Abstract
In this study, glutathione S-transferase (GST) enzyme was purified from nontumour and tumour human gastric tissue and in vitro effects of heavy metals on the enzyme were examined. GST was purified 3089 fold with a specific activity of 20 U/mg and a yield of 78% from gastric tumour tissue; and 1185 fold with a specific activity of 5.69 U/mg and a yield of 50% from nontumour tissue by using glutathione−agarose affinity column, respectively. Enzyme purity was verified by SDS-PAGE and subunit molecular mass was calculated around 26 kDa. The molecular weight of the enzyme was calculated as 52 kDa by using Sephadex G-75 gel filtration column. Then, inhibitory effects of metal ions on the enzymes were investigated. Mg2+ and Cd2+ had inhibitory effect on the enzymes activities. Other kinetic properties of the enzymes were also determined.
Keywords::
Introduction
Gastric cancer is the second most common cause of cancer connected deaths in the world. In the developed countries, there are patients with advanced disease and die from metastasesCitation1,Citation2. When we look from a global viewpoint, we can see that gastric cancer is the fourth most common malignant tumour and the second most common cause of cancer-associated death, accounting for a guessed 1.066.543 new cases and 800.230 deaths annually. Despite advances in surgery and chemotherapy, the prognosis of gastric cancer is poor, with an estimated relative 5-year survival proportion of 10–20% in USA and European countriesCitation3,Citation4.
Glutathione S-transferases are the member of detoxification enzyme family and play a vital role in drug resistance in various diseasesCitation5,Citation6. Glutathione S-transferases (EC 2.5.1.18; GSTs), the family of multifunctional enzymes, are involved in the detoxification of both endogenous and xenobiotic compounds, in intracellular transport, biosynthesis of hormones, and protection against oxidative stressCitation7. Mammalian GST is divided into seven classes. Five of this enzyme classes is cytosolic, two of them are membrane-bound. Cytosolic enzyme classes contain 13 subunitsCitation8. GSTs are found in mammals, insects, fish, birds, annelids, mollusca, and many microorganisms. GSTs are placed in the cytosols and membranes of various tissues primarily in the liver, small intestine, large intestine, kidney, lung, breast, muscle, spleen, testis and placentaCitation9. In humans, GST enzymes can be separated into five main classes: Alpha (GSTA), Mu (GSTM), Pi (GSTP), Theta (GSTT), and Zeta (GSTZ). Each class made up of one or more isoenzymes (i.e., A1-A4, M1-M5, P1, T1- T2 and Z1), with a different but sometimes overlapping substrate specificityCitation10. Mitochondria also includes GSTs which are believed to be similar to their cytosolic counterpartsCitation11. Harris et al. were previously purified mitochondrial GSTs from rat liverCitation11,Citation12.
Glutathione S-transferase (GST) enzyme families are formed in many cytosolic, mitochondrial, and microsomal (now designated as MAPEG) proteins. GSTs exist in eukaryotes and prokaryotes, where they catalyze several of reactions and admit endogenous and xenobiotic substratesCitation13. The mammalian GST superfamily consists of cytosolic dimeric isoenzymes of 45–55 kDa sizeCitation14.
GSTs are important for the fight against the cancer because of their interactions with carcinogens and chemotherapeutic agents. They are the target of antiasthmatic and antitumour drugsCitation15. Production of excessive amounts of GST enzyme in mammalian tumour cells leads to the formation of a resistance to some anticancer drugs and chemical carcinogensCitation16. Erythrocyte GST activity of hemolytic anaemia was investigated in 513 patients and no significant correlation between the lack of GST and anaemia was detected. It is reported that decrease in GST levels, related with protecting cells from cellular damage in erythrocytes, causes a low stability of the cells and increased risk of anaemiaCitation17.
Population growth and industrial activity lead an increase in environmental pollution continuously in many parts of the world. Industrial waste is frequently emptied to the surroundings without any prerefining. As a result, in some areas, the natural habitat of animals and balance of nature are threatened by pollution in freshwaters, seas and atmosphereCitation18. In the environment, heavy metal concentration is increased by quick industrialization, poor emission control, disordered urbanization, and risen motor traffic. Plants and other organisms in the habitat are influenced by the contamination of soil, water, and atmosphere with heavy metalsCitation19,Citation20.
Therefore, in our study we wondered the effects of some heavy metals on the GSTs. For this purpose, we purified the enzyme from nontumour and tumour human stomach. Then we investigated inhibitory effects of Pb2+, Cu2+, Fe2+, Cr2+, Al3+, Ni2+, Mn2+, Cd2+, Zn2+, and Mg2+ on the enzyme.
Materials and methods
The specimens of tumour and nontumour tissues of the human stomach were obtained from the Pathology Departments of Research Hospital of Ataturk University after operation and stored at −80°C until usage. Twenty grams of thawed samples of human stomach were separately cut into small pieces with a knife. The fragments were homogenized with liquid nitrogen. The homogenate was taken to 2 volumes of buffer solution (10 mM KH2PO4 /150 mM NaCl, pH = 7.0) and 30 mL hexane was added into solution to solve the lipids. The homogenate was filtered through four layers of cheesecloth. Then, lipid fraction has been removed from the homogenate by using a separatory funnel. Next, it is centrifuged at 13.000 rpm for 1 h. The pellet was discarded. The homogenate was applied to the prepared Glutathione−Agarose affinity column equilibrated with 10 mM KH2PO4/150 mM NaCl, (pH = 7.0). The affinity gel was washed with 10 mM KH2PO4/0.1 M KCl, (pH = 8.0). The cancerous human tumour and non tumour stomach Glutathione S-Transferases enzymes were eluted with 50 mM Tris-HCl/1.25–10 mM GSH (pH = 9.5). All the purification steps were performed at 4°C.
Determination of GST activity
Glutathione S-transferase activity was performed by using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate according to Habig et al. Among its many other substrates, the aromatic electrophile 1-chloro-2,4-dinitrobenzene is one that is the most often used substrate in GST enzyme activity determination. The reaction product, dinitrobenzene S-glutathione (DNB-SG), displays maximum absorbance at 340 nm. Activity measurements were thus carried out by measuring the absorbance increase at this wavelengthCitation9.
Protein determination
During each purification steps, protein determination was performed spectrophotometrically at 595 nm according to the Bradford method, by using bovine serum albumin as the standardCitation21.
SDS polyacrylamide gel electrophoresis
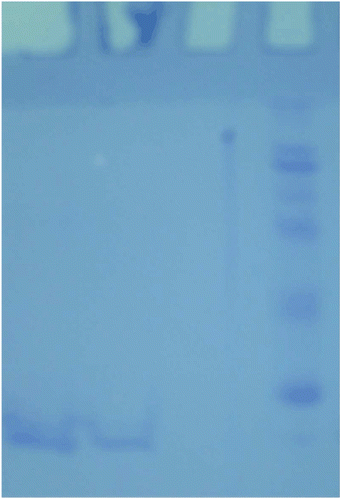
After the purification steps, SDS polyacrylamide gel electrophoresis was performed to verify enzyme purity. It was carried out in 8% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedure. A 20 µg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without dyeCitation22 ().
Figure 1. Lane (1) human tumour gastric GST. Lane (2) human nontumour gastric GST. Lane (3) standard proteins (E. coli β-galactosidase (116 kDa)), rabbit phosphorylase B (97.4 kDa), bovine serum albumin (66 kDa), chicken ovalbumin (45 kDa) and bovine carbonic anhydrase (29 kDa) SDS-PAGE analysis of purified.
In vitro effects of compounds
Inhibitory effects of the metals were analysed at different inhibitor concentrations. Compounds showing inhibitory effects were tested in triplicate at each concentration used. We measured Glutathione S-transferase activity in the presence of metal concentrations. Control activity in the absence of inhibitor was taken as 100%. For each metal, activity (%) vs. inhibitor concentration graphs were drawn. In Ki studies, we measured enzyme activities at three different inhibitor concentrations for five different substrate concentrations. Lineweaver–Burk curves were used to determine the Ki values and inhibitor type of each inhibitor.
Results and discussion
Gastric cancer is a disease in which malignant cells of the gastric tissue increases in numberCitation23. Although the incidence and the mortality rate of this disease have been decreasing significantly (approximately 5–6 fold) for last 30 years, it is the fourth most common cancer in the world. Prognosis of gastric cancer is evil and death ratio of this cancer ranked as second after lung cancerCitation24.
The aim of this study was to purify GST enzyme from tumour and nontumour human gastric tissue and investigate the effects of some heavy metals on enzyme activity. Cancerous human stomach and non cancerous tissues were chosen, because gastric cancer is the fourth highest cancer in the world. Tumour and nontumour GST enzymes were purified by using glutathione−agarose affinity column with high yields and purification folds ( and ). Enzyme purity was verified by SDS-PAGE and subunit molecular mass was calculated around 26 kDa. The molecular weight of the enzyme was calculated as 52 kDa by using Sephadex G-75 gel filtration column. (). The apparent Km and Vmax values of tumour stomach were calculated as 3.46 µM, 43.7 (EU/mL·min) for GSH and 250 µM, 114.7 (EU/mL·min) for CDNB. These values for nontumour human stomach were calculated as 25.4 µM, 31 (EU/mL·min) for GSH and 79 µM, 25 (EU/mL·min) for CDNB, respectively. Inhibitory effects of metals were examined under in vitro conditions. Ki values for each inhibitor were defined ().
Table 1. Summary of purification procedure for tumour human gastric GST.
Table 2. Summary of purification procedure for nontumour human gastric GST.
Table 3. Ki values and inhibition types for two metal ions of tumour and nontumour GST.
All living organisms are subjected to frequent toxic chemicals. Metals come to the people’s body with nutrition and bioaccumulation and they cannot be metabolized by the body because of their stability. The most well-known harmful metals are Al, Ar, Cd, Cu, Pb, Hg and Ni. Other heavy metals are not as harmful as these ones. Usually they do not have basic tasks in the body and they are very toxic. They are found in drinking water, food and thousands of man-made chemical substances and products. They are taken to the body by respiratory, digestion and skin absorption. If metals are taken to the body faster than detoxification, they are stored in the body and their toxic effects are formed. The harmful effects of toxic metals are formed by the generation of oxidative free radicals. Intense uptake of toxic substances and antioxidant deficiency triggers an uncontrolled free radical production. Uncontrolled free radicals in the body create tissue damage and lead to a number of degenerative diseases. The pH of the blood is decreased by toxic heavy metals. Body takes out Ca2+ ions from the bones to balance the pH and an inflammatory condition occurs due to this Ca2+ decrease in arteries and tissuesCitation25.
In cell homeostasis, glutathione is an important modulator for the detoxification of oxiradical and carcinogens. Thus, in the absence of GSH, organism becomes sensitive to the pollutants. This situation shows the importance of GSH’s ability of organisms fight against heavy metals which are known to inhibit antioxidant enzymes and increases the consumption of intracellular glutathione. When glutathione level increases, it stops increasing lipid peroxidation effects of Cd, Rb, Fe, Hg together with Zn or SeCitation26.
Wright et al.Citation27 suggested that Glutathione S-transferase enzyme is a tissue biomarker against Pb poisoning. Moreover, Glutathione plays an important role in arsenic toxicity. As the thiols are quite abundant in the cell, arsenic poisoning is known to be formed by thiol deficiency in the proteins. Lack of thiol causes toxicity and detoxification. Glutathione has been reported to increase the resistance of cells against As and SbCitation28. Cd, has basically non-essential task, is a toxic heavy metal even in very small doses. Enzymatic activity in respiratory and urinary systems is impaired by Cadmium toxicity, and it causes kidney stones formation. Carcinogenic effects of this metal in epidemiological studies are also reportedCitation29.
Fujian in China, Zn, Cu, Se levels in serum were investigated in people of 294 healthy and 109 gastric cancer. Quantities of these elements were found to be as 0.905 mg/L, 0.914 mg/L, 83.22 mg/L for healthy ones, while for the 109 gastric cancer individuals, the values were obtained as 0.843 mg/L, 1.045 mg/L and 73.58 mg/L, respectivelyCitation30.
Recent studies show that GST isoforms in cancer tissue were being overexpressed compared to normal ones. Tsuchida and his friends demonstrated that GST-Pi content in cancer colon mucosa was 6-fold higher than the level found in normal mucosa by using an enzyme-linked immunosorbent assay (ELISA) (in-house method)Citation31. In addition, concentration of GSTs was found to be higher in the lavage fluid taken from the area of tumour lung when compared with the normal area of tissueCitation32. Besides, in 19 lung, 27 colon and 9 gastric cancer patients, it was determined by chromatography that GST-Pi concentration was significantly higher in tumour tissue according to normal tissue samplesCitation33. Similar studies in various malignancies such as stomach, colon, breast and ovarian cancers, cancer GST levels were detected to be higher than in normal tissuesCitation33,Citation34.
In this study, we studied the effects of some metals on GST enzymes of tumour and nontumour tissues. Inhibitory effects of Mg2+ and Cd2+ metals were observed in both tumour and nontumour tissue. Ki values were calculated for both metals. Ki values of tumour tissue for Mg2+ and Cd2+ metals were calculated as 48.096 mM and 2.01 mM, respectively. Ki values of Mg2+ and Cd2+ metals from nontumour tissue were calculated as 49.2 mM and 7.38 mM, respectively. According to the data we obtained, metal ions had an inhibition at a lower concentration on GST enzyme purified from tumour tissue than that of nontumour tissues. We demonstrated that metals had toxic effects at lower concentrations in cancerous tissues.
It is known that GST removes reactive oxygen species in metabolism. We observed in our study that metals inhibit enzymes of tumour tissues at lower concentrations. Ki value of the tumour GST enzyme was lower than nontumour one. Nontumour and tumour tissues exposed to metals like Cd and Mg, it is expected nontumour tissues have more reactive oxygen species. Thus reactive oxygen species may have more negative effects on tumour tissues. As a result metals, Cd and Mg were found to have inhibitory effects on both tumour and nontumour GST enzyme. They may increase the spread of tumour tissues. Hence, exposure to metals of humans should be limited because of their harmful effects in the metabolism.
Acknowledgements
Research ethics committee approval needed for the cancerous tissue used in this study was taken from Ataturk University, Faculty of Medicine.
Declaration of interest
The authors report no conflicts of interest.
References
- Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1–9.
- Matharu G, Tucker O, Alderson D. Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg 2011;98:1225–1235.
- Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol 2009;71:127–164.
- Wang J, Yuan W, Chen Z, Wu S, Chen J, Ge J et al. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour Biol 2012;33:95–101.
- Rathaur S, Yadav M, Gupta S, Anandharaman V, Reddy MV. Filarial glutathione-S-transferase: A potential vaccine candidate against lymphatic filariasis. Vaccine 2008;26:4094–4100.
- Yadav M, Liebau E, Haldar C, Rathaur S. Identification of major antigenic peptide of filarial glutathione-S-transferase. Vaccine 2011;29:1297–1303.
- Enayati AA, Ranson H, Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol Biol 2005;14:3–8.
- Vessey DA, Boyer TD. Characterization of the activation of rat liver glutathione S-transferases by nonsubstrate ligands. Toxicol Appl Pharmacol 1988;93:275–280.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
- Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 1999;49:156–164.
- Harris JM, Meyer DJ, Coles B, Ketterer B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J 1991;278 (Pt 1):137–141.
- Raza H, Robin MA, Fang JK, Avadhani NG. Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J 2002;366:45–55.
- Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J 2009;276:58–75.
- Wilce MC, Parker MW. Structure and function of glutathione S-transferases. Biochim Biophys Acta 1994;1205:1–18.
- Matshushita N, Aritake K, Takada A, Hizue M, Hayashi K, Mitsui K. et al. Pharmacological studies on the novel antiallergenic drug HQL-79: 2. Elucidation of mechanisms for antiallergic and antiasthmatic effects. Jpn J Pharmacol 1998;78:11–22.
- Hayes JD, McLellan LI, Stockman PK, Chalmers J, Beckett GJ. Glutathione S-transferases in man: The relationship between rat and human enzymes. Biochem Soc Trans 1987;15:721–725.
- Beutler E, Dunning D, Dabe IB, Forman L. Erythrocyte glutathione S-transferase deficiency and hemolytic anemia. Blood 1988;72:73–77.
- Soyut H, Beydemir S, Hisar O. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 2008;123:179–190.
- Onder S, Dursun S. Air borne heavymetal pollution of Cedrus libani (A. Rich.) in the city centre of Konya (Turkey). Atmos Envıron 2006;40:1122–1133.
- Demirdag R, Yerlikaya E, Kufrevioglu OI. Purification of carbonic anhydrase-II from sheep liver and inhibitory effects of some heavy metals on enzyme activity. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.615744.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- LaemmLi DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–683.
- Fei SJ, Xiao SD. Diet and gastric cancer: A case-control study in Shanghai urban districts. Chin J Dig Dis 2006;7:83–88.
- Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: A meta-analysis. J Natl Cancer Inst 2006;98:1078–1087.
- Anonymous 2005. Available at: http://tuberose.com/Heavy_Metal_Toxicity.html.
- Shukla GS, Srivastava RS, Chandra SV. Glutathione status and cadmium neurotoxicity: Studies in discrete brain regions of growing rats. Fundam Appl Toxicol 1988;11:229–235.
- Wright LS, Kornguth SE, Oberley TD, Siegel FL. Effects of lead on glutathione S-transferase expression in rat kidney: A dose-response study. Toxicol Sci 1998;46:254–259.
- Lorico A, Bertola A, Baum C, Fodstad O, Rappa G. Role of the multidrug resistance protein 1 in protection from heavy metal oxyanions: Investigations in vitro and in MRP1-deficient mice. Biochem Biophys Res Commun 2002;291:617–622.
- Dara SS. (1997). Text book of Environmental Chemistry and its Pollution Control. 2nd edition. New Delhi: S. Chand & Co. Ltd.
- Lu HD, Wang ZQ, Pan YR, Zhou TS, Xu XZ, Ke TW. Comparison of serum Zn, Cu and Se contents between healthy people and patients in high, middle and low incidence areas of gastric cancer of Fujian Province. World J Gastroenterol 1999;5:84–86.
- Tsuchida S, Sekine Y, Shineha R, Nishihira T, Sato K. Elevation of the placental glutathione S-transferase form (GST-pi) in tumor tissues and the levels in sera of patients with cancer. Cancer Res 1989;49:5225–5229.
- Howie AF, Bell D, Hayes PC, Hayes JD, Beckett GJ. Glutathione S-transferase isoenzymes in human bronchoalveolar lavage: A possible early marker for the detection of lung cancer. Carcinogenesis 1990;11:295–300.
- Howie AF, Forrester LM, Glancey MJ, Schlager JJ, Powis G, Beckett GJ et al. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis 1990;11:451–458.
- Abou Ghalia AH, Fouad IM. Glutathione and its metabolizing enzymes in patients with different benign and malignant diseases. Clin Biochem 2000;33:657–662.
