Abstract
A new series of four biologically active triazole derived Schiff base ligands (L1–L4) and their cobalt(II), nickel(II), copper(II) and zinc(II) complexes (1–16) have been synthesized and characterized. The ligands were prepared by the condensation reaction of 3-amino-5-methylthio-1H-1,2,4-triazole with chloro-, bromo- and nitro-substituted 2-hydroxybenzaldehyde in an equimolar ratio. The antibacterial and antifungal bioactivity data showed the metal(II) complexes to be more potent antibacterial and antifungal than the parent Schiff bases against one or more bacterial and fungal species.
Introduction
Triazoles and their derivatives occupy a central positionCitation1 amongst the most significant compounds that constitute pharmaceutically and medicinally important drug centersCitation2. A group of triazoles such as () terconazole (1) has been widely used for the control of vulvovaginal moniliasis caused by Candida albicans and other Candida species, fluconazole (2) is a broad spectrum antifungalCitation3 and trazodone (3) is used as an antidepressantCitation5. Similarly, vorozole (4), anastrozole (5) and letrozole (6) potentially inhibit breast cancerCitation6,Citation7 properties. Many triazole based Schiff bases have also been reported to possess antibacterialCitation8, antifungalCitation9, antitumorCitation10, plant growth regulatingCitation11 and cytotoxicCitation12 activities. Furthermore, the process of chelation/coordination plays an important role in the biological systemCitation13. It has been suggested that biological activity of many compounds used as drugs is enhancedCitation14 upon coordination with the metal atoms. In view of the significant structural and biological applications of triazole compounds, we wish to report the synthesis of a new class of triazole Schiff base derivatives (L1–L4) and their cobalt(II), nickel(II), copper(II) and zinc(II) metal chelates (1–16). These compounds have been investigated for in vitro antibacterial activity against four Gram-negative (Escherichia coli, Shigella sonnei, Pseudomonas aeruginosa, Salmonella typhi) and two Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains, and antifungal activity against six fungal strains (Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata). In vitro Brine shrimp bioassay has also been conducted to study the cytotoxic properties of these compounds.
Materials and methods
All chemicals used were of reagents grade. All metal salts were used as chloride. Melting points were recorded on Fisher Johns melting point apparatus. Infrared spectra were recorded on SHIMADZU FT-IR spectrometer. The C, H and N analyses was carried out using a Perkin Elmer, USA model. The NMR spectra of all compounds were recorded as δ (ppm) in DMSO-d6 using TMS as internal standard on a Bruker Spectrospin Avance DPX-500 spectrometer. Electron impact mass spectra (EIMS) were recorded on JEOL MS Route Instrument. In vitro antibacterial, antifungal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Centre for Chemical Sciences, University of Karachi, Pakistan and Department of Chemistry, The Islamia University, of Bahawalpur, Pakistan.
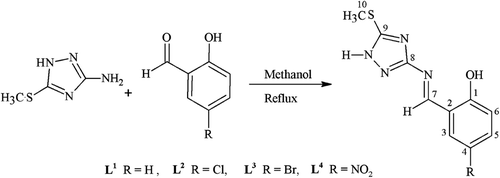
General procedure for the synthesis of ligands (L1–L4)
2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol (L1)
To a hot magnetically stirred solution of 3-amino-5-methylthio-1H-1,2,4-triazole (1.31 g, 10 mmol) in methanol (15 mL) was added a solution of 2-hydroxybenzaldehyde (1.22 g, 1.06 mL, 10 mmol) in methanol (25 mL). The resultant mixture was refluxed for 5 h. The completion of reaction was monitored by TLC. The reaction mixture was cooled to room temperature. On cooling, a light yellow solid product was formed which was filtered and washed with methanol, then with ether and dried. It was recrystallized from a solution mixture of hot ethanol–methanol (1:1). The purity of product was checked by TLC. The same method was applied for the preparation of other ligands.
Physical, analytical and spectral data of triazole Schiff bases
2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol (L1)
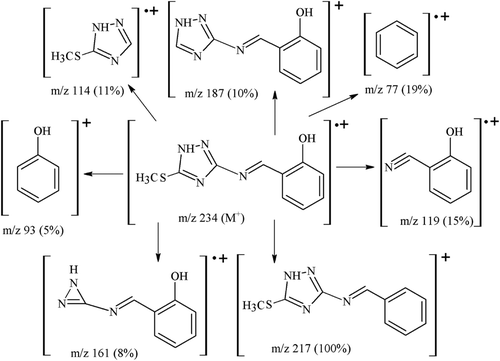
Yield: 72% (1.7 g). Colour (light-yellow). M.p. 183–185°C. IR (KBr, cm−1): 3265 (OH), 3175 (NH), 1625 (HC=N), 1606 (C=N, triazole), 1030 (N–N), 810 (S–C). 1H NMR 14.10 (s, 1H, triazole NH), 12.00 (s, 1H, OH), 9.35 (s, 1H, azomethine C7–H), 7.80 (dd, 1H, J = 7.81, 2.35 Hz, ph C3–H), 7.45 (ddd, 1H, J = 7.90, 7.84, 2.35 Hz, ph C5–H), 7.1 (dd, 1H, J = 7.84, 2.4 Hz, ph C6–H), 6.95 (ddd, 1H, J = 7.90, 7.81, 2.4 Hz, ph C4–H), 2.6 (s, 3H, triazole –SCH3). 13C NMR 163.22 (C1), 161.93 (C7), 158.73 (C9), 156.23 (C8), 134.53 (C5), 131.71 (C3), 119.58 (C4), 119.30 (C2), 116.74 (C6), 13.88 (C10). EIMS (70eV) m/z (%): 234 ([M]+, 57), 217 (100), 187 (10), 161 (8), 119 (15), 114 (11), 93 (5), 77 (19). Anal. Calcd. for C10H10N4SO [234.29]: C: 51.27; H: 4.30; N: 23.91; S: 13.69; Found: C: 50.72; H: 4.30; N: 23.88; S: 13.77%.
4-Chloro-2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol (L2)
Yield: 74% (2.0 g). Colour (off-white). M.p. 214–215°C. IR (KBr, cm−1): 3285 (OH), 3180 (NH), 1630 (HC=N), 1607 (C=N, triazole), 1030 (N–N), 825 (C–Cl), 815 (S-C). 1H NMR 14.00 (s, 1H, triazole NH), 11.90 (s, 1H, OH), 9.15 (s, 1H, azomethine C7–H), 7.70 (d, J = 2.5 Hz, ph C3–H), 7.30 (dd, 1H, J = 7.94, 2.5 Hz, ph C5–H), 6.85 (d, 1H, J = 7.92 Hz, ph C6–H), 2.45 (s, 3H, triazole –SCH3). 13C NMR 163.24 (C1), 162.11 (C7), 158.82 (C9), 156.23 (C8), 133.64 (C5), 131.92 (C3), 127.58 (C4), 120.80 (C2), 118.74 (C6), 13.90 (C10). EIMS (70 eV) m/z (%): 270/268 ([M]+, 33/54), 253/251 (69/100), 233 (10), 197/195 (5/7), 155/153 (14/21), 114 (11), 77 (19). Anal. Calcd. for C10H9N4SClO [268.72]: C: 44.70; H: 3.38; N: 20.85; S: 11.93; Cl: 13.19; Found: C: 44.34; H: 3.34; N: 20.68; S: 12.04; Cl: 13.23%.
4-Bromo-2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol (L3)
Yield: 76% (2.38 g). Colour (greenish-yellow). M.p. 210–212°C. IR (KBr, cm−1): 3275 (OH), 3185 (NH), 1627 (HC=N), 1605 (C=N, triazole), 1025 (N–N), 805 (S–C), 680 (C–Br). 1H NMR 14.15 (s, 1H, triazole NH), 12.20 (s, 1H, OH), 9.35 (s, 1H, azomethine C7–H), 8.00 (d, 1H, J = 2.44 Hz, ph C3–H), 7.60 (dd, 1H, J = 7.89, 2.44 Hz, ph C5–H), 6.95 (d, 1H, J = 7.89 Hz, ph C6–H), 2.60 (s, 3H, triazole –SCH3). 13C NMR 163.36 (C1), 162.13 (C7), 158.05 (C9), 156.37 (C8), 136.27 (C5), 135.21 (C3), 121.42 (C2), 119.11 (C6), 115.71 (C4), 13.95 (C10). EIMS (70 eV) m/z (%): 314/312 ([M]+, 46/51), 297/295 (94/100), 267/265 (9/12), 240/238 (8/11), 233 (14), 199/197 (11/15), 174/172 (9/12), 114 (16), 75 (14). Anal. Calcd. for C10H9N4OSBr [313.17]: C: 38.35; H: 2.90; N: 17.89; S: 10.24; Br: 25.51; Found: C: 38.33; H: 2.83; N: 17.63; S: 10.19; Br: 25.64%.
2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]-4-nitrophenol (L4)
Yield: 75% (2.1 g). Colour (deep-yellow). M.p. 222–224°C. IR (KBr, cm−1): 3290 (OH), 3182 (NH), 1632 (HC=N), 1606 (C=N, triazole), 1360 (C–NO2), 1025 (N–N), 815 (S-C). 1H NMR 14.10 (s, 1H, NH), 12.6 (s, 1H, OH), 9.40 (s, 1H, azomethine C7–H), 8.45 (d, 1H, J = 2.45 Hz, ph C3–H), 8.30 (dd, 1H, J = 7.86, 2.45 Hz, ph C5–H), 7.15 (d, 1H, J = 7.86 Hz, ph C6–H), 2.72 (s, 3H, C10–H). 13C NMR 163.34 (C1), 162.28 (C7), 158.44 (C9), 156.27 (C8), 140.53 (C4), 127.71 (C3), 125.58 (C5), 120.30 (C2), 117.74 (C6), 13.98 (C10). EIMS (70 eV) m/z (%): 279 ([M]+, 54), 262 (100), 233 (23), 166 (18), 141 (15), 123 (10), 120 (6), 114 (11), 76 (19). Anal. Calcd. for C10H9N5 O3S [279.29]: C: 43.01; H: 3.25; N: 25.08; S: 11.48; Found: C: 42.45; H: 2.98; N: 25.35; S: 11.64%.
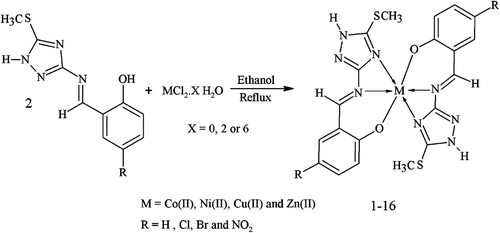
General procedure for the synthesis of complexes (1–16)
Preparation of cobalt(II) complex with 2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol (L1) (1)
To a hot magnetically stirred solution of 2-[(E)-{[5-(methylsulfanyl)-1H-1,2,4-triazol-3-yl]imino}methyl]phenol L1 (1.87 g, 8 mmol) in ethanol (30 mL) was added dropwise a solution of CoCl2·6H2O (0.95 g, 4 mmol) in ethanol (20 mL). The resultant mixture was refluxed for 2 h. The colored precipitated product formed during refluxing was collected by filtration, thorough washing with ethanol followed by ether and dried. Recrystallization in hot aqueous–ethanol (1:2) afforded TLC pure product. The same method was used for the preparation of all other complexes. Physical, analytical and spectral data are given in Supplementary Tables S1 and S2.
NMR Data of Zn (II) Complexes
[Zn (L1-H)2] (4)
1H NMR of Zn (II) complex; 2.72 (s, 6H, triazole –SCH3), 7.12 (ddd, 2H, J = 7.90, 7.81, 2.4 Hz, ph C4–H), 7.24 (dd, 2H, J = 7.84, 2.4 Hz, ph C6–H), 7.58 (ddd, 2H, J = 7.90, 7.84, 2.35 Hz, ph C5–H), 7.98 (dd, 2H, J = 7.81, 2.35 Hz, ph C3–H), 9.69 (s, 2H, C7–H), 14.21 (s, 2H, triazole NH). 13C NMR of Zn(II) complex; 14.03 (C10), 117.04 (C6), 119.77 (C2), 119.79 (C4), 131.99 (C3), 134.77 (C5), 158.29 (C8), 159.98 (C9), 163.35 (C7), 164.17 (C1).
[Zn (L2-H)2] (8)
1H NMR of Zn (II) complex; 2.49 (s, 6H, triazole -SCH3), 7.03 (d, 2H, J = 7.94 Hz, ph C6–H), 7.48 (dd, 2H, J = 7.94, 2.5 Hz, ph C5–H), 7.86 (d, 2H, J = 2.5 Hz, ph C3–H), 9.49 (s, 2H, C7–H), 14.11 (s, 2H, triazole NH). 13C NMR of Zn(II) complex; 14.13 (C10), 118.99 (C6), 121.15 (C2), 127.85 (C4), 132.25 (C3), 133.92 (C5), 157.59 (C8), 160.0 (C9), 163.55 (C7), 164.33 (C1).
[Zn (L3-H)2] (12)
1H NMR of Zn (II) complex; 2.72 (s, 6H, triazole –SCH3), 7.12 (d, 2H, J = 7.89 Hz, ph C6–H), 7.79 (dd, 2H, J = 7.89, 2.44 Hz, ph C5–H), 8.18 (d, 2H, J = 2.44 Hz, ph C3–H), 9.74 (s, 2H, C7–H), 14.26 (s, 2H, triazole NH). 13C NMR of Zn(II) complex; 14.12 (C10), 115.96 (C4), 119.39 (C6), 121.74 (C2), 135.53 (C3), 136.66 (C5), 158.00 (C8), 159.51 (C9), 163.66 (C7), 164.87 (C1).
[Zn (L4-H)2] (16)
1H NMR of Zn (II) complex; 2.83 (s, 6H, triazole –SCH3), 7.30 (d, 2H, J = 7.86 Hz, ph C6–H), 8.47 (dd, 2H, J = 7.86, 2.45 Hz, ph C5–H), 8.61 (d, 2H, J = 2.45 Hz, ph C3–H), 9.81 (s, 2H, C7–H), 14.21 (s, 2H, triazole NH); 13C NMR of Zn(II) complex; 14.1 (C10), 117.99 (C6), 120.59 (C2), 125.83 (C5), 127.99 (C3), 140.84 (C4), 157.42 (C8), 159.59 (C9), 163.78 (C7), 164.75 (C1).
Biological activity
Antibacterial studies
The synthesized Schiff bases L1–L4 and their respective metal(II) complexes 1–16 were tested against four Gram-negative (Escherichia coli, Shigella sonnei, Pseudomonas aeruginosa, Salmonella typhi) and two Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains by the disk diffusion methodCitation15. The test compounds (ligand/complex) were dissolved in DMSO to get 10 mg/mL solution. A known volume (10 µL) of the solution was applied with the help of a micropipette onto the sterilized filter paper discs. The discs were dried at room temperature over night and stored in sterilized dry containers. Discs soaked with 10 µL of DMSO and dried in air at room temperature were used as the negative control. The standard antibiotic discs used as positive control were prepared as mentioned above in the laboratory by applying a known concentration of the standard antibiotic solution. Ampicillin was used as standard antibiotic. Bacterial culture was grown in nutrient broth medium at 37°C overnight and spread onto the solidified nutrient agar medium in Petri plates using sterilized cotton swabs. Test and control disks were then applied to the medium surface with the help of sterilized forceps. The plates were incubated at 37°C for 24–48 h. The results were recorded by measuring the zone of inhibition in millimeter against each compoundCitation15. The experiments were carried out in triplicate and the values obtained were statistically analyzed.
Antifungal activity (in vitro)
Antifungal activities of all compounds were studied against six fungal strains (Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata), according to literature protocolCitation16. Sabouraud dextrose agar (Oxoid, Hampshire, England, UK) was seeded with 105 (cfu) mL−1 fungal spore suspensions and transferred to Petri plates. Discs soaked in 20 mL (200 µg/mL in DMSO) of test compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for 7 days. The results were recorded as percentage of inhibition and compared with standard drugs miconazole and amphotericin B15.
Minimum inhibitory concentration (MIC)
Compounds containing significant antibacterial activity (over 80%) were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 µg/mL of the test compounds and applying the protocolCitation17.
In vitro cytotoxicity
In vitro cytotoxic activity of all synthesized ligands and their metal(II) complexes were studied using the protocol of Meyer et al.Citation18 Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled waterCitation18. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, in dark while the matter compartment was opened to ordinary light. After two days, Nauplii were collected by a pipette from the side in ordinary light. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMSO. From this stock solutions, 500, 50 and 5 µg/mL were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMSO only to determine its participating role. The solvent was allowed to evaporate overnight. After 2 days, when shrimp larvae were ready, sea water (1 mL) and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with sea water to 5 mL per vial. After 24 h, the number of survivors was counted. Data were analyzed by Finney computer program to determine the LD50 valuesCitation18.
Results and discussion
Chemistry
The triazole derived Schiff bases (L1–L4) have been prepared by the reaction of 3-amino-5-methylthio-1H-1,2,4-triazole with a series of chloro-, bromo-, and nitro- substituted 2-hydroxybenzaldehyde under reflux ().
All derivatives were soluble in ethanol, dioxane, DMF, and DMSO at room temperature and in methanol on heating only. The metal complexes (1–16) were obtained by stoichiometric reaction of the corresponding ligand with metal [Co(II), Ni(II), Cu(II) and Zn(II)] as chlorides in a molar ratio M:L (1:2) ().
All metal complexes were air and moisture stable. They were insoluble in common organic solvents and only soluble in DMF and DMSO. Physical and analytical data of the complexes (1–16) are given in Supplementary Tables S1 and S2.
Molar conductivity and magnetic properties
The molar conductance values of the metal complexes (1–16) in DMF (Supplementary Table S2) fall in the range 11.7–19.8 Ω−1·cm2·mol−1 showing their non-electrolytic natureCitation19. The room temperature magnetic moment values of the Co(II), Ni(II) and Cu(II) complexes are given in Supplementary Table S2. The observed magnetic moment of Co(II) complexes were in the range of 4.43–4.74 B.M indicating the high-spin Co(II) complexes with three unpaired electrons in octahedral environmentCitation20. The Ni(II) complexes showed µeff values in the range of 3.24–3.51 B.M indicating two unpaired electrons per Ni(II) ion suggesting their octahedral geometryCitation21. The measured values 1.39–1.74 B.M for Cu(II) complexes were indicative of one unpaired electron per Cu(II) ion for d9-system suggesting distorted octahedralCitation20 configuration. All the Zn(II) complexes were found to be diamagneticCitation21 as expected.
IR spectra
The characteristic bands of IR spectra of the ligands and their metal complexes are reported in experimental section and in Supplementary Table S2. The IR spectra of all the ligands exhibitedCitation22 the bands at 3175–3185, 1605–1607, 1025–1030 and 805–815 cm−1 respectively, due to (N–H), (C=N), (N–N) and (–S–C) vibrations of triazole moiety. The stretching bands in the spectra of the ligands at 1715 and 3325 cm−1 due to carbonyl v(C=O) and amino v(NH2) groups disappeared and in turn, a new strong band at 1625–1632 cm−1 assigned to azomethine (–C=N) linkageCitation22 appeared providing a clue for condensation of amino (NH2) group of triazole with carbonyl (C=O) group of aldehydes. The vibration of ν(–OH) at low values, reflected the existenceCitation22 of intramolecular hydrogen bonding between (–OH) and azomethine (–C=N) group. The ligands, L2 and L3 exhibited the vibrations at 825 and 680 cm−1 assignedCitation22 to ν(C–Cl) and ν(C–Br), respectively. The ligand, L4 showedCitation22 band at 1360 cm−1 owing to vibrations of ν(C–NO2) group. A strong band appeared in the spectra of all metal complexes at 1612–1617 cm−1 due to azomethine (HC=N) group that shifted to lower frequency by 10–17 cm−1 indicating the participationCitation22 of the azomethine nitrogen with the metal ion. Similarly, the triazole ring (C=N) band originally appearing at 1605–1607 cm−1 in the spectra of the ligands shifted to lower frequency at 1590–1594 cm−1 in the spectra of the metal complexes by 12–17 cm−1 indicativeCitation22 of the involvement of triazole ring nitrogen in the complexes. In all the metal(II) complexes, a new band appearedCitation22 at 525–535 cm−1 due to ν(M–N) vibrations. However, the band appearing at 1025–1030 cm−1 due to ν(N-N) mode of triazole ring in all the ligands remained unchanged in the spectra of the metal(II) complexes indicating non-involvement of the nitrogen of (N–N) of triazole ring with the metal ion. The disappearanceCitation22 of broad band of ν(OH) at 3265–3290 cm−1 in spectra of all metal complexes and appearance of new band of (C–O) at 1380–1385 cm−1 revealed deprotonation and coordination of hydroxyl-O to the metal atom. This metal to oxygen coordination was further justified by the appearance of new band at 450–465 cm−1 due to (M–O).
1H NMR Spectra
The 1H NMR spectral data of the ligands (L1–L4) and their diamagnetic Zn(II) complexes are recorded in the experimental part. The exhibited signals of all the protons due to heteroaromatic/aromatic groups were foundCitation23 as to be in their expected region. The spectra of all the Schiff base ligands displayed a singletCitation23, due to proton of azomethine C7–H and hydroxyl group (–OH) of phenol moieties at 9.15–9.40 and 11.90–12.60 ppm, respectively. The strong down field shift of the hydroxyl proton indicatedCitation23 its participation in intramolecular hydrogen bonding. The Schiff base ligands L2–L4 displayed C3–H and C6–H protons of phenol as doublet at 7.70–8.45 and 6.85–7.15 ppm, respectively and the same ligands showed C5–H proton of phenol as double of the doublet at 7.30–8.30 ppm. The L1 exhibited the phenol C4–H and C5–H protons as doublet of the double doublet at 6.95 and 7.45 ppm, respectively. However, the phenol C3–H and C6–H protons appeared as double of the doublet at 7.80 and 7.1 ppm. Moreover, all the Schiff base ligands displayed (–S–CH3) methyl protons of triazole as singlet at 2.45–2.72 ppm. A broad singlet at 14.00–14.15 ppm displayedCitation23 due to tautomerism of NH proton of triazole in all the ligands. The coordination of the azomethine (CH=N) nitrogen are assignedCitation23 by the downfield shifting of azomethine (C7–H) proton signal from 9.15–9.40 ppm in the free ligands to 9.49–9.81 ppm in their zinc(II) complexes. Furthermore, signal of hydroxyl (OH) protons appearing at 11.90–12.60 ppm in the spectra of free ligands, disappeared in spectra of their corresponding zinc complexes indicatingCitation23 deprotonation and coordination of the hydroxyl-O with zinc metal. All other protons overall underwent downfield shift by 0.11–0.25 ppm due to the increased conjugationCitation23 in the spectra of the Zn(II) complexes.
13C NMR Spectra
The 13C NMR spectra of the Schiff base ligands L1–L4 and their Zn(II) complexes were done in DMSO-d6. The 13C NMR spectral data are reported along with their possible assignments in the experimental section and all the carbons were found in the expected regionsCitation23. The conclusion obtained from these studies provides further support to the mode of bonding explained in their IR and 1H NMR spectral data. The 13C NMR spectra of all the Schiff base ligands, displayedCitation23 the azomethine carbon (C7) and methyl carbon (C10) of triazole (–S–CH3) at 161.93–162.28 and 13.88–13.98 ppm, respectively. The carbons of phenol moiety of all the Schiff base ligands were appearedCitation23 in the range from 115.71–163.36 ppm. The downfield peak in all the ligands at 163.22–163.36 ppm is due to (C1). This downfield shift of (C1) signal is due to attachment of hydroxyl (OH) group at (C1) position. Similarly, the ligand L2 showed downfield peak at 127.58 ppm of (C4) owing to the electronegative effect of chloro group at this position, as compared to ligand L1 which showed upfield peak at 119.58 ppm due to absence of chloro group at this position. The triazole carbons (C8) and (C9) of all the ligands appearedCitation23 in the region of 156.23–158.82 ppm. The spectra of Zn(II) complexes of all the ligands exhibitedCitation23 a downfield shifting of azomethine carbon (C7) from 161.93–162.28 ppm in free ligands to 163.35–163.78 ppm in the zinc(II) complexes, revealing the coordination of azomethine nitrogen to the Zn(II) ion. Also the downfield shifting of triazole carbon (C9) from 158.05–158.82 ppm in Schiff base ligands to 159.51–160.0 ppm in Zn(II) complexes, displayingCitation23 the coordination of triazole nitrogen to the zinc ion. Similarly, the phenol carbon (C1) of the ligands existed near the coordination sites (C1–O–M) showed downfield shift by 0.9–2.1 ppm from 163.22–163.36 ppm in the spectra of ligands to 164.17–164.87 ppm in the spectra of the zinc(II) complexes. Furthermore, all other carbons of the all the ligands in Zn(II) complexes underwentCitation23 downfield shifting by 0.24–0.8 ppm due to the increased conjugation and coordination with the metal atoms.
Mass spectra
The mass spectral data and fragmentation pattern of all the Schiff base ligands L1–L4 clearly justify the formation of the ligands possessing proposed structures and their bonding pattern. The mass spectra of ligand L1 showed molecular ion peak m/z 234 (calcd. 234.29) of [C10H10N4SO].+, which loses a hydroxyl group (OH) as radical to give most stable fragment at m/z 217 of [C10H9N4S]+. Similarly the mass spectra of ligand, L2 showed molecular ion peak at m/z 268 (calcd. 268.72) of [C10H9N4SOCl].+, with base peak fragment of [C10H8N4SCl]+ at m/z 251. The ligand, L3 exhibited molecular ion peak at m/z 314.0/312 (calcd. 313.17) of [C10H9N4SOBr].+ with base peak fragment [C10H8N4SBr]+ at m/z 298/296. In the same way the ligand, L4 displayed molecular ion peak at m/z 279.0 (calcd. 279.29) of [C10H9N5SO3].+, with stable fragment [C10H8N5SO2]+ as base peak at m/z 262. The fragmentation pattern followed the cleavage of C=N (exocyclic as well as endocyclic), C–OH, C–C, C–N and C–S bonds. Fragmentation pattern of one ligand L1 is shown as .
Electronic spectra
The electronic spectral values of Co(II), Ni(II), Cu(II) and Zn(II) complexes are recorded in Supplementary Table S2. The Co(II) complexes in general, exhibited three absorption bands in the region at 8560–8880, 17,680–17,945 and 29,684–29,940 cm−1 are respectively assigned to the transitions 4T1g → 4T2g(F), 4T1g → 4A2g(F) and 4T1g → 4Tg(P), showing an octahedral geometryCitation24 around the Co(II) ion. The spectra of Ni(II) complexes showed bands in the region at 9694–10,095, 15,860–16,213 and 29,456–29,750 cm−1 which are assignedCitation24 due to the transitions of 3A2g → 3T2g(F)(ν1), 3A2g → 3T1g(F) (ν2) and 3A2g → 3T1g(P) (ν3), respectively, consistent with their well-defined octahedral configuration. The electronic spectra of Cu(II) complexes exhibited low-energy absorption band at 14,670–14,880 cm−1 assigned to the transitions 2Eg → 2T2g. The high-energy band at 25,590–25,860 cm−1 is due to forbidden ligand → metal charge transfer. On the basis of which a distorted octahedral geometry is suggested for Cu(II) complexesCitation24. The diamagnetic Zn(II) complexes did not show any d–d transitions and their spectra were dominatedCitation24 only by the charge transfer band at 28,675–28,930 cm−1.
Biological activity
Antibacterial bioassay
Antibacterial activity data of newly synthesized Schiff bases and their corresponding metal(II) complexes was determined against four Gram-negative (Escherichia coli, Shigella sonnei, Pseudomonas aeruginosa, Salmonella typhi) and two Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains () according to literature protocolCitation15. The obtained results were compared with standard drug ampicillin and 3-amino-5-methylthio-1H-1,2,4-triazole (Supplementary Figures S1 and S3). The percentage of activity was compared with the activity of the standard drug (ampicillin) considering its activity as 100 %. The obtained results indicated that the Schiff base ligand, L1 exhibited moderate antibacterial activity (46–53 %) against all bacterial strains whereas the Schiff base ligand, L2 showed significant activity (55.17 %) against (f) and moderate activity (46–53 %) against (a)–(e). The antibacterial results from indicated that the Schiff base ligand, L3 possessed significant activity (57.14 %) against (d) and moderate activity (43–52 %) against (a)–(c), (e) and (f) while the ligand, L4 exhibited significant activity (55.17–61.53 %) against (a) and (f) and moderate activity (48–52 %) against (b)–(e). The antibacterial data of metal(II) complexes indicated that metal(II) complexes, 1, 3–8, 10 and 12–14 exhibited significant activity (54–82 %) against all observed bacterial strains. However, the metal(II) complexes, 2 and 15 showed significant activity (57–81 %) against (a) and (c)–(f) and moderate activity (53 %) against (b). Similarly, the metal(II) complexes, 9 possessed significant activity (59–79 %) against (b) and (d)–(f) and moderate activity (47–52 %) against (a) and (c), compound 11 also showed significant activity (61–71 %) against (a), (b) and (d)–(f) and moderate activity (47 %) against (c). Compound 16 displayed significant activity (57–75 %) against (a)-(d) and (f) and moderate activity (52 %) against (e). The comparative studies of data, exhibited that the Schiff base ligands L1-L4 were more active than 3-amino-5-methylthio-1H-1,2,4-triazole against tested bacterial strains which provided evidences that the activity of 3-amino-5-methylthio-1H-1,2,4-triazole increased upon Schiff base preparation. Moreover, the synthesized Schiff base ligands possessed smaller average activity values (14.3 mm) than the average values (17.9 mm) of metal(II) complexes which showed that the activity was enhancedCitation25 upon coordination.
Table 1. Antibacterial bioassay (concentration used 1 mg/mL of DMSO) of ligands L1–L4 and the metal(II) complexes 1–16.
Antifungal bioassay
In vitro antifungal bioassay
Antifungal screening of all the newly synthesised Schiff base ligands, and their metal(II) complexes, was carried out against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata fungal strains according to the literatureCitation16 protocol and their results were compared with standard drugs Miconazole and Amphotericin B. The antifungal data () exhibited that the Schiff base ligand, L1 showed significant activity (57–61 %) against (b) and (c), moderate (38–46 %) against (a), (d) and (f) and weak (33 %) against (e) fungal strains. The Schiff base ligands, L2 and L4 showed significant activity (57–60 %) against (c) and (b), while the ligand L2 exhibited moderate activity (43–48 %) against (b) and (f), and weak (33 %) against (a) and (d) fungal strains. Schiff base ligand L4 showed moderate activity (39–51 %) against (a), (d) and (f) and weak (33 %) against (b) fungal strains. The activity of Schiff base ligand, L3 was found to be significant (54–67 %) against (e) and (f) moderate (38–47 %) against (a)–(c) and weak (33 %) against (d) fungal strains. The results of antifungal activity exhibited that the metal(II) complexes 10, 13, 14 and 16 showed significant activity (54–57 %) against (a) the complexes 1–4 showed significant activity (57–66 %) against (b) and the complexes 1–8 and 13–16 showed significant activity (56–66 %) against (c) fungal strains. Similarly, the metal(II) complexes, 5–9 and 11–16 possessed significant activity (56–65 %) against (e) and the complexes 9–12 showed significant activity (69–76 %) against (f) fungal strains. The metal(II) complexes 1-5, 8, 9, 11, 12 and 15 showed moderate activity (34–53 %) against (a), and the same activity shown by complexes 5–12 against (b), 9–12 against (c) and 1–5, 8, 13–16 against (d). In the same way, the metal(II) complex 10 showed moderate activity (34–53 %) against (e) and the complexes 1–8 and 12–16 possessed same activity against (f) fungal strains. The metal (II) complexes, 1–4 and 6 showed weaker activity (19–31 %) respectively against (e) and (a) and the complex 7 also showed weaker activity (29–30 %) against (a) and (d) fungal strains. Similarly the metal (II) complexes, 9–12 showed weaker activity (20–24 %) against (d), and also the complexes, 13 and 16 possessed weaker activity (30–31 %) against (b) fungal strains. The average activity data comparison showed that the metal(II) complexes exhibited greater average activity value (47.4 %) than the average activity value of the ligands (44.4 %). The above discussions concluded that the antifungal activity of the Schiff base ligands increasedCitation26 upon chelation/coordination with the metal ions. The comparative activity data of the ligands and their metal(II) complexes is presented in Supplementary Figures S2 and S4.
Table 2. Antifungal bioassay (concentration used 200 µg/mL) of ligands L1–L4 and the metal(II) complexes 1–16.
Minimum inhibitory concentration (MIC)
The synthesized Schiff base ligands and their metal(II) complexes, having antibacterial activity more than 80 % were selected for MIC studies and obtained results are recorded in . It was investigated from the data that the synthesized compounds exhibited varying degree of inhibitory effect on the growth of tested bacterial strains. The preliminary antibacterial screening results of all the compounds exhibited that the metal(II) complexes 5, 8, 12, 14, and 15 were found to be the most (above 80%) active compounds. Therefore, these compounds were selected for minimum inhibitory concentration (MIC) studies (). The MIC values of these compounds fall in the range 58.62–90.33 µg/mL. The MIC result, however, showed that the compound 8 was the most active showing maximum inhibition against bacterial strain S.sonnei.
Table 3. Minimum inhibitory concentration (µg/mL) of the selected compounds 5, 8, 12, 14 and 15 against selected bacteria
In vitro cytotoxic bioassay
The synthesized ligands and their metal (II) complexes were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et alCitation18. The cytotoxic data recorded in Supplementary Table S3, revealed that only two compounds, 3 and 11 displayed potent cytotoxic activity having LD50 as 1.10 × 10−4–1.67 × 10−4 M against Artemia salina while all other compounds can be considered as almost inactive in this assay. It was interesting to note that metal complexes showed potent cytotoxicity as compared to ligands. This activity relationship can serve as a useful tool for future studies in designing and development of cytotoxic agents.
Conclusion
All the newly synthesized Schiff bases (L1–L4) act as tridentate ligands through the coordination of triazole (C=N) and azomethine (CH=N) nitrogens and, phenolic oxygen with the metal ion. The bonding of ligands to the metal ion and octahedral geometry observed by all the metal complexes was confirmed by the analytical, IR, 1H NMR, electronic and magnetic studies. From the in vitro antibacterial and antifungal activity data shown against representative bacterial and fungal strains is evident that the Schiff bases and their Co(II), Ni(II), Cu(II) and Zn(II) complexes were found to possess good antibacterial and antifungal activities however, the metal complexes showed more activity as compared to the simple Schiff bases.The average activity data ( and ) of the Schiff base ligands revealed that halo- and nitro-substituted ligands showed overall more activity than the unsubstituted ligand. Amongst the substituted ones, the nitro-substituted ligand (L4) possessed higher activity than the chloro-substituted ligand (L2), which in turn shows more activity than the bromo-substituted ligand (L3). It was also observed that amongst the metal ions, the Zn(II) complexes showed overall more antibacterial activity than other metals. Similarly, Co(II) complexes were overall found to be more antifungal than the other metal ions.
Acknowledgment
One of us (M.H.) is grateful to Higher Education Commission (HEC), Government of Pakistan for the award to carry out this research. We are also thankful to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for providing help in undertaking NMR, mass spectral and antibacterial/antifungal data.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents.
References
- Noël R, Song X, Jiang R, Chalmers MJ, Griffin PR, Kamenecka TM. Efficient methodology for the synthesis of 3-amino-1,2,4-triazoles. J Org Chem 2009;74:7595–7597.
- Al-Masoudi IA, Al-Soud YA, Al-Salihi NJ, Al-Masoudi NA. 1,2,4-Triazoles: synthetic approaches and pharmacological importance. Chem Heterocycl Compd 2006;42:1377.
- Nakata A, Hashimoto S, Ikura K, Katsuura K. Development of a new fungicide, triflumizole. J Pestic Sci 1991;16:301–314.
- Propst T, Vogel W, Propst A, Dietze O, Braunsteiner H. Successful treatment of recurrent hepatic Candida infection with fluconazole after chemotherapy for acute leukemia: a case report. Clin Investig 1992;70:55–58.
- Stahl SM. Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr 2008;13:1027–1038.
- Dalloul HM, Al-Abadla NS, El-Nwairy KA. Synthesis of some new substituted 4,5-dihydro-1H-1,2,4-triazoles. Chem Heterocyclic Comps 2007;43:314–319.
- Capranico G, Zagotto G, Palumbo M. Development of DNA topoisomerase-related therapeutics: a short perspective of new challenges. Curr Med Chem Anticancer Agents 2004;4:335–345.
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff bases derived from fluorobenzaldehyde and triazoles. J Enzyme Inhib Med Chem 2006;21:557–562.
- Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem 2009;44:3064–3067.
- Guo-Qiang H, Li-Li H, Song-Qiang X, Wen-Long H. Design, synthesis and antitumor activity of asymmetric bis(s-triazole Schiff-base)s bearing functionalized side-chain Chin J Chem 2008;26:1145–1149.
- Jin JY, Zhang LX, Zhang AJ, Lei XX, Zhu JH. Synthesis and biological activity of some novel derivatives of 4-amino-3-(D-galactopentitol-1-yl)-5-mercapto-1,2,4-triazole. Molecules 2007;12:1596–1605.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur J Med Chem 2008;43:2639–2649.
- Kharodawala MJ, Rana AK, Synthesis, characterization and biological activity of some transitionn metal chelates of 4-acyloxime-2-pyrazolin-5-ones. Synth React Inorg Metal-org Chem 2003;33:1483–1504.
- Banerjee P, Pandey OP, Sengupta SK. Microwave assisted synthesis, spectroscopic and antibacterial studies of titanocene chelates of Schiff bases derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles. Trans Met Chem 2008;33:1047–1052.
- Chohan ZH, Iqbal MS, Aftab SK, Rauf A. Antibacterial dimeric copper(II) complexes with chromone-derived compounds. J Enzyme Inhib Med Chem 2012;27:223–231.
- Chohan ZH, Supuran CT. Organometallic compounds with biologically active molecules: In vitro antibacterial and antifungal activity of some 1,1′- (dicarbohydrazono)ferrocenes and their Co (II), Cu (II), Ni (II) and Zn (II) complexes. Appl Organomet Chem 2005;19:1207–1218.
- McLaughlin JL, Chang CJ, Smith DL. (1991). Studies in natural products chemistry,’’bentch-top’’ bioassays for the discovery of bioactive natural products, an update, structure and chemistry (Part-B), (Ed.: Atta-ur-Rahman), Vol 9. Amsterdam: Elsevier Science, 383.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982;45:31–34.
- Chohan ZH, Supuran CT. Structure and biological properties of first row d-transition metal complexes with N-substituted sulfonamides. J Enzyme Inhib Med Chem 2008;23:240–251.
- Chohan ZH, Pervez H, Rauf A, Scozzafava A, Supuran CT. Antibacterial Co(II), Cu(II), Ni(II) and Zn(II) complexes of thiadiazole derived furanyl, thiophenyl and pyrrolyl Schiff bases. J Enzyme Inhib Med Chem 2002;17:117–122.
- Chohan ZH. Metal-based antibacterial and antifungal sulfonamides: Synthesis, characterization and biological properties. Trans Met Chem 2009;34:153–161.
- Chohan ZH, Sumrra SH. Synthesis, characterization and biological studies of oxovanadium(IV) complexes with triazole-derived Schiff bases. Appl Organomet Chem 2010;24:122–130.
- Levitt M. (2001). Spin dynamics: basics of nuclear magnetic resonance. John Wiley and Sons.
- Chohan ZH, Rau A, Noreen S, Scozzafava A, Supuran CT. Antibacterial cobalt(II), nickel(II) and zinc(II) complexes of nicotinic acid-derived Schiff-bases. J Enzyme Inhib Med Chem 2002;17:101–106.
- Chohan ZH, Supuran CT. Metalloantibiotics: synthesis, characterization and in-vitro antibacterial studies on cobalt (II), copper (II), nickel (II) and zinc (II) complexes with cloxacillin. J Enzyme Inhib Med Chem 2006;21:441–448.
- Chohan ZH, Mahmood-Ul-Hassan, Khan KM, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide–derived Schiff’s bases and their metal complexes. J Enzyme Inhib Med Chem 2005;20:183–188.