Abstract
A series of substituted phenylethylidenehydrazinylpyridinium derivatives bearing methyl, ethyl, propyl, and propylphenyl groups on the pyridinium nitrogen were synthesized and evaluated for in vitro antileishmanial activity against Leishmania tropica by using the microdilution method. Among the tested compounds, 3d, 5c, 3b, and 3c were found to be the most active derivatives against the promastigotes of L. tropica (IC50 values are 6.90, 9.92, 11.69 and 12.03 µM, respectively) and to be more active than reference drug meglumine antimonaite (glucantime) (IC50 value: 20.49 µM). The derivatives investigated in this study may have the potential to be lead compound against leishmanial infection.
Introduction
Leishmaniasis is a serious global public health problem causing significant morbidity and mortality in the world. Leishmania species are classified under the kingdom Protozoa, phylum Sarcomastigophora and are widely distributed in nature. The most common transmission of the Leishmania parasite occurs through the bites of the infected female phlebotomine sandfly. Transmission is also possible by parenteral, sexual and occupational routes as well as by blood transfusionCitation1. The clinical forms of leishmaniasis are visceral, cutaneous, mucocutaneous and post kala azar dermal leishmaniasisCitation2. The currently available drugs are limited and involve the pentavalent antimony compounds (sodium stibogluconate and meglumine antimonate), amphotericin B, pentamidine and miltefosine. These drugs have several disadvantages such as high toxicity, serious side effects, drug resistance, high treatment costCitation3–5. Therefore, the development of effective new agents against leishmaniasis is needed.
In recent years, a great number of natural and synthetic compounds have been tested against leishmaniasisCitation6–8. These compounds comprising a diverse group of chemical structures have been reported as antileishmanial agents. Most of them include nitrogen heterocycle rings such as quinolinesCitation9, quinazolinesCitation10, acridinesCitation11, pyrimidinesCitation12, pyrazolsCitation13, pyridinesCitation8, benzothiazolesCitation14, imidazolesCitation15, thiadiazolesCitation16 and include functional structures such as alkyl phospholipidsCitation17, ether phospholipidsCitation18, chalconesCitation19, amidinesCitation20, oximesCitation21, amidoximeCitation22, hydrazonesCitation23 and hydrazidesCitation24,Citation25.
On the other hand, a number of quaternized amine derivatives such as thiadiazolium-phenylamineCitation26,Citation27, arylisoquinoliniumCitation28, imidazo-pyridiniumCitation29, alkyl-ammonium-phenothiazinesCitation30, indolo-quinoliniumCitation31 and naphthalimide-amonniumCitation32 were previously reported to exert antiprotozoal properties. Antiprotozoal activity of quaternary ammonium compounds against Acanthamoeba spp. was mainly studied in connection with the disinfection of contact lenses. Benzalkonium chloride having quaternary nitrogen atom is used in commercial contact lens solutionsCitation33. Arylisoquinolinium salts and imidazo-pyridinium derivatives possessed good activity results against leishmania and tripanosoma parasites respectively. Their activity results suggested that the activity may depend on their cationic structureCitation28,Citation29. In addition, some researchers have reported that the N+ charge of the quaternary nitrogen at indolo-quinolinium iodide compounds is required for binding to DNA fragments for antiinfective activityCitation31. Besides, mono- and bisquaternary pyridinium compounds were reported to have antimicrobial, antimalarial and antileishmanial activitiesCitation34. This study suggests that the aliphatic chain attached to pyridinium skeleton may be important for retaining antimicrobial/antiprotozoal activity and the presence of pyridinium skeleton does not suffice requirements to inhibit microbes or parasites.
In our previous study, oxime-ether and hydrazone derivatives with quaternary nitrogen on pyridine ring were synthesized and evaluated for their antimicrobial activities. Some of these compounds exhibited remarkable antimicrobial activitiesCitation35. In this study, we synthesized 16 phenylethylidenehydrazinylpyridinium salts bearing different alkyl side chains on pyridinium nitrogen and evaluated them for their antileishmanial activity.
Materials and methods
Chemistry
Melting points were determined with an Electrothermal IA9100 melting point apparatus and were not corrected. 1H NMR spectra was recorded on a Varian AS 400 Mercury Plus NMR instrument. Abbreviations for data quoted are: s, singlet; br s, broadsinglet; d, doublet; t, triplet; quin, quintet; dd, doublet of doublets; m, multiplet. IR spectra of compounds were recorded as potassium bromide pellets on a Jasco FT/IR-400 spectrometer. High resolution mass spectrum (HRMS) of the title compounds were recorded on a HPLC-TOF Waters Micromass LCT Premier XE (Milford, MA, USA) mass spectrometer using an electrospray ion source (ESI). Reagents and solvents used for synthesis were purchased from Aldrich, Fluka, and Merck companies. Thin-layer chromatographies were carried out on precoated silica gel 60 F254 plates (Merck). The spots were visualized with UV light or iodine.
General procedure for synthesis of phenylethylidenehydrazinylpyridine derivatives (2–5)
4-Hydrazinylpyridine (0.01 mol) and appropriate acetophenone derivatives (0.01 mol) were refluxed in ethanol for 10–18 h. The precipitate was filtered and washed with cool ethanol and recrystallized from ethanol.
4-[2-(1-Phenylethylidene)hydraziny]pyridine (2)
Yield 54%, mp: 185°C IR (KBr) vmaks (cm−1): 3203, 1602, 1515, 1492, 1444, 1419, 815, 763, 686; 1H NMR (400 MHz, DMSO-d6), δ (ppm): 11.14 (1H, s, N-H), 8.22 (2H, d, J = 6.2 Hz, H-2, H-6), 7.82 (2H, d, J = 7.8 Hz, H-2′, H-6′), 7.43-7.33 (3H, m, H-3′, H-4′, H-5′), 7.14 (2H, d, J = 5.8 Hz, H-3, H-5), 2.29 (3H, s, C-CH3).
4-[2-(1-[4′-Methylphenyl]ethylidene)hydrazinyl]pyridine (3)
Yield 73%, mp: 221°C IR (KBr) vmaks (cm−1): 3205, 1594, 1504, 819, 730; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.68 (1H, s, N-H), 8.19 (2H, d, J = 6.2 Hz, H-2, H-6), 7.69 (2H, d, J = 8.2 Hz, H-2′, H-6′), 7.19 (2H, d, J = 8.2 Hz, H-3′, H-5′), 7.10 (2H, dd, J = 1.6, 5.1 Hz, H-3, H-5), 2.31 (3H, s, -CH3), 2.24 (3H, s, -CH3).
4-[2-(1-[4′-Methoxyphenyl]ethylidene)hydrazinyl]pyridine (4)
Yield 65%, mp: 210°C IR (KBr) vmaks (cm−1): 3201, 1602, 1506, 1423, 1249, 825, 730; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.66 (1H, s, N-H), 8.18 (2H, d, J = 5.8 Hz, H-2, H-6), 7.74 (2H, d, J = 8.6 Hz, H-2′, H-6′), 7.08 (2H, d, J = 6.2 Hz, H-3, H-5), 6.94 (2H, d, J = 8.6 Hz, H-3′, H-5′), 3.77 (3H, s, OCH3), 2.24 (3H, s, C-CH3).
4-[2-(1-[4′-Chlorophenyl]ethylidene)hydrazinyl]pyridine (5)
Yield 60%, mp: 241°C IR (KBr) vmaks (cm−1): 3209, 1602, 1484, 1421, 823, 775, 688; 1H NMR (400 MHz, DMSO-d6) δ (ppm) 9.81 (1H, s, N-H), 8.21 (2H, d, J = 6.2 Hz, H-2, H-6), 7.83-7.80 (2H, m, H-2′, H-6′), 7.45-7.42 (2H, m, H-3′, H-5′), 7.12 (2H, d, J = 6.2 Hz, H-3, H-5), 2.26 (3H, s, C-CH3).
General procedure for synthesis of the pyridinium compounds (2a–5d)
A mixture of compound 2–5 (0.01 mol) and corresponding alkyl halide (0.02 mol) were refluxed in ethanol for 6–50 h. The mixture was cooled to room temperature or 0°C and the obtained precipitate was filtered and washed with cool ethanol. The crude products were recrystallized from ethanol to give compounds 2a–5d.
1-Methyl-4-[2-(1-phenylethylidene)hydrazinyl]pyridinium iodide (2a)
Yield 71%; mp: 279°C; IR (KBr) vmaks (cm−1): 3411, 3018, 2963, 2819, 1644, 1577, 1536, 1442 821, 763, 690; 1H NMR (DMSO-d6) δ (ppm): 11.14 (1H, s, N-H), 8.38 (1H, br s, H-2 or H-6), 8.34 (1H, br s, H-2 or H-6), 7.94-7.90 (2H, m, H-2′, H-6′), 7.69 (1H, br s, H-3 or H-5), 7.48-7.45 (3H, m, H-3′, H-4′, H-5′), 7.34 (1H, br s, H-3 or H-5), 4.01 (3H, s, +N-CH3), 2.43 (3H, s, C-CH3); HRMS (ESI+) calcd. for C14H16N3+ 226.1344, Found: 226.1336.
1-Ethyl-4-[2-(1-phenylethylidene)hydrazinyl]pyridinium bromide (2b)
Yield 62%; mp: 222°C; IR (KBr) vmaks (cm−1): 3426, 3019, 2987, 2851, 1644, 1579, 1538 848, 757, 688; 1H NMR (DMSO-d6) δ (ppm) 11.21 (1H, s, N-H), 8.46 (1H, br s, H-2 or H-6), 8.43 (1H, br s, H-2 or H-6), 7.91-7.89 (2H, m, H-2′, H-6′), 7.67 (1H, br s, H-3 or H-5), 7.45-7.44 (3H, m, H-3′, H-4′, H-5′), 7.39 (1H, br s, H-3 or H-5), 4.27 (2H, q, J = 7.4 Hz, +N-CH2), 2.43 (3H, s, C-CH3), 1.42 (3H, t, J = 7.4 Hz, +N-CH2-CH3); HRMS (ESI+) calcd. for C15H18N3+ 240.1501, Found: 240.1501.
4-[2-(1-Phenylethylidene)hydrazinyl]-1-propyl pyridinium bromide (2c)
Yield 69%; mp: 225°C; IR (KBr) vmaks (cm−1): 3400, 3008, 2903, 2879, 1644, 1579, 1538, 1440, 809, 757, 688; 1H NMR (DMSO-d6) δ (ppm): 11.25 (1H, s, N-H), 8.47 (1H, d, J = 6.6 Hz, H-2 or H-6), 8.42 (1H, d, J = 6.6 Hz, H-2 or H-6), 7.93-7.91 (2H, m, H-2′, H-6′), 7.68 (1H, d, J = 6.2 Hz, H-3 or H-5), 7.48-7.46 (3H, m, H-3′, H-4′, H-5′), 7.41 (1H, d, J = 5.8 Hz, H-3 or H-5), 4.23 (2H, t, J = 7.0 Hz, +N-CH2), 2.45 (3H, s, C-CH3), 1.86-1.81 (2H, m, +N-CH2-CH2-CH3), 0.87 (3H, t, J = 7.4 Hz, +N-CH2-CH2-CH3); HRMS (ESI+) calcd. for C16H20N3+ 254.1657, Found: 254.1656.
4-[2-(1-Phenylethylidene)hydrazinyl]-1-(3-phenylpropyl)pyridinium bromide (2d)
Yield 50%; mp: 106°C; IR (KBr) vmaks (cm−1): 3480, 3010, 2990, 2860, 1644, 1538, 1445, 840, 754, 692; 1H NMR (DMSO-d6) δ (ppm): 11.28 (1H, s, N-H), 8.47 (1H, d, J = 6.6 Hz, H-2 or H-6), 8.43 (1H, d, J = 7.0 Hz, H-2 or H-6), 7.91-7.89 (2H, m, H-2′, H-6′), 7.65 (1H, d, J = 6.6 Hz, H-3 or H-5), 7.46-7.43 (4H, m, Ar-H), 7.30-7.26 (2H, m, Ar-H), 7.21-7.16 (3H, m, Ar-H), 4.29 (2H, t, J = 7.4 Hz, +N-CH2), 2.60 (2H, t, J = 7.8 Hz, +N-CH2CH2-CH2), 2.44 (3H, s, -CH3), 2.13 (2H, quin, J = 7.4 Hz, +N-CH2-CH2-CH2); HRMS (ESI+) calcd. for C22H24N3+ 330.1970, Found: 330.1967.
1-Methyl-4-[2-(1-[4-methylphenyl]ethylidene)hydrazinyl]pyridinium iodide (3a)
Yield 64%; mp: 268°C; IR (KBr) vmaks (cm−1): 3396, 3029, 2965, 2856, 1644, 1579, 1538, 1511, 840, 819; 1H NMR (DMSO-d6) δ (ppm): 11.09 (1H, s, N-H), 8.33 (1H, d, J = 7.0 Hz, H-2 or H-6), 8.27 (1H, d, J = 7.8 Hz, H-2 or H-6), 7.80 (2H, d, J = 8.2 Hz, H-2′, H-6′), 7.64 (1H, d, J = 5.8 Hz, H-3 or H-5), 7.26-7.24 (3H, m, H-3 or H-5, H-3′, H-5′), 3.97 (3H, s, +N-CH3), 2.38 (3H, s, -CH3), 2.34 (3H, s, -CH3); HRMS (ESI+) calcd. for C15H18N3+ 240.1501, Found: 240.1501.
1-Ethyl-4-[2-(1-[4′-methylphenyl]ethylidene)hydrazinyl]pyridinium bromide (3b)
Yield 43%; mp: 205°C; IR (KBr) vmaks (cm−1): 3409, 3014, 2925, 2869, 2767, 1644, 1579, 1538, 1509, 852, 817; 1H NMR (DMSO-d6) δ (ppm): 11.16 (1H, s, N-H), 8.46 (1H, d, J = 7.8 Hz, H-2 or H-6), 8.41 (1H, d, J = 7.0 Hz, H-2 or H-6), 7.81 (2H, d, J = 8.2 Hz, H-2′, H-6′), 7.66 (1H, d, J = 7.8 Hz, H-3 or H-5), 7.34 (1H, d, J = 5.8 Hz, H-3 or H-5), 7.27 (2H, d, J = 8.2 Hz, H-3′, H-5′), 4.28 (2H, q, J = 7.4 Hz, +N-CH2), 2.41 (3H, s, -CH3), 2.36 (3H, s, -CH3), 1.43 (3H, t, J = 7.4 Hz, +N-CH2-CH3); HRMS (ESI+) calcd. for C16H20N3+ 254.1657, Found: 254.1661.
4-[2-(1-[4-Methylphenyl]ethylidene)hydrazinyl]-1-propylpyridinium bromide (3c)
Yield 41%; mp: 238°C; IR (KBr) vmaks (cm−1): 3442, 3055, 2921, 2852, 1644, 1540, 1511, 815; 1H NMR (DMSO-d6) δ (ppm): 11.20 (1H, s, N-H), 8.45 (1H, d, J = 7.2 Hz, H-2 or H-6), 8.40 (1H, d, J = 7.8 Hz, H-2 or H-6), 7.82 (2H, d, J = 8.3 Hz, H-2′, H-6′), 7.66 (1H, d, J = 7.2 Hz, H-3 or H-5), 7.37 (1H, d, J = 5.2 Hz, H-3 or H-5), 7.27 (2H, d, J = 8.0 Hz, H-3′, H-5′), 4.22 (2H, t, J = 7.4 Hz, +N-CH2), 2.41 (3H, s, -CH3), 2.36 (3H, s, -CH3), 1.85-1.80 (2H, m, +N-CH2-CH2-CH3), 0.86 (3H, t, J = 7.4 Hz, +N-CH2-CH2-CH3); HRMS (ESI+) calcd. for C17H22N3+ 268.1814, Found: 268.1812.
4-[2-(1-[4-Methylphenyl]ethylidene)hydrazinyl]-1-(3-phenylpropyl)pyridinium bromide (3d)
Yield 40%; mp: 128°C; IR (KBr) vmaks (cm−1): 3463, 3023, 2912, 2870, 1644, 1581, 1536, 1490, 817, 750; 1H NMR (DMSO-d6) δ (ppm): 11.25 (1H, s, N-H), 8.57 (1H, d, J = 7.4 Hz, H-2 or H-6), 8.43 (1H, d, J = 7.0 Hz, H-2 or H-6), 7.82 (2H, d, J = 8.2 Hz, H-2′, H-6′), 7.65 (1H, d, J = 6.6 Hz, H-3 or H-5), 7.44 (1H, d, J = 5.5 Hz, H-3 or H-5), 7.31-7.20 (7H, m, Ar-H), 4.31 (2H, t, J = 7.4 Hz, +N-CH2), 2.62 (2H, t, J = 7.4 Hz, +N-CH2CH2-CH2), 2.43 (3H, s, -CH3), 2.36 (3H, s, -CH3), 2.15 (2H, quin, J = 7.4 Hz, +N-CH2-CH2-CH2); HRMS (ESI+) calcd. for C23H26N3+ 344.2127, Found: 344.2126.
4-[2-(1-[4-Methoxylphenyl]ethylidene)hydrazinyl]-1-methylpyridinium iodide (4a)
Yield 79%; mp: 295°C; IR (KBr) vmaks (cm−1): 3374, 3052, 2996, 2965, 2931, 1644, 1610, 1573, 1536, 1506, 1467, 1243, 827, 717; 1H NMR (DMSO-d6) δ (ppm): 11.07 (1H, s, N-H), 8.35 (1H, d, J = 6.6 Hz, H-2 or H-6), 8.28 (1H, d, J = 6.2 Hz, H-2 or H-6), 7.88 (2H, d, J = 8.9 Hz, H-2′, H-6′), 7.64 (1H, d, J = 5.1 Hz, H-3 or H-5), 7.29 (1H, d, J = 5.1 Hz, H-3 or H-5), 7.00 (2H, d, J = 8.6 Hz, H-3′, H-5′), 3.99 (3H, s, +N-CH3), 3.82 (3H, s, OCH3), 2.39 (3H, s, C-CH3); HRMS (ESI+) calcd. for C15H18N3O+ 256.1450, Found: 256.1451.
1-Ethyl-4-[2-(1-[4-methoxylphenyl]ethylidene)hydrazinyl]pyridinium bromide (4b)
Yield 60%; mp: 241°C; IR (KBr) vmaks (cm−1): 3426, 3018, 2961, 2832, 1642, 1578, 1540, 1511, 1251, 836, 711; 1H NMR (DMSO-d6) δ (ppm): 11.13 (1H, s, N-H), 8.45 (1H, d, J = 7.0 Hz, H-2 or H-6), 8.39 (1H, d, J = 6.2 Hz, H-2 or H-6), 7.88 (2H, d, J = 8.6 Hz, H-2′, H-6′), 7.64 (1H, d, J = 5.5 Hz, H-3 or H-5), 7.34 (1H, d, J = 5.1 Hz, H-3 or H-5), 7.01 (2H, d, J = 8.6 Hz, H-3′, H-5′), 4.27 (2H, q, J = 7.4 Hz, +N-CH2), 3.82 (3H, s, OCH3), 2.40 (3H, s, C-CH3), 1.43 (3H, t, J = 7.4 Hz, +N-CH2-CH3); HRMS (ESI+) calcd. for C16H20N3O+ 270.1606, Found: 270.1601.
4-[2-(1-[4-Methoxylphenyl]ethylidene)hydrazinyl]-1-propylpyridinium bromide (4c)
Yield 51%; mp: 220°C; IR (KBr) vmaks (cm−1): 3426, 3018, 2962, 2923, 2867, 1643, 1540, 1511, 1251, 833, 711; 1H NMR (DMSO-d6) δ (ppm): 11.17 (1H, s, N-H), 8.42 (1H, d, J = 7.4 Hz, H-2 or H-6), 8.36 (1H, d, J = 7.0 Hz, H-2 or H-6), 7.86 (2H, d, J = 8.9 Hz, H-2′, H-6′), 7.62 (1H, d, J = 5.1 Hz, H-3 or H-5), 7.35 (1H, d, J = 5.1 Hz, H-3 or H-5), 6.98 (2H, d, J = 8.9 Hz, H-3′, H-5′), 4.19 (2H, t, J = 7.4 Hz, +N-CH2), 3.79 (3H, s, OCH3), 2.39 (3H, s, C-CH3), 1.83-1.77 (2H, m, +N-CH2-CH2-CH3), 0.85 (3H, t, J = 7.4 Hz, +N-CH2-CH2-CH3); HRMS (ESI+) calcd. for C17H22N3O+ 284.1763, Found: 284.1756.
4-[2-(1-[4-Methoxylphenyl]ethylidene)hydrazinyl]-1-(3-phenylpropyl)pyridinium bromide (4d)
Yield 45%; mp: 173°C; IR (KBr) vmaks (cm−1): 3432, 3045, 2942, 2862, 1644, 1538, 1509, 1461, 833, 761; 1H NMR (DMSO-d6) δ (ppm): 11.21 (1H, s, N-H), 8.46 (1H, d, J = 7.4 Hz, H-2 or H-6), 8.41 (1H, d, J = 7.4 Hz, H-2 or H-6), 7.89 (2H, d, J = 8.9 Hz, H-2′, H-6′), 7.63 (1H, d, J = 7.0 Hz, H-3 or H-5), 7.41 (1H, d, J = 7.0 Hz, H-3 or H-5), 7.32-7.28 (2H, m, Ar-H), 7.23-7.18 (3H, m, Ar-H), 7.01 (2H, d, J = 8.9 Hz, H-3′, H-5′), 4.09 (2H, t, J = 7.4 Hz, +N-CH2), 3.80 (3H, s, OCH3), 2.62 (2H, t, J = 7.4 Hz, +N-CH2CH2-CH2), 2.42 (3H, s, -CH3), 2.15 (2H, quin, J = 7.4 Hz, +N-CH2-CH2-CH2); HRMS (ESI+) calcd. for C23H26N3O+ 360.2076, Found: 360.2081.
4-[2-(1-[4-Chlorophenyl]ethylidene)hydrazinyl]-1-methylpyridinium iodide (5a)
Yield 65%; mp: 305°C; IR (KBr) vmaks (cm−1): 3425, 3020, 2951, 2832, 1643, 1583, 1538, 1513, 1482, 823, 763, 680; 1H NMR (DMSO-d6) δ (ppm): 11.17 (1H, s, N-H), 8.39 (1H, br s, H-2 or H-6), 8.34 (1H, br s, H-2 or H-6), 7.96-7.94 (2H, m, H-2′, H-6′), 7.70 (1H, br s, H-3 or H-5), 7.53-7.50 (2H, m, H-3′, H-5′), 7.33 (1H, br s, H-3 or H-5), 4.01 (3H, s, +N-CH3), 2.41 (3H, s, C-CH3); HRMS (ESI+) calcd. for C14H15N3Cl+ 260.0955, Found: 260.0942.
4-[2-(1-[4-Chlorophenyl]ethylidene)hydrazinyl]-1-ethylpyridinium bromide (5b)
Yield 51%; mp: 267°C; IR (KBr) vmaks (cm−1): 3421, 3014, 2941, 2820, 1641, 1536, 1490, 833; 1H NMR (DMSO-d6) δ (ppm): 11.22 (1H, s, N-H), 8.47 (1H, br s, H-2 or H-6), 8.44 (1H, br s, H-2 or H-6), 7.94-7.91 (2H, m, H-2′, H-6′), 7.68 (1H, br s, H-3 or H-5), 7.52-7.49 (2H, m, H-3′, H-5′), 7.37 (1H, br s, H-3 or H-5), 4.28 (2H, q, J = 7.4 Hz, +N-CH2), 2.41 (3H, s, C-CH3), 1.42 (3H, t, J = 7.0 Hz, +N-CH2-CH3); HRMS (ESI+) calcd. for C15H17N3Cl+ 274.1111, Found: 274.1117.
4-[2-(1-[4-Chlorophenyl]ethylidene)hydrazinyl]-1-propylpyridinium bromide (5c)
Yield 43%; mp: 228°C; IR (KBr) vmaks (cm−1): 3425, 3006, 2911, 2825, 1644, 1540, 1486, 854, 828; 1H NMR (DMSO-d6) δ (ppm): 11.29 (1H, s, N-H), 8.48 (1H, br s, H-2 or H-6), 8.43 (1H, br s, H-2 or H-6), 7.94-7.89 (2H, m, H-2′, H-6′), 7.68 (1H, d, J = 5.5 Hz, H-3 or H-5), 7.50 (2H, d, J = 8.9 Hz, H-3′, H-5′), 7.43 (1H, d, J = 5.8 Hz, H-3 or H-5), 4.22 (2H, t, J = 7.0 Hz, +N-CH2), 2.42 (3H, s, C-CH3), 1.84-1.78 (2H, m, +N-CH2-CH2-CH3), 0.85 (3H, t, J = 7.0 Hz, +N-CH2-CH2-CH3); HRMS (ESI+) calcd. for C16H19N3Cl+ 288.1268, Found: 288.1263.
4-[2-(1-[4-Chlorophenyl]ethylidene)hydrazinyl]-1-(3-phenylpropyl)pyridinium bromide (5d)
Yield 38%; mp: 115°C; IR (KBr) vmaks (cm−1): 3465, 3021, 2905, 2879, 1644, 1585, 1536, 1484, 831, 748, 694; 1H NMR (DMSO-d6) δ (ppm): 11.37 (1H, s, N-H), 8.53 (1H, d, J = 6.6 Hz, H-2 or H-6), 8.49 (1H, d, J = 7.0 Hz, H-2 or H-6), 7.95 (2H, d, J = 8.5 Hz, H-2′, H-6′), 7.68 (1H, d, J = 5.9 Hz, H-3 or H-5), 7.53-7.50 (3H, m, H-3′, H-5′, H-3 or H-5), 7.32-7.28 (2H, m, Ar-H), 7.24-7.18 (3H, m, Ar-H), 4.34 (2H, t, J = 7.4 Hz, +N-CH2), 2.63 (2H, t, J = 7.4 Hz, +N-CH2CH2-CH2), 2.46 (3H, s, -CH3), 2.16 (2H, quin, J = 7.4 Hz, +N-CH2-CH2-CH2); HRMS (ESI+) calcd. for C22H23N3Cl+ 364.1581, Found: 364.1581.
Antileishmanial activity
Parasite
Promastigotes of Leishmania tropica (MHOM/TR/10/CBU52) isolated from Manisa/Turkey, were used to evaluate the antileishmanial effect of the compounds.
Saline (0.5 ml) was injected to the lesions of the patients by applying a small incision at the merger of the lesions with intact tissue or by entering with a syringe. After a few minutes, aspirated liquid was inoculated into NNN (Novyi MacNeal Nicolle) medium and the medium was kept in an incubator at 25°C for 1 week. After observing the presence of promastigotes, these were cultured in liquid RPMI medium containing 10% fetal-calf serum. It was observed that the promastigotes entered the logarithmic phase on days of the eighth-ninth. Then, they were transferred to cryo-eppendorf tubes containing 12% DMSO and placed in liquid nitrogen tank. They were stored in the tank until use. When to use eppendorf tubes were taken from the liquid nitrogen tank and thawed rapidly by placing in water bath at 37°C. After the promastigotes (MHOM/TR/10/CBU/52) were recultured in liquid RPMI medium containing 10% FCS for approximately 10 days, they were used in the study.
In vitro antileishmanial activity test
In vitro activity of the compounds against L. tropica was investigated by microdilution method. Stock solutions of the compounds were prepared in DMSO/distilled water (1/1) and serial dilutions were made to achieve the final concentrations (0.244 mg/L 500 mg/L) in the wells of microplates. Promastigotes that were cultivated in RPMI-1640 medium with 5% fetal-calf serum, were counted in hemocytometer. Final concentrations of the promastigotes were adjusted to 1 × 106 cell/ml in 200 µl RPMI + 5% FCS medium. Microplates were incubated in 27°C for 48 h. Viable promastigotes were counted by hemocytometer after 48 h and IC50 were determined. Meglumine antimonate (glucantime) which is employed in treatment of cutaneous leishmaniasis (CL), was used as a reference agent in concentrations between 5 and 40 mg/L. The experiments were performed triplicate and average values were calculated. DMSO was studied alone (without compounds) in order to evaluate the any possible antileishmanial activity. The highest concentration of DMSO was 6.25% in the first wells (corresponding to 500 µg/ml concentrations of substances) of the microplates.
Results and discussion
Chemistry
Phenylethylidenehydrazinylpyridine salts were prepared in two steps according to the procedure described in our previous studyCitation35, as shown in . In the first step, 4-hydrazinylpyridineCitation(1) was condensed with various substituted acetophenones to furnish the corresponding hydrazone derivatives 2–5. In the second step, the final compounds 2a–5d were obtained by quaternization of hydrazone derivatives 2–5 with the appropriate substituted alkyl halide. All the title compounds 2a–5d and the spectral data of the intermediate compounds 2–5 were reported for the first time in this study.
Scheme 1. The synthesis pathway and the structures of the compounds. (a) substituted acetophenon, C2H5OH, reflux and (b) RX, C2H5OH, reflux.
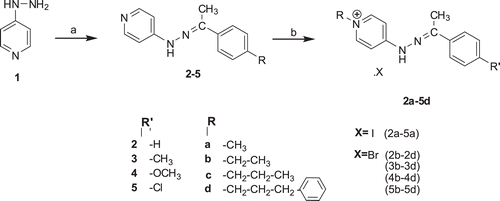
We have previously reported that the molecules containing the same scaffold with these compounds were the E isomer according to the X-ray crystallographyCitation36,Citation37. X-ray interpretations suggested all of our compounds have E isomer. In addition, E configuration of a representative compound, 4d, analyzed by 2D NOESY NMR spectroscopy and the assignment of stereochemistry of compound 4d were consistent with the observed crosspeak between N-H (δ 11.21) and CH3 (δ 2.42, CH3-C=N-NH).
The structures of the final compounds were confirmed by spectral analyses and the spectroscopic properties were in accord with the proposed structures. The IR spectra of the final compounds showed intense absorption bands within 3374–3480 cm−1 range that attributed to NH vibrations. The assessment of the chemical shifts in 1H NMR spectrum demonstrated that the aromatic and aliphatic protons were observed in the expected regions with expected multiplicities confirming the substitution patternCitation38. The proton signals due to the NH group were recorded between 11.37 and 11.07 ppm. The high resolution mass spectrum results of final compounds (2a–5d) confirmed for purposed structures.
Antileishmanial activity
Phenylethylidenehydrazinylpyridine salts without any substituent and also having methyl, methoxyl, chloro substituents at para position of phenyl ring and methyl, ethyl, propyl and 3-phenylpropyl side chains in quaternary pyridinium ring were investigated for in vitro antileishmanial activity against promastigotes of Leishmania tropica, a causative agent of cutaneous leishmaniasis. The antileishmanial activities of phenylethylidenehydrazinylpyridine salts were assessed by microdilution methodCitation39. Stock solutions of the compounds were prepared in DMSO/distilled water (1/1) and serial dilutions were made to achieve the final concentrations (0.244 mg/L 500 mg/L ) in the wells of microplates. DMSO was studied without compounds in order to evaluate the any possible antileishmanial activity. The highest concentration of DMSO was 6.25% in the first wells (corresponding to 500 µg/ml concentrations of substances) of the microplates. This concentration of DMSO partially inhibited the growth of L. tropica, however there was no effect of DMSO on the growth of the micro-organism starting from the second wells.
In vitro biological activities of phenylethylidenehydrazinylpyridine salts displayed encouraging results compared to reference drug glucantime. The results are expressed as IC50 values of the compounds and summarized in .
Table 1. Structures and in vitro antileishmanial activities of compounds (2a–5d).
All compounds exhibited antileishmanial activity with different values. According to the antileishmanial activity results, in general, methyl substituent on phenyl ring seemed to increase the activity whereas chloro substituent decreased the activity with the exception of 5c. Based on the Hammett constants of these substituents on phenyl ring, only chlorine substituent has positive Hammett value. On the other hand, 2a, 4a and 5a compounds having methyl group on pyridine ring and H, OMe, Cl substituents on phenyl ring, respectively, manifested close IC50 values whereas compound 3a bearing methyl substituent on phenyl ring displayed weakest activity. Due to these findings, methyl group on phenyl ring is favoured while methyl group on pyridinium ring is unfavoured for bioactivity. Different Hammett values of H, OMe and Cl groups (σ = 0.00, −0.27, +0.23, respectively) of the these compounds with close IC50 values suggest us electronic properties of phenyl substituents do not have a direct effect on the activity. However, when alkyl substituents on pyridine nitrogen are ethyl and the substituents on phenyl ring are H (2b) and OMe (4b), the IC50 values remain close to the reference compound’s IC50 value. However in compound 3b (R:Me), the IC50 value is twice as the reference compound while compound 5b having chloro substituent displays four times less activity compared to the reference compound. Depending on the data obtained from N-ethyl derivatives, substituents donating electrons to phenyl ring causing negative σ value might be preferred for the activity. No relationship between the bioactivity of compounds and π values of alkyl groups on pyridine ring was detected.
Considering the side chain on pyridinium nitrogen, compounds having ethyl and propyl side chains showed higher activity with the exception for 5b. Moreover, for the compounds bearing propyl and phenylpropyl side chains, addition of the phenyl ring to the propyl side chain seemed to decrease the activity except for 3d. The most active compounds were 3d>5c>3b>3c with IC50 values of 6.90, 9.92, 11.69, and 12.03 µM, respectively and their antileishmanial activities were approximately two times more effective than glucantime (20.49 µM).
However, the exact mode of action has not been elucidated yet, it can be speculated that the cationic pyridinium moiety of the compounds may have an interaction potency with DNA and/or other biological nucleophillesCitation40,Citation41. These final compounds are capable of making ionic and hidrogen bonds as well as ion-dipol, dipol-dipol, hydrophobic interactions with biological componentsCitation40.
A number of studies reported that the activities of the quaternary ammonium compounds were attributed to their effect on the cell wall resulting in a direct or indirect lethal effect on the cell viabilityCitation42,Citation43. Our compounds, bearing unsaturated quaternary salts might disturb the cell wall of micro-organism. In addition, trypanosomes and/or leishmanias are important targets for redoxactive compoundsCitation44. On the other hand, pyridinium salts are also known as redox targettorsCitation43. According to Bodor there is a redox mechanism between dihydropyridine and pyridinium salts and dihydropyridine shows an equilibrium with pyridinium salt in this type of redox systemCitation45–47. In conclusion, the pyridinium structure of our title compounds includes redox potency, which could give rise to antileishmanial activity.
Conclusion
In conclusion, we have synthesized 16 phenylethylidenehydrazinylpyridine salts 2a–5d and evaluated for their antileishmanial activity. The synthesis and antileishmanial activity of the title compounds 2a–5d have been reported for the first time in this study. The most active compound was found to be 4-[2-(1-[4-methylphenyl]ethylidene)hydrazinyl]-1-(3-phenylpropyl)pyridinium bromide (3d) having IC50 value of 6.90 µM against L. tropica. The results indicate that all the compounds are active with varying values; the derivatives having methyl substituent on phenyl ring exhibit better activity and the length of the chain on nitrogen orient the antileishmanial activity. The obtained preliminary results suggest that some of these compounds (3b, 3c, 3d, 5c) might serve as potential candidates for antileishmanial agents and will be used as basis strategy point for the design of new molecules with improved antileishmanial activity. Further investigations are in progress in our laboratory.
Acknowledgments
The authors thank Prof. Ahmet Ozbilgin, Department of Parasitology, Faculty of Medicine, Celal Bayar University, Manisa, Turkey for providing the L. tropica promastigotes.
Declaration of interest
This study was supported by research grants from Ege University (project number: 09/Ecz/012).
References
- Otero ACS, Da-Silvia VO, Luz, KG, Palatnik M, Pirmez C, Fernandes O, De-Sousa CBP. Short report: Occurence of Leishmania donovani DNA in donated blood from seroreactive Brazilian blood donors. Am J Trop Med Hyg 2000;62:128–131.
- Pearson RD, Sousa AQ. (1995). Leishmania species: Visceral (kala-azar), cutaneous and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone, 2428–2442.
- Murray HW. Treatment of visceral leishmaniasis in 2004. Am J Trop Med Hyg 2004;71:787–794.
- Croft SL. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol Sci 1988;9:376–381.
- Berman JD. Chemotherapy for leishmaniasis: Biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis 1988;10:560–586.
- Khan F, Choudhary M, Rasheed M, Ullah Z, Hayat S, Kaukab F et al. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogues. Bioorg Med Chem 2003;11:1381–1387.
- Zhai L, Chen M, Blom J, Theander TG, Christensen SB, Kharazmi A. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother 1999;43:793–803.
- de Mello H, Echevarria A, Bernardino AM, Canto-Cavalheiro M, Leon LL. Antileishmanial pyrazolopyridine derivatives: Synthesis and structure-activity relationship analysis. J Med Chem 2004;47:5427–5432.
- Chakrabarti G, Basu A, Manna PP, Mahato SB, Mandal NB, Bandyopadhyay S. Indolylquinoline derivatives are cytotoxic to Leishmania donovani promastigotes and amastigotes in vitro and are effective in treating murine visceral leishmaniasis. J Antimicrob Chemother 1999;43:359–366.
- Agarwal KC, Sharma V, Shakya N, Gupta S. Design and synthesis of novel substituted quinazoline derivatives as antileishmanial agents. Bioorg Med Chem Lett 2009;19:5474–5477.
- Delmas F, Avellaneda A, Di Giorgio C, Robin M, De Clercq E, Timon-David P et al. Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives. Eur J Med Chem 2004;39:685–690.
- Chandra N, Ramesh, Ashutosh, Goyal N, Suryawanshi SN, Gupta S. Antileishmanial agents part-IV: Synthesis and antileishmanial activity of novel terpenyl pyrimidines. Eur J Med Chem 2005;40:552–556.
- Rathelot P, Azas N, El-Kashef H, Delmas F, Di Giorgio C, Timon-David P et al. 1,3-Diphenylpyrazoles: Synthesis and antiparasitic activities of azomethine derivatives. Eur J Med Chem 2002;37:671–679.
- Delmas F, Di Giorgio C, Robin M, Azas N, Gasquet M, Detang C et al. In vitro activities of position 2 substitution-bearing 6-nitro- and 6-amino-benzothiazoles and their corresponding anthranilic acid derivatives against Leishmania infantum and Trichomonas vaginalis. Antimicrob Agents Chemother 2002;46:2588–2594.
- Bhandari K, Srinivas N, Marrapu VK, Verma A, Srivastava S, Gupta S. Synthesis of substituted aryloxy alkyl and aryloxy aryl alkyl imidazoles as antileishmanial agents. Bioorg Med Chem Lett 2010;20:291–293.
- Poorrajab F, Ardestani SK, Emami S, Behrouzi-Fardmoghadam M, Shafiee A, Foroumadi A. Nitroimidazolyl-1,3,4-thiadiazole-based anti-leishmanial agents: Synthesis and in vitro biological evaluation. Eur J Med Chem 2009;44:1758–1762.
- Croft SL, Snowdon D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother 1996;38:1041–1047.
- Coghi P, Vaiana N, Pezzano MG, Rizzi L, Kaiser M, Brun R et al. Parallel synthesis and antileishmanial activity of ether-linked phospholipids. Bioorg Med Chem Lett 2008;18:4658–4660.
- Aponte JC, Castillo D, Estevez Y, Gonzalez G, Arevalo J, Hammond GB et al. In vitro and in vivo anti-Leishmania activity of polysubstituted synthetic chalcones. Bioorg Med Chem Lett 2010;20:100–103.
- Mayence A, Vanden Eynde JJ, LeCour L Jr, Walker LA, Tekwani BL, Huang TL. Piperazine-linked bisbenzamidines: A novel class of antileishmanial agents. Eur J Med Chem 2004;39:547–553.
- Mäntylä A, Rautio J, Nevalainen T, Vepsälainen J, Juvonen R, Kendrick H et al. Synthesis and antileishmanial activity of novel buparvaquone oxime derivatives. Bioorg Med Chem 2004;12:3497–3502.
- Paloque L, Bouhlel A, Curti C, Dumètre A, Verhaeghe P, Azas N et al. Synthesis and evaluation of monoamidoxime derivatives: Toward new antileishmanial compounds. Eur J Med Chem 2011;46:2984–2991.
- Visbal G, Marchán E, Maldonado A, Simoni Z, Navarro M. Synthesis and characterization of platinum-sterol hydrazone complexes with biological activity against Leishmania (L.) mexicana. J Inorg Biochem 2008;102:547–554.
- Rando DG, Avery MA, Tekwani BL, Khan SI, Ferreira EI. Antileishmanial activity screening of 5-nitro-2-heterocyclic benzylidene hydrazides. Bioorg Med Chem 2008;16:6724–6731.
- Saudi MNS, El-Semary MMA, Elbayaa RY, Jaeda MI, Eissa MM, Amer EI et al. Synthesis and biological evaluation of a novel classas antileishmanial agent. Med Chem Res 2012;21:257–267.
- da Silva EF, Canto-Cavalheiro MM, Braz VR, Cysne-Finkelstein L, Leon LL, Echevarria A. Synthesis, and biological evaluation of new 1,3,4-thiadiazolium-2-phenylamine derivatives against Leishmania amazonensis promastigotes and amastigotes. Eur J Med Chem 2002;37:979–984.
- Rodrigues RF, da Silva EF, Echevarria A, Fajardo-Bonin R, Amaral VF, Leon LL et al. A comparative study of mesoionic compounds in Leishmania sp. and toxicity evaluation. Eur J Med Chem 2007;42:1039–1043.
- Ponte-Sucre A, Gulder T, Wegehaupt A, Albert C, Rikanovic C, Schaeflein L et al. Structure-activity relationship and studies on the molecular mechanism of leishmanicidal N,C-coupled arylisoquinolinium salts. J Med Chem 2009;52:626–636.
- Sundberg RJ, Dahlhausen DJ, Manikumar G, Mavunkel B, Biswas A, Srinivasan V et al. Cationic antiprotozoal drugs. Trypanocidal activity of 2-(4′-formylphenyl)imidazo[1,2-a]pyridinium guanylhydrazones and related derivatives of quaternary heteroaromatic compounds. J Med Chem 1990;33:298–307.
- Khan MO, Austin SE, Chan C, Yin H, Marks D, Vaghjiani SN et al. Use of an additional hydrophobic binding site, the Z site, in the rational drug design of a new class of stronger trypanothione reductase inhibitor, quaternary alkylammonium phenothiazines. J Med Chem 2000;43:3148–3156.
- Zhu XY, Mardenborough LG, Li S, Khan A, Zhang W, Fan P et al. Synthesis and evaluation of isosteres of N-methyl indolo[3,2-b]-quinoline (cryptolepine) as new antiinfective agents. Bioorg Med Chem 2007;15:686–695.
- Tischer M, Sologub L, Pradel G, Holzgrabe U. The bisnaphthalimides as new active lead compounds against Plasmodium falciparum. Bioorg Med Chem 2010;18:2998–3003.
- Zanetti S, Fiori PL, Pinna A, Usai S, Carta F, Fadda G. Susceptibility of Acanthamoeba castellanii to contact lens disinfecting solutions. Antimicrob Agents Chemother 1995;39:1596–1598.
- Bharate SB, Thompson CM. Antimicrobial, antimalarial, and antileishmanial activities of mono- and bis-quaternary pyridinium compounds. Chem Biol Drug Des 2010;76:546–551.
- Alptüzün V, Parlar S, Tasli H, Erciyas E. Synthesis and antimicrobial activity of some pyridinium salts. Molecules 2009;14:5203–5215.
- Alptüzün V, Prinz M, Hörr V, Scheiber J, Radacki K, Fallarero A et al. Interaction of (benzylidene-hydrazono)-1,4-dihydropyridines with beta-amyloid, acetylcholine, and butyrylcholine esterases. Bioorg Med Chem 2010;18:2049–2059.
- Aydın A, Akkurt M, Alptuzun V, Buyukgungor O, Holzgrabe U, Radacki K. 4-[(2E)-2-(4-Chlorobenzylidene)hydrazinylidene]-1-methyl-1,4-dihydropyridine monohydrate. Acta Cryst Sec E 2010;66:1324–1325.
- Giger R, Meier HP, Küpfer U. [Length of gestation of Freiberger mares with mule and horse foals]. Schweiz Arch Tierheilkd 1997;139:303–307.
- Ostan I, Saglam H, Limoncu ME, Ertabaklar H, Toz SO, Ozbel Y et al. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol 2007;30:439–445.
- Chan C, Yin H, Garforth J, McKie JH, Jaouhari R, Speers P et al. Phenothiazine inhibitors of trypanothione reductase as potential antitrypanosomal and antileishmanial drugs. J Med Chem 1998;41:148–156.
- Gao YG, Sriram M, Denny WA, Wang AH. Minor groove binding of SN6999 to an alkylated DNA: Molecular structure of d(CGC[e6G]AATTCGCG)-SN6999 complex. Biochemistry 1993;32:9639–9648.
- Kourai H, Yabuhara T, Shirai A, Maeda T, Nagamune H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur J Med Chem 2006;41:437–444.
- Madaan P, Tyagi VK. Quaternary pyridinium salts: A review. J Oleo Sci 2008;57:197–215.
- Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A. Trypanothione: A novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 1985;227:1485–1487.
- Prókai-Tátrai K, Pop E, Anderson W, Lin JL, Brewster ME, Bodor N. Redox derivatives of tranylcypromine: Syntheses, properties, and monoamine oxidase inhibitor activity of some chemical delivery systems. J Pharm Sci 1991;80:255–261.
- Bodor N, AbdelAlim AM. Improved delivery through biological membranes XIX: Novel redox carriers for brain-specific chemical delivery systems. J Pharm Sci 1985;74:241–245.
- Bodor N, Farag HH. Improved delivery through biological membranes. 13. Brain-specific delivery of dopamine with a dihydropyridine in equilibrium with pyridinium salt type redox delivery system. J Med Chem 1983;26:528–534.
- Finney DG. (1971). Probit analysis. 3rd ed. London: Cambridge University Press.
