 50.51, 40.82, 32.34, 31.27, 29.14, 26.92 ppm. Anal. Calcd. for C30H29N3O8: C, 64.39; H, 5.22; N, 7.51. Found: C, 64.31; H, 5.18 N, 7.46.
N-(4-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)acetamide (8d) This compound was recrystallized from ethanol as yellow solid to give 0.17 g (82%), mp 298°CCitation19.
N-(3-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8e) This compound was recrystallized from ethanol as white solid to give 0.1 g (52%), mp 199–201°C; ir: 3364, 3019, 2957, 1650, 1606, 1362, 1198 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.92 (s, 6H, 2xCH3), 1.09 (s, 6H, 2xCH3), 2.09 (d, 2H, J = 16.2 Hz, CH2), 2.27 (d, 2H, J = 16.2 Hz, CH2), 2.48-2.61 (m, 4H, 2xCH2), 4.52 (s, 1H, CH), 6.88 (d, 1H, J = 7.8 Hz, Ar-H), 7.19 (t, 1H, J = 7.8 Hz, Ar-H), 7.48-7.68 (m, 5H, Ar-H), 7.94 (d, 2H, J = 8.3 Hz, Ar-H), 10.19 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.51, 165.89, 163.35, 145.28, 139.31, 135.48, 133.32, 131.93, 129.72, 129.02, 123.73, 120.85, 118.70, 114.88, 50.55, 40.83, 32.34, 31.78, 29.12, 27.03 ppm. Anal. Calcd. for C30H31NO4: C, 76.73; H, 6.65; N, 2.98. Found: C, 76.66; H, 6.61; N, 2.95.
4-Nitro-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8f) This compound was recrystallized from ethanol as white solid to give 0.20 g (84%), mp 298°C; ir: 3361, 3042, 2962, 1670, 1645, 1607, 1389, 1192 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.94 (s, 6H, 2xCH3), 1.08 (s, 6H, 2xCH3), 2.09 (d, 2H, J = 16.2 Hz, CH2), 2.28 (d, 2H, J = 16.2 Hz, CH2), 2.48–2.73 (m, 4H, 2xCH2), 4.52 (s, 1H, CH), 6.91 (d, 1H, J = 7.8 Hz, Ar-H), 7.22 (t, 1H, J = 7.8 Hz, Ar-H), 7.62-7.67 (m, 2H, Ar-H), 8.17 (d, 2H, J = 8.7 Hz, Ar-H), 8.37 (d, 2H, J = 8.7 Hz, Ar-H), 10.51 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.52, 164.24, 163.39, 149.58, 145.41, 141.14, 138.83, 129.69, 128.61, 124.17, 123.95, 121.00, 118.80, 114.82, 50.53, 40.84, 32.34, 31.81, 29.12, 27.02 ppm. Anal. Calcd. for C31H33NO4: C, 76.99; H, 6.88; N, 2.90. Found: C, 76.88; H, 6.83; N, 2.86.
3,5-Dinitro-N-(3-(3,3,6,6-tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)benzamide (8g) This compound was recrystallized from ethanol as white solid to give 0.25 g (90%), mp 290°C; ir: 3351, 3095, 2958, 1669, 1648, 1611, 1352, 1194 cm-1. 1H nmr (CDCl3, 300 MHz): δ 1.01 (s, 6H, 2xCH3), 1.12 (s, 6H, 2xCH3), 2.21 (d, 2H, J = 15.9 Hz, CH2), 2.28 (d, 2H, J = 16.9 Hz, CH2), 2.51 (s, 4H, 2xCH2), 4.73 (s, 1H, CH), 6.95 (d, 1H, J = 7.8 Hz, Ar-H), 7.18 (t, 1H, J = 7.7 Hz, Ar-H), 7.49 (d, 2H, J = 6.8 Hz, Ar-H), 7.65 (s, 1H, Ar-H), 8.55 (br, 1H, NH), 9.13 (s, 1H, Ar-H), 9.21 (s, 1H, Ar-H) ppm. 13C nmr (CDCl3, 75 MHz): δ 197.06, 162.90, 160.87, 148.59, 144.98, 138.66, 136.97, 128.62, 127.76, 124.16, 121.37, 121.02, 118.48, 115.14, 50.77, 40.86, 32.28, 32.16, 29.18, 27.41 ppm. Anal. Calcd. for C30H29N3O8: C, 64.39; H, 5.22; N, 7.51. Found: C, 64.27; H, 5.19; N, 7.45.
N-(3-(3,3,6,6-Tetramethyl-1,8-dioxo-2,3,4,5,6,7,8,9-octahydro-1H-xanthen-9-yl)phenyl)acetamide (8h) This compound was recrystallized from ethanol as white solid to give 0.16 g (77%), mp 214°C; ir: 3312, 3051, 2959, 1661, 1610, 1362, 1199 cm-1. 1H nmr (DMSO-d6, 300 MHz): δ 0.98 (s, 6H, 2xCH3), 1.09 (s, 6H, 2xCH3), 2.00 (s, 3H, CH3), 2.08 (d, 2H, J = 16.2 Hz, CH2), 2.26 (d, 2H, J = 16.2 Hz, CH2), 2.48 (d, 2H, J = 17.3 Hz, CH2), 2.58 (d, 2H, J = 17.6 Hz, CH2), 4.48 (s, 1H, CH), 6.79 (d, 1H, J = 7.6 Hz, Ar-H), 7.10 (t, 1H, J = 7.6 Hz, Ar-H), 7.37 (s, 1H, Ar-H), 7.46 (d, 1H, J = 8.1 Hz, Ar-H), 9.85 (br, 1H, NH) ppm. 13C nmr (DMSO-d6, 75 MHz): δ 196.49, 168.58, 163.32, 145.22, 139.43, 128.52, 123.01, 119.49, 117.35, 114.84, 50.52, 40.83, 32.32, 31.70, 29.12, 27.00, 24.43 ppm. Anal. Calcd. for C25H29NO4: C, 73.68; H, 7.17; N, 3.44. Found: C, 73.56; H, 7.12; N, 3.40.
Biological screening
Antibacterial activities of the compounds were determined by using the disc diffusion methodCitation20. The antimicrobial screening was performed using Nutrient Agar (NA) for bacteria and YEPD Agar (YEPDA) for fungus. The culture suspensions were prepared and adjusted by comparing against 0.5 Mc Farland turbidity standard tubes. NA and YEPDA (20 mL) was poured into each sterile Petri dish after injecting cultures (100 µL) of microorganisms and distributing medium in Petri dish homogeneously. Compounds were filtered with a pore size of 0.45 µm. Compounds were dissolved in DMSO of 20 mg/mL. Empty sterilized discs of 6 mm were each impregnated with 20 µL of compounds. Discs were placed on agar plates, and the plates were incubated at 37°C for 24 h. Inhibition zones formed on the medium were evaluated in mm. Studies performed in duplicate and the inhibition zones were compared with those of reference discs. The solvents control (DMSO) did not show any antimicrobial activity.
Result and discussion
The designed compounds have been synthesized as indicated in . The precursors 3,3,6,6-tetramethyl-9-(4-nitrophenyl)-3,4,5,6,7,9-hexahydro-1Hxanthene-1,8(2H)-dione (3a) and 3,3,6,6-tetramethyl-9-(3-nitrophenyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (3b) were obtained in a high yield and purity via condensation of dimedone (1), with m-nitrobenzaldehyde (2a) and p-nitrobenzaldehyde (2b) through the modification of the reported proceduresCitation21,Citation22 ().
This starting compounds 3a, 3b were reduced by using tin/HCl in ethanol mixture and 9-(4-aminophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (4a), 9-(3-aminophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (4b) compounds were obtained. Structure of the xanthene amine compounds 4a, 4b were established by the spectral data. The IR spectra of 4a, 4b displayed sharp peaks for the carbonyl band at 1657 and 1660 cm-l, aliphatic C–H stretching bands at 2960 and 2956 cm-1 and aromatic C–H stretching bands at 3009 and 3029 cm-1, in addition to two absorption bands at 3445 and 3359 cm-1 and 3475 and 3378 cm-1 indicative of NH2 group, respectively. The 1H NMR spectra of the 4a, 4b shows immediately that both compounds contain a broad band of amino group protons at approximately 3.5 ppm. The 13C NMR spectra of 4a, 4b exhibited a carbonyl carbon signal at about 196 ppm which is in agreement with the suggested structures.
Furthermore, the xanthenesulfonamide derivatives 6a–n were synthesized via reaction of amino xanthenes 4a, 4b with the sulphonyl chloride 5a–g derivatives in tetrahydrofuran. The structure assignments of new compounds were based on their elemental analysis and spectral data. The IR spectra of 6a–n showed sharp peaks for the carbonyl band at 1650–1688 cm-l, aliphatic C–H stretching bands at 2951–2969 cm-1 and aromatic C–H stretching bands at 3012–3093 cm-1, one absorption band at 3162–3288 cm-1 indicative of NH group, in addition to the strong bands at 1331–1397 cm-1 and 1132–1293 cm-1 for the SO2NH moietyCitation23. The 1H NMR spectra of the 6a–n displayed all compounds contain an amino group proton a broad 6.43–10.21 ppm. The 13C NMR spectra of 6a–n exhibited a carbonyl carbon signal at about 196 ppm which is in agreement with the suggested structures.
Finally, the xanthene carboxamide derivatives 8a–h were synthesized via reaction of amino xanthenes 4a, 4b with the acyl chloride 7a–d derivatives in tetrahydrofuran. The structure assignments of new compounds were based on their elemental analysis and spectral data. The IR spectra of 8a–h showed sharp peaks for the carbonyl band at 1640–1669 cm-l, aliphatic C–H stretching bands at 2956–2962 cm-1 and aromatic C–H stretching bands at 3012–3097 cm-1, in addition to one absorption band at 3189–3364 cm-1 indicative of NH group. The 1H NMR spectra of the 8a–h displayed all compounds contain a amino group proton a broad at 8.55-11.23 ppm. The 13C NMR spectra of 8a–h exhibited a carbonyl carbon signal at about 196 ppm which is in agreement with the suggested structures.
All of the products 4a, 4b, 6a–n and 8a–h were confirmed by the elemental analyses that displayed found with theorical of the %C, H, N, S which matches with their molecular formula.
As seen in , the synthesized xanthene sulfonamide/carboxamide derivatives showed antibacterial activity against the Gram-positive bacteria Staph. aureus and B. subtilis, the Gram-negative bacteria S. enteritidis and E. coli, and antifungal activity to the pathogenic fungi C. albicans and C. glabrata. The reference antibiotic Erytromycin and fungicide Nystatin were used as positive controls for comparison. Nitro and amino xanthene compounds (3a,b and 4a,b) were weakly antifungal activity to the pathogenic fungi C. albicans and C. glabrata. Also, 3a showed no effect against the Gram-positive bacteria Staph. aureus and B. subtilis. 4a,b Compounds were weakly effect against Gram-negative bacteria E. coli. All the xanthene sulfonamide/carboxamide compounds 6a–n and 8a–h exhibited moderate activity against Gram-negative bacteria (S. enteritidis and E.coli) and most compounds activities could reach the conventional reference antibiotic. 6c Compound was highly inhibition effect against S. enteritidis, but 6g compound was showed weakly antibacterial activity. 6m, 8b Compounds were highly activity against Gram-positive bacteria Staph. Aureus, but 6h compound was showed weakly antibacterial activity. All the compounds (except 6h, 6m, 8b compounds) were moderate antibacterial activity bacteria Staph. Aureus. While compounds 6a–n and 8a–h were good activity against Gram-positive bacteria Staph. aureus, they showed weakly inhibition effect against Gram-positive bacteria B. subtilis. 6f, 6m compounds showed moderate against Gram-positive bacteria B. subtilis. All the compounds 6a–n and 8a–h (except for 8d compound) were observed to have moderate antifungal activity against C. glabrata. 8d compound was not inhibition effect against C. glabrata. Also, all the compounds 6a–n and 8a–h (except for 6c, 8f compounds) was observed to have weakly antifungal activity against C. albicans. 6c, 8f Compounds were showed not antifungal activity against C. albicans.
Table 1. Inhibition zones of compounds against to the test microorganisms.
Conclusion
A series of novel sulfonamides and carboxamides containing xanthene ring were synthesized and their structural features were identified. The prepared compounds containing both xanthene ring and sulfonamide/carboxamide group are thought to be very interesting because of the combination of sulfonamide and carboxamide can be used in the treatment of some diseases i.e. antimicrobial, antimalarial, antibacterial agents. Therefore, the synthesized compounds are considered to be important both in the production of some drugs and in the synthesis of more complex molecules.
Acknowledgements
The authors wish to thank Assoc. Prof. Dr. Gökçen Yuvalı Çelik, Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Erciyes for his technical support in achieving and characterization of antimicrobial studies.
Declaration of Interest: The authors are very grateful to Dumlupınar University Research Fund for providing financial support for this project (Grant No. 2011-16).
References
- Wang H, Lu L, Zhu S, Li Y, Cai W. The phototoxicity of xanthene derivatives against Escherichia coli, Staphylococcus aureus, and Saccharomyces cerevisiae. Curr Microbiol 2006;52:1–5.
- Qiao YF, Okazaki T, Ando S, Mizoue K, Kondo K, Eguchi T. et al. Isolation and characterization of a new pyrano[4′,3′:6,7]naphtho[1,2-b]xanthene antibiotic FD-594. J Antibiot 1998;51:282–287.
- Limsuwan S, Tripc EN, Kouwen TRHM, Piersma S, Hiranrat A, Mahabusarakam W. et al. Rhodomyrtone: a new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 2009;16:645–651.
- Kaya M, Basar E, Colak F. Synthesis and antimicrobial activity of some bisoctahydroxanthene-1,8-dione derivatives. Med Chem Res 2011; 20:1214–1219.
- Ion RM, Planner A, Wiktorowicz K, Frackowiak D. The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy. Acta Biochim Pol 1998;45:833–845.
- Poupelin JP, Saintruf G, Perche JC, Roussey JC, Laude B, Narcisse G, Bakrilogeais F, Hubert F. 2-Hydroxy-1-3 indandione derivatives. 2.condensation of ninhydrine with polyphenois and their o-methylated derivatives. J Med Chem 1980; 15:253–262.
- Jamison JM, Krabill K, Hatwalkar A, Jamison E, Tsai CC. Potentiation of the antiviral activity of poly r(A-U) by xanthene dyes. Cell Biol Int Rep 1990;14:1075–1084.
- Krasnoff SB, Faloon D, Williams JE, Gibson DM. Toxicity of xanthene dyes to entomopathogenic fungi. Biocontrol Sci Techn 1999; 9:215–225.
- Saint-Ruf G, Huynh-Trong-Hieu, Poupelin JP. The effect of dibenzoxanthenes on the paralyzing action of zoxazolamine. Naturwissenschaften 1975;62:584–585.
- Hatakeyama S, Ochi N, Numata H, Takano S. A new route to substituted 3-methoxycarbonyldıhydropyrans–enantioselective synthesis of (-)-methyl elenolate. Chem Commun 1988; 1202–1204.
- Faidallah HM, Khan KA, Asiri AM. Synthesis and biological evaluation of new 3,5-di(trifluoromethyl)-1,2,4-triazolesulfonylurea and thiourea derivatives as antidiabetic and antimicrobial agents. J Fluorine Chem 2011;132:870–877.
- Yildirir Y, Us MF, Colak N, Özkan H, Yavuz S, Dişli A, Öztürk Ş, Türker L. The synthesis and investigation of the antimicrobial activity of some new phenylselanyl-1-(toluene-4sulfonyl)-1H-tetrazole derivatives. Med Chem Res 2009;18:91–97.
- Alyar S, Karacan N. Synthesis, characterization, antimicrobial activity and structure-activity relationships of new aryldisulfonamides. J Enzyme Inhib Med Chem 2009;24:986–992.
- Nessaib M, Djahoudi A, Seridi A, et al. Synthesis, crystal structure and antibacterial evaluation of N-Substituted perhdro-1-3-oxazin-2-ones containing N-phenylsulfonamide. Heterocyles 2011; 83:1041–1056.
- Aytemir MD, Hider RC, Erol DD, et al. Synthesis of new antimicrobial agents; Amide derivatives of pyranones and pyridinones. Turk J Chem 2003; 27:445–452.
- Uchida T, Kagoshima Y, Konosu T. Amide analogs of antifungal dioxane-triazole derivatives: synthesis and in vitro activities. Bioorg Med Chem Lett 2009;19:2013–2017.
- Aytemir MD, Erol DD, Hider RC, et al. Synthesis and evaluation of antimicrobial activity of new 3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxamide derivatives. Turk J Chem 2003;27:757–764.
- Kumar N, Khan SI, Atheaya H, Mamgain R, Rawat DS. Synthesis and in vitro antimalarial activity of tetraoxane-amine/amide conjugates. Eur J Med Chem 2011;46:2816–2827.
- Darviche F, Balalaie S, Chadegani F, Salehi P. Diammonium hydrogen phosphate as a neutral and efficient catalyst for synthesis of 1,8-dioxo-octahydroxanthene derivatives in aqueous media. Synthetic Commun 2007; 37:1059–1066.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically Fifth edition. Approved standard. NCCLS Document M7-A5, 20, No. 2, NCCLS, Wayne, PA, January 2000.
- Jin TS, Zhang JS, Xiao JC, Wang AQ, Li TS. Clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenezenesulfonic acid in aqueous media. Synlett 2004; 5:866–870.
- Kaya M. Aldol Condensation and Michael Addition of 4,4-Dimethyl-cyclohexane-1,3-dione and Aromatic Aldehydes. Unconventional Substituent Effects. Chin J Chem 2011; 29:2355–2360.
- Kaya M, Basar E, Cakir E, Tunca E, Bülbül M. (2011) J Enzym Inhib Med Chem. doi: 10.3109/14756366.2011.599029.