Abstract
In this work, we reported the synthesis and evaluation of antibacterial and antifungal activities of three new compound series obtained from 6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazole-3-acetic acid hydrazide: 2-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]acetyl}-N-alkyl/arylhydrazinecarbothioamides (2a–d), 4-alkyl/aryl-2,4-dihydro-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-3H-1,2,4-triazole-3-thiones (3a–n), and 2-alkyl/arylamino-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-1,3,4-thiadiazoles (4a–g). The newly synthesized compounds were characterized by IR, 1H NMR, 13C NMR (APT), mass and elemental analysis. Their antibacterial and antifungal activities were evaluated against Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Candida albicans ATCC 10231, C. parapsilosis ATCC 22019, C. krusei ATCC 6258, Trichophyton mentagrophytes var. erinacei NCPF 375, Microsporum gypseum NCPF 580, and T. tonsurans NCPF 245. 3c, 3f, 3m, 3n, and 4e showed the highest antibacterial activity. Particularly 3c, 3f, 3g, 3k, 3n, 4a, 4e, and 4g showed the highest antifungal activity against tested fungi.
Introduction
The rapidly expanding population of immunocompromised patients results in a corresponding increase of diseases caused by bacteria, yeasts, and other fungi. Although not life-threatening, superficial mycosis and infections of keratinized tissues such as nails, skin, and hair cause prolonged periods of distress. Dermatophytoses which are most prevalent among superficial mycosis are currently treated by the imidazole derivatives clotrimazole, miconazole, econazole, () and other azole antifungals which interfere with fungal ergosterol synthesis by inhibiting lanosterol 14-demethylaseCitation1. Meanwhile, fusion of imidazole and thiazole moieties into a single drug gave a potent immunomodulating drug called levamisole, which is 2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole () Citation2. The imidazo[2,1-b]thiazole derivatives have been reported in the literature as antibacterialCitation3, antifungalCitation4, and antitumourCitation5 agents. On the other hand, there are a number of antimicrobial compounds containing a 1,2,4-triazole ring in their structures such as fluconazole and ravuconazole, that are important antifungal drugs ()Citation6.
Heterocycles containing a 1,2,4-triazole or 1,3,4-thiadiazole moiety, and the compounds consisting of 1,2,4-triazole and 1,3,4-thiadiazole condensed nucleus systems constitute a class of compounds possessing a wide spectrum of biological activities such as antibacterialCitation7, antifungalCitation8, antitubercularCitation9, anticonvulsantCitation10, anticancerCitation11 activities.
In view of these facts and as a continuation of our research on the biological properties of 1,2,4-triazole- and 1,3,4-thiadiazole containing derivativesCitation12–18, we have designed and synthesized a number of imidazo[2,1-b]thiazole substituted fused 1,2,4-triazole and 1,3,4-thiadiazole systems, as potential antibacterial and antifungal agents.
Materials and methods
Chemistry
All chemicals for synthesis were commercially available. Melting points were determined by using a Büchi 530 melting point apparatus (Flawil, Switzerland) in open capillary tubes and are uncorrected. Elemental analyses were performed on a Carlo Erba 1106 elemental analyzer (Milano, Italy). IR spectra were recorded on KBr discs, using a Perkin Elmer 1600 FT-IR spectrophotometer (Waltham, MA, USA). 1H NMR (DMSO-d6/TMS) and 13C NMR (Attached Proton Test) (DMSO-d6/TMS) spectra were measured on Bruker AC 200 (200 MHz), VarianUNITY INOVA (500 MHz) spectrometer. Electron impact mass spectra were recorded on a VG Zab Spec (70 eV) instrument. The starting materials were either commercially available or synthesized according to the references cited.
General procedure of the synthesis of 2-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]acetyl}-N-alkyl/arylhydrazinecarbothioamides (2a–d)
To a solution of 6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazole-3-acetic acid hydrazide (0.005 mol) (1) in EtOH (30 mL), an appropriate isothiocyanate (0.005 mol) was added. The resulting mixture was heated under reflux for 3 h. After cooling, the precipitate was separated and purified by washing with hot EtOH.
2-{[6-Phenylimidazo[2,1-b]thiazol-3-yl]acetyl}-N-phenylhydrazinecarbothioamide (2a)
IR (KBr, ν, cm−1): 3215 (N-H), 1670 (C=O); 1H NMR (200 MHz, δ, ppm, DMSO-d6): 3.88 (s, 2H, CH2CO), 7.09 (s, 1H, C2-H), 7.14–7.57 (m, 8H, ar), 7.79 (d, 2H, J = 7.3 Hz, ar), 8.22 (s, 1H, C5-H), 9.64 (s, 1H, NH), 9.77 (s, 1H, NH), 10.36 (s, 1H, NH); EIMS (70 eV) m/z (%): 407 (M+, 0.3), 272 (58), 241 (16), 214 (44), 135 (100), 93 (37), 77 (92).
General procedure of the synthesis of 4-alkyl/aryl-2,4-dihydro-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-3H-1,2,4-triazole-3-thiones (3a–n)
A solution of an appropriate hydrazinecarbothioamide (0.005 mol) (2) in 2 N aqueous NaOH (20 mL) was heated under reflux for 2 h. After cooling the reaction mixture was acidified by the addition of 12.5% aqueous HCl. The precipitate thus obtained was collected by filtration, washed with water several times and purified by washing with hot C2H5OH.
4-Methyl-2,4-dihydro-5-{[6-phenylimidazo[2,1-b]thiazol-3-yl]methyl}-3H-1,2,4-triazole-3-thione (3a)
IR (KBr, ν, cm−1): 3432 (N-H), 1602, 1579, 1513, 1491 (C=N, C=C); 1H NMR (500 MHz, δ, ppm, DMSO-d6): 3.49 (s, 3H, CH3), 4.40 (s, 2H, CH2), 7.15 (s, 1H, C2-H), 7.23–7.26 (m, 1H, ar), 7.36–7.39 (m, 2H, ar), 7.79 (d, 2H, J = 8.3 Hz, ar), 8.19 (s, 1H, C5-H), 13.60 (s, 1H, NH). 13C NMR (APT) (125 MHz, δ, ppm, DMSO-d6): 24.98 (CH2), 30.69 (CH3), 109.27 (C5), 111.42 (C2), 125.78 (C3), 125.31, 127.74, 129.36, 134.87 (ar C), 146.70 (C6), 149.29 (C7a), 148.75 (triazole C5), 167.86 (C=S).
4-Phenyl-2,4-dihydro-5-{[6-(4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-3H-1,2,4-triazole-3-thione (3n)
IR (KBr, ν, cm−1): 3480, 3103 (O-H/N-H), 1592, 1579, 1535, 1496 (C=N, C=C); 1H NMR (500 MHz, δ, ppm, DMSO-d6): 4.13 (s, 2H, CH2), 6.94 (s, 1H, C2-H), 7.43–7.45 (m, 2H, ar), 7.48–7.53 (m, 3H, ar), 7.55–7.58 (m, 2H, ar), 7.81 (d, 2H, J = 8.4 Hz, ar), 8.17 (s, 1H, C5-H), 13.82 (s, 1H, NH).
General procedure of the synthesis of 2-alkyl/arylamino-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-1,3,4-thiadiazoles (4a–g)
The appropriate hydrazinecarbothioamide (0.005 mol) (2) was dissolved in 5.3 ml of H2SO4 (96%) and allowed to stand for 30 min. The solid then was poured in crushed ice and neutralized with Na2CO3. The precipitate thus obtained was filtered and recrystallized from C2H5OH-H2O.
2-Methylamino-5-{[6-phenylimidazo[2,1-b]thiazol-3-yl]methyl}-1,3,4-thiadiazoles (4a)
IR (KBr, ν, cm−1): 3284 (N-H), 1543, 1470, 1440, 1400 (C=N, C=C); 1H NMR (500 MHz, δ, ppm, DMSO-d6): 2.83 (s, 3H, CH3), 4.49 (s, 2H, CH2), 7.10 (s, 1H, C2-H), 7.23–7.27 (m, 1H, ar), 7.36–7.40 (m, 2H, ar), 7.62 (q, 1H, J = 4.8 Hz, NH), 7.80 (d, 2H, J = 8.3 Hz, ar), 8.14 (s, 1H, C5-H). 13C NMR (APT) (125 MHz, δ, ppm, DMSO-d6): 28.74 (CH2), 31.87 (CH3), 108.77 (C5), 110.61 (C2), 128.83 (C3), 125.39, 127.80, 129.37, 134.79 (ar C), 146.86 (C6), 149.45 (C7a), 152.63 (thiadiazole C5), 170.73 (thiadiazole C2).
Microbiology
All compounds to be tested were dissolved in DMSO at a stock concentration of 3200 µg.cm−3. The final desired concentration were prepared with RPMI 1640 medium for Candida species and dermatophytes and with Mueller-Hinton broth of bacteria. The final DMSO concentration was reduced to 1%.
Antibacterial activity
Minimum inhibitory concentrations (MICs) were determined by the microbroth dilution method using the National Committee for Clinical Laboratory Standards (NCCLS) recommendationsCitation19. Mueller-Hinton broth (Oxoid, Hemakim, Turkey) was used as the test medium. An inoculum of ~5 × 105 CFU.cm−3 was delivered per well. Serial twofold dilutions of the test compounds (64–0.25 µg.cm−3) and extra dilutions (0.12–0.015 µg cm−3) for antibiotic standards were prepared. Plates were incubated for 16–20 h at 35°C in an ambient air incubator. The lowest concentration of the test compounds inhibiting visible growth was taken as the MIC value.
Antifungal activity
Antifungal activity for Candida species
MICs were determined by the microbroth dilution method using the NCCLS recommendationsCitation20. RPMI broth was prepared from RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 0.3 g of glutamine/dm3, bufferred with 3-(N-morpholino)-propanesulfonic acid (MOPS), and adjusted to pH 7.0. A working suspension of the inoculum was prepared by a 1:100 dilution of the 0.5 McFarland standards yeast suspension in 0.85% saline followed by a 1:20 dilution in RPMI broth.
Twofold dilutions of test compounds from 64 to 0.25 µg cm−3 were prepared with the working suspension of the inoculum. Extra dilutions (0.12–0.015 mg.cm−3) were added for itraconazole. The plates were incubated at 35°C for 48 h in ambient air. The MIC is the lowest concentration of a compound that inhibits growth of the organism as detected visually.
Antifungal activity for dermatophytes
Microdilution method was used according to a standard protocol by NCCLSCitation19. RPMI 1640 broth with l-glutamine without sodium bicarbonate was and 0.165 M MOPS buffer (34.54 g/lt) and used. The medium was adjusted to pH 7.0 at 25°C. Preparation of inoculum suspensions of dermatophytes were based according to the NCCLS guidelinesCitation21 and previously described procedureCitation22.
The isolates were subcultured on to potato dextrose agar plates at 28°C, during 7–14 days. The fungal colonies were covered with 1 mL of sterile 0.85% saline, and suspensions were made by gently probing the surface with the tip of Pasteur pipette. The resulting mixture of conidia and hyphal fragments was withdrawn and transferred to a sterile tube. Heavy particles were allowed to settle for 15–20 min at room temperature; the upper suspension was mixed with a vortex for 15 s. The turbidity of supernatants was measured spectrophotometrically at a wavelength of 530 nm, and transmission was adjusted to 65–75 %. These stock suspensions were diluted to 1:50 in RPMI medium to obtain the final inoculum sizes, which range from 0.4 × 104 to 5 × 104 CFU/mL. Microdilution plates were prepared and frozen at −70°C until needed. Rows from 2 to 12 contained the series of drug dilutions in 100 μL volumes and first row contained 100 μL of drug-free medium, which served as the growth control. Each well was inoculated on the day of the test with 100 μL of the corresponding inoculum. This step brought the drug dilutions and inoculum size to the final test concentrations given above. The microplates of dermatophytes were incubated at 28°C during 7 days. The microplates were read visually with the aid of an inverted reading mirror after 7 days for dermatophytes. For all drugs, the MIC was defined as the lowest concentration showing 100% inhibition of growth.
Results and discussion
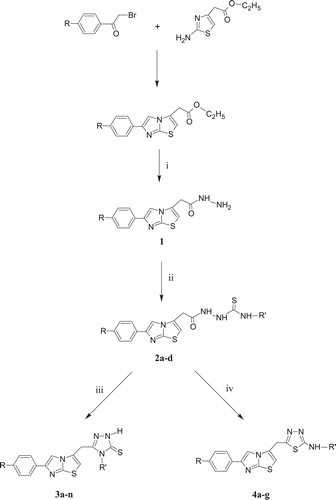
The target compounds were prepared from 6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazole-3-acetic acid hydrazide (1) Citation2, by a three step synthesis as shown in . 2-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]acetyl}-N-alkyl/arylhydrazinecarbothioamides (2a–d) were obtained from 1 and the corresponding alkyl/arylisothiocyanates.
Alkaline cyclisation of the compounds 2 using sodium hydroxide afforded the corresponding 4-alkyl/aryl-2,4-dihydro-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-3H-1,2,4-triazole-3-thiones (3a–n). The reaction of hydrazinecarbothioamide (2) with concentrated sulphuric acid at room temperature resulted in the formation of the corresponding 2-alkyl/arylamino-5-{[6-(phenyl/4-chlorophenyl)imidazo[2,1-b]thiazol-3-yl]methyl}-1,3,4-thiadiazoles (4a–g).
The structures of the synthesized compounds were confirmed by analytical (Supplementary ) and spectral data (IR, 1H NMR, 13C NMR, EIMS). The IR spectra of 2a–d, 3a–n, and 4a–g exhibited N-H bands in the 3500-3195, 3500-3102, and 3284-3181 cm−1, respectively. The absorption bands at 1613-1436 and 1624-1400 cm−1 are due to the presence of -C=N- stretch of the triazole and thiadiazole ring system, respectively. The C=O stretchings of 2a–d were observed at 1676-1670 cm−1. Absence of the C=O absorptions in 3a–n and 4a–g provided definitive proof for the formation of new products. The three 1H NMR resonances located in the region of 10.40-8.08 ppm were assigned to the NH protons of the carbothioamides and supported the structures of 2a–dCitation23. The 1H NMR of 3a–n (except 3c, 3e, and 3i) showed single NH resonances in the region of 13.93-13.57 ppm. In the 1H NMR spectra of 3c, 3e, and 3i, the NH protons were not observed due to rapid proton deuteron exchange reaction in deuterated dimethyl sulfoxide solvent. In the 1H NMR spectra of 4b, 4f, 4g, and 4e, the NH proton at 2-position of 1,3,4-thiadiazole ring appeared at 7.63, 8.19, 10.32 ppm as a singlet and 7.48-7.44 ppm together with Ar-H as a multiplet, respectively. In the 1H NMR spectra of 4a, 4c, and 4d, the NH proton appeared at 7.62 and 7.67 ppm as a quartet and triplet, respectively. The exocyclic -CH2- protons of 3a–n and 4a–g resonated at 4.13–4.51 and 4.48–4.60 ppm, respectively. The protons of the imidazo[2,1-b]thiazole nucleus and the other protons resonated at the expected regionsCitation24. In the APT 13C NMR spectra of 3a and 4a chosen as prototypes, all the carbons resonated in the expected regionsCitation24. The EIMS of compounds 2a, 2d, 3f, 3k, 4b, and 4e displayed molecular ions which confirmed their molecular weights. Fragmentation followed the route in accordance with literatureCitation25.
Table 1. Antifungal activity of compounds 2a–d, 3a–n, and 4a–g (MIC μg/mL).
Compounds 2a–d, 3a–n, and 4a–g were evaluated for in vitro antibacterial activity against Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, and Escherichia coli ATCC 25922 as well as for antifungal activity against Candida albicans ATCC 10231, C. parapsilosis ATCC 22019, C. krusei ATCC 6258, Trichophyton mentagrophytes var. erinacei NCPF 375, Microsporum gypseum NCPF 580 and, T. tonsurans NCPF 245 using the microbroth dilution methodCitation19. As can be seen in Supplementary Table 2, 3f (R = H, R′ = C6H5), 3n (R = Cl, R′ = C6H5), and 4e (R = Cl, R′ = C3H7), showed the highest activity against S. aureus ATCC 29213 and E. coli ATCC 25922 (MIC = 32 μg.cm−3). 3c (R = H, R′ = C3H7), showed the highest activity against E. coli ATCC 25922 (MIC = 32 μg.cm−3). 3m (R = Cl, R′ = allyl), showed the highest activity against P. aeruginosa ATCC 27853 and E. coli ATCC 25922 (MIC = 32 μg.cm−3). Derivatives 3c (R = H, R′ = C3H7), were most active against M. gypseum NCPF 580 (MIC = 8 μg.cm−3, ).
Compounds 3c (R = H, R′ = C3H7) and 3n (R = Cl, R′ = C6H5) showed the highest activity against C. parapsilosis ATCC 22019 (MIC = 16 μg.cm−3). Compounds 3g (R = H, R′ = 4-ClC6H4) and 3n (R = Cl, R′ = C6H5) showed the highest activity against C. krusei ATCC 6258 (MIC = 16 μg.cm−3). Compounds 3f (R = H, R′ = C6H5), 3k (R = Cl, R′ = C3H7), 3n (R = Cl, R′ = C6H5), 4a (R = H, R′ = CH3), 4e (R = Cl, R′ = C3H7), and 4g (R = Cl, R′ = C6H5) showed the highest activity against T. mentagrophytes var. erinacei NCPF 375 (MIC = 16 μg.cm−3). Compounds 3f (R = H, R′ = C6H5), 3k (R = Cl, R′ = C3H7), 3n (R = Cl, R′ = C6H5) showed the highest activity against M. gypseum NCPF 580 (MIC = 16 μg.cm−3). Compounds 3c (R = H, R′ = C3H7), 3f (R = H, R′ = C6H5), 3g (R = H, R′ = 4-ClC6H4), 3k (R = Cl, R′ = C3H7), 3n (R = Cl, R′ = C6H5), 4a (R = H, R′ = CH3), 4e (R = Cl, R′ = C3H7), and 4g (R = Cl, R′ = C6H5) also showed the highest activity against T. tonsurans NCPF 245 (MIC = 16 μg.cm−3). As can be seen from Supplementary Table 2 and , triazole (3a–n) and thiadiazole derivatives (4a–g) were generally more active than the hydrazinecarbothioamide derivatives (2a–d). The antimicrobial activity results indicated that some of the tested compounds showed the most promising antibacterial and antifungal activities.
In summary, a new series of hydrazinecarbothioamides 2a–d, 1,2,4-triazoles 3a–n, and 1,3,4-thiadiazoles 4a–g have been synthesized and evaluated for their antibacterial and antifungal activities. Derivatives 3c (R = H, R′ = C3H7), were most active against M. gypseum NCPF 580 (MIC = 8 μg.cm−3). Triazole (3a–n) and thiadiazole derivatives (4a–g) were generally more active than the hydrazinecarbothioamide derivatives 2a–d. The antimicrobial activity results indicated that some of the tested compounds showed the most promising antibacterial and antifungal activities. Further studies are in progress in our laboratories and will be reported upon in the future.
Acknowledgements
This work was supported by the Research Fund of Istanbul University (Project Numbers: 283/05012005.
Declaration of interest
The authors report no conflicts of interest.
References
- Mutschler E, Derendorf H. (1995). Drug actions: basic principles and therapeutic aspects. Stuttgart: Medpharm Scientific Publishers, 559.
- Harraga S, Nicod L, Drouhin JP, Xicluna A, Panouse JJ, Seilles E et al. Imidazo[2,1-b]thiazole derivatives. XI. Modulation of the CD2-receptor of human T trypsinized lymphocytes by several imidazo[2,1-b]thiazoles. Eur J Med Chem 1994;29:309–315.
- Mahfouz AAA, Elhabashy FM. New synthesis of 2-substituted imidazo[2,1-b]thiazoles and their antimicrobial activities. Arch Pharm Res 1990;3:9–13.
- Mohan J, Kiran K. Novel bridgehead nitrogen heterocycles: Synthesis and antimicrobial activity of 2H-imidazo[2,1-a]pyrazolo[3,4-d]thiazoles. Indian J Chem 1991;30B:898–900.
- Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R et al. Synthesis and antitumor activity of guanylhydrazones from 6-(2,4-dichloro-5-nitrophenyl)imidazo[2,1-b]thiazoles and 6-pyridylimidazo[2,1-b]thiazoles(1). J Med Chem 2006;49:7897–7901.
- Castellano S, Stefancich G, Chillotti A, Poni G. Synthesis and antimicrobial properties of 3-aryl-1-(1,1′-biphenyl-4-yl)-2-(1H-imidazol-1-yl)propanes as ‘carba-analogues’ of the N-arylmethyl-N-[(1,1′-biphenyl)-4-ylmethyl])-1H-imidazol-1-amines, a new class of antifungal agents. Farmaco 2003;58:563–568.
- Aggarwal N, Kumar R, Dureja P, Khurana JM. Synthesis, antimicrobial evaluation and QSAR analysis of novel nalidixic acid based 1,2,4-triazole derivatives. Eur J Med Chem 2011;46:4089–4099.
- Jalilian AR, Sattari S, Bineshmarvasti M, Shafiee A, Daneshtalab M. Synthesis and in vitro antifungal and cytotoxicity evaluation of thiazolo-4H-1,2,4-triazoles and 1,2,3-thiadiazolo-4H-1,2,4-triazoles. Arch Pharm (Weinheim) 2000;333:347–354.
- Zahajská L, Klimešová V, Kočí J, Waisser K, Kaustová J. Synthesis and antimycobacterial activity of pyridylmethylsulfanyl and naphthylmethylsulfanyl derivatives of benzazoles, 1,2,4-triazole, and pyridine-2-carbothioamide/-2-carbonitrile. Arch Pharm 2004;337:549–555.
- Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A et al. Synthesis and anticonvulsant activity of new 2-substituted-5- [2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett 2004;14:6057–6059.
- Shivarama Holla B, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–767.
- Ulusoy Güzeldemirci N, Küçükbasmacı Ö. Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo[2,1-b]thiazole moiety. Eur J Med Chem 2010;45:63–68.
- Ulusoy N, Gürsoy A, Otük G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001;56:947–952.
- Ulusoy N, Ergenç N, Otük G, Kiraz M. Synthesis of some 4-(alkylidene/arylidene)amino-2,4-dihydro-5- (2-thienyl)-3H-1,2,4-triazole-3-thiones tested for antimicrobial activity. Boll Chim Farm 2001;140:417–421.
- Günay NS, Capan G, Ulusoy N, Ergenç N, Otük G, Kaya D. 5-Nitroimidazole derivatives as possible antibacterial and antifungal agents. Farmaco 1999;54:826–831.
- Ilhan E, Ergenc N, Ulusoy N, Otük-Sanis G. [Synthesis and antimicrobial action of 4-arylideneamino-3-(alpha,alpha- diphenyl-alpha-hydroxmethyl)-1,4-dihydro-5H-1,2,4-triazino-5-thiones and 6-arene-3-(alpha,alpha-diphenyl-alpha-hydroxymethyl)-7H-s-triazolo(3,4- b) (1,2,4)thiadiazine]. Pharmazie 1996;51:123–124.
- Ergenç N, Ulusoy N, Çapan G, Ötük Sanış G, Kiraz, M. Synthesis and antimicrobial properties of new 4-(alkylidene/arylidene)-amino-5-(2-furanyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones and 6-aryl-3-(2-furanyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Arch Pharm Pharm Med Chem 1996;329:427–430.
- Ergenç N, Ulusoy N, Ekinci AC. Synthesis and anticonvulsant activity of some new 1,1-bis(4-substituted 1,2,4-triazoline-5-thione-3-yl)-2-methylbutanes. Farmaco 1995;50:189–192.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial testing, 15th informational supplement. M100-S15. Wayne, PA: Clinical and Laboratory Standards Institute, 2005.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard-2nd Edition. M27-A2. Wayne, PA, 2002.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution an antifungal susceptibility testing filamentous fungi; Approved standard M38-A2. Wayne, Pennsylvania:National Commitee for Clinical Laboratory Standards, 2002.
- Fernández-Torres B, Cabañes FJ, Carrillo-Muñoz AJ, Esteban A, Inza I, Abarca L et al. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microbiol 2002;40:3999–4003.
- Trotsko N, Dobosz M, Jagiello-Wójtowicz E. Cyclization of thiosemicarbazide derivatives of 5-arylidene-2,4-dioxothiazolidine-3-acetic acids to 1,3,4-thiadiazoles and their pharmacological properties. Acta Pol Pharm 2007;64:227–231.
- Gürsoy E, Güzeldemirci NU. Synthesis and primary cytotoxicity evaluation of new imidazo[2,1-b]thiazole derivatives. Eur J Med Chem 2007;42:320–326.
- Zamani K, Faghihi K, Sangi MR, Zolgharnein J. Synthesis of some new substituted 1,2,4-triazole and 1,3,4-thiadiazole and their derivatives. J Turk J Chem 2003;27:119–125.