Abstract
A new cinnamic acid derivative was isolated from the whole plant of Viola betonicifolia as off white needle. On the basis of various modern spectroscopic techniques including HREI–MS and 1D and 2D NMR, its structure was elucidated as 2,4-dihydroxy, 5-methoxy-cinnamic acid. It showed marked inhibition against DPPH (diphenyl-2-picryl hydrazyl) free radicals with IC50 = 124 ± 5.76 µM. The antioxidant property of the compound was compared with α-tocopherole and vitamin C having IC50 values 96 ± 0.46 and 90 ± 0.56 µM, respectively. In case of antiglycation assay, the compound exhibited moderate activity (IC50 = 355 ± 7.56 µM) similar to standard compound, rutin (IC50 = 294 ± 0.56 µM). However, it was non-toxic to PC-3 cell line. It is concluded that 2,4-dihydroxy, 5-methoxy-cinnamic acid has antiglycation potential which was further augmented by its antioxidant activity and thus offered an ideal natural therapeutic option for the effective management of diabetes.
Introduction
Viola betonicifolia locally known as Banafsha belongs to family Violaceace. It is perennial herb of 8–20 cm height. The stem of the plant is absent and leaves are triangular or obtuse and petiole is longer than lamina. Roots are slender, unbranched and rhizome is short. V. betonicifolia is available in various countries of the world like Pakistan, India, Nepal, Sri-lanka, China, Malaysia and AustraliaCitation1. In Pakistan, it is available in Swat, Hazara and Dir districts. In Pakistani traditional medicines system, it is used as antipyretic, sedative, astringent, diaphoretic, anticancer and purgative; it is also used in epilepsy and nervous disordersCitation2. It is also recommended for the treatment of sinusitis, skin and blood disorders and in pharyngitisCitation3. Roots are used to cure kidney diseases, pneumonia and bronchitis. Flowers are used in the treatment of lung troubles, cough and cold, while leaves are useful for boilsCitation4.
Cinnamic acid and its derivatives are phenolic compounds reported from several parts of the plantsCitation5–8. Several pharmacological activities of cinnamic acid and its derivatives have been reported, such as hepatoprotectiveCitation9,Citation10, antioxidantCitation10–12, anticancerCitation13–15 and antidiabetic activitiesCitation16–18. In continuation of our research work on Pakistani medicinal plantsCitation19–22 and keeping in view these reported work on cinnamic acid derivatives and biological potential (antioxidant and anticancer), we tested our isolated new cinnamic derivative for its antioxidant, antiglycation and anticancer activities. Recently we have tested various solvent fraction of this plant for different biological activities like antioxidant, cytotoxic, phytotoxic and neuropharmacological propertiesCitation23–25.
Material and methods
General techniques
The structures of the isolated compound was elucidated using different spectroscopic techniques including IH NMR, 13C-NMR, HMBC, HMQC, NOESY, COSY, HREI–MS and IR. IR spectra were recorded on a Vector 22 (Bruker) Fourier-Transform Infrared (FT-IR) spectrometer, using KBr windows with CH2Cl2 as solvent against an air background. 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance spectrometer. The 2D-NMR spectra were recorded on a Bruker Avance NMR spectrometer. Mass spectra (EI and HREI–MS) were measured in an electron impact mode on Finnigan MAT-312 and MAT-95 XP spectrometers and ions are given in m/z (%). TLC was performed on precoated silica gel F-254 plates (E. Merck); the detection was done at 254 nm and by spraying with ceric sulphate reagent. Column Silica gel (E. Merck, 70-230 mesh) and flash silica gel (E. Merck, Germany, 230-400 mesh) was used for column chromatography.
Plant material
Whole plant of V. betonicifolia was collected from Swat, Khyber Pakhtunkhawa, Pakistan, in April 2010. The plant specimen was identified by Taxonomy Section, Department of Botany, University of Peshawar and a specimen was deposited there in the herbarium under voucher number 6410/Bot.
Extraction, fractionation and isolation
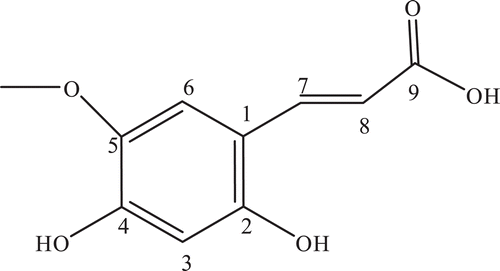
The collected whole plant (12 kg) was air dried and powdered. The powder was extracted by maceration with methanol at room temperature for 14 days with occasional shaking. The methanolic extract was filtered and concentrated by rotary evaporator at low temperature (45°C) resulting crude methanolic extract (1.98 kg, 22 % w/w). The crude methanolic extract (1.60 kg) was dissolved in distilled water and further fractionated into various solvent fractions such as n-hexane, chloroform, ethyl acetate, butanol and aqueous yielding n-hexane (706 g, 44.13 % w/w), chloroform (9 g, 0.56% w/w), ethyl acetate (16 g, 1.00% w/w), butanol (265 g, 16.56% w/w), and aqueous (498 g, 31.13% w/w). The chloroform and ethyl acetate fractions were combined and subjected to column chromatography. The column was eluted with chloroform: n-hexane solvent system, starting from pure n-hexane and then chloroform was used in various percentages with n-hexane which yielded fractions 1–12. The fraction no. 9 was rechromatographed over the silica gel and eluted with ethyl acetate and n-hexane. The compound 1 was purified as 2,4-dihydroxy, 5-methoxycinnamic acid from this fraction using solvent system 35% ethyl acetate and n-hexane.
2,4-Dihydroxy, 5-methoxy-cinnamic acid (1)
Off white needle. M. P. = 187 °C, UV (λmax) 229, 345 nm. IR (KBr) λmax: 567 cm−1 (olefin), 1514 cm−1 (aromatic), 2500–3500 cm−1 (COOH). EIMS: m/z [rel.int.]: 210, 192, 177 and 149. 1H NMR (CDCl3500 MHz): . 13C NMR (CDCl3, 125 MHz): .
Table 1. 1H-NMR and 13C-NMR chemical shift values of 1.
Chemicals
All reagents, chemicals, and solvents used were of analytical grade. Bovine serum albumin (BSA) was purchased from Merck Marker Pvt. Ltd (Karachi, Pakistan). Rutin, methyl glyoxal (MGO, 40% aqueous solution), p-nitrophenyl-α-d-glucopyranoside, 1-deoxynojirimycin, trichloroacetic acid (TCA) sodium azide (NaN3), dimethyl sulfoxide (DMSO), sodium dihydrogen phosphate (NaH2PO4), sodium chloride (NaCl), disodium hydrogen phosphate (Na2HPO4), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich. Sodium phosphate buffer (pH = 7.4), was prepared by mixing Na2HPO4 and NaH2PO4 (67 mM) containing sodium azide (3 mM), phosphate buffer saline (PBS) pH 10 was prepared by mixing NaCl (137 mM) + Na2HPO4 (8.1 mM) + KCl (2.68 mM) + KH2PO4 (1.47 mM) + pH 10 was adjusted with NaOH (0.25 mM), while BSA (10 mg/mL) and anhydrous glucoses (50 mg/mL) solutions were prepared in sodium phosphate buffer.
DPPH free radical scavenging assay
The compound was dissolved in DMSO and diluted up to 50 mL with distilled water. From this stock solution, various concentrations of 2–50 µg/mL were prepared by dilution method. Five millilitre of each solution was taken in a test tube and 1 mL of 0.001 M of DPPH solution was added to it. All these solutions were kept in dark for 30 min. Five millilitre of methanol and 1 mL of DPPH solution was added, for control solution. At the end of incubation period, the mixtures were examined for the antioxidant activity using Optima UV-Visible spectrophotometer (Agilent Technologies (Pvt) Ltd, Karachi, Pakistan) at wavelength of 517 nmCitation26,Citation27. The experiments were performed in triplicate and the activity against DPPH (%) was determined using formula:
Antiglycation assay
In this assay, 96-well plate was used. Each well contained glucose–BSA (10 mg/mL), MGO (14 mM), and various concentrations of the compounds (prepared in DMSO, 10% final) in 0.1 M phosphate buffer (pH 7.4) containing sodium azide (30 mM). The reaction mixture was incubated under aseptic conditions at 37°C for 9 daysCitation28. After 9 days of incubation, each sample was examined for the development of specific fluorescence (excitation, 330 nm; emission, 440 nm), against sample blank using a spectrofluorimeter (RF-1500, Shimadzu, Japan). Test compound was replaced by DMSO (10% final) as control. Rutin was used as the standard antiglycating agentCitation29. The percent inhibition of advanced glycation endproduct (AGE) formation was calculated as:
While IC50 was calculated as concentration of test compounds that caused 50% inhibition of AGEs.
Cytotoxic assay
Cytotoxicity of the compounds was assayed by MTT assay using PC3 cell line. The cell was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 2% antibiotics (penicillin and streptomycin) at 37°C with 5% CO2. After 80% growth confluence, growth, 104 cells/well were transferred in 96-well plate and incubated for 24 h. Then media was replaced with test compound (50 mM prepared in DMEM and DMSO). After 48 h the medium was again replaced with (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT (0.5 mg/mL, prepared in PBS and DMEM), and incubated for 4 h. Then 100% DMSO was added and left for 15 min and reading was taken at 570 nm. Test compound was replaced with DMSO (final, 0.7%) as controlCitation29. Doxorubicin was used as the standard inhibitor. The percentage of inhibition was calculated as:
Results and discussion
Compound 1 was isolated as off white needle from chloroform and ethyl acetate combined fraction of methanolic extract. UV spectrum displayed absorption maximum (λmax) at 229 and 345 nm, IR spectrum showed absorptions at 567 cm−1 (olefin), 1514 cm−1 (aromatic) and 2500–3500 cm−1 (COOH). EIMS showed molecular ion peak at m/z 210 and fragment peaks at m/z 192, 177 and 149. Molecular formula of compound 1, C10H10O5, was determined from EIMS and 13C-NMR (BB and DEPT).
1H-NMR spectrum () exhibited resonances at δ 3.90 (s, H–OMe), 6.20 (d, J = 9.5 Hz, H-8), 6.77 (s, H-3), 7.11 (s, H-6), 7.85 (d, J = 9.5 Hz, H-7). Geometry of the double bond of the side chain was trans on the basis of coupling constant of the protons H-7 and H-8, both showed coupling constant value 9.5 HzCitation30. Position of the hydroxyl and methoxy group in ring was determined with the help of 13C-NMR values, and HMBC interactions. Protons H-3 and H-6 were in para positions to each other, confirmed by HMBC and their presence as singlet in 1H-NMR spectrum. 13C-NMR spectrum (BB, and DEPT) () showed resonances for all ten carbons including one methyl, four methine and five quaternary carbons. Structure of the compound was further confirmed by using 2D-NMR technique (HSQC, HMBC and COSY). Key HMBC and COSY interactions in compound 1 are shown in . Compound 1 was reported as synthetic derivative of sclopolitin (natural compound)Citation31. However, it is isolated from any natural source for the first time and complete NMR data has also reported for the first time.
Compound 1 showed significant inhibition against DPPH free radicals when used in the concentration range of 2–50 µg/mL with IC50 124 ± 5.76 µM (). The antioxidant property of the compound was compared with α-tocopherole and vitamin C having IC50 values 96 ± 0.46 and 90 ± 0.56 µM. The antioxidant profile of any chemical/plant can be simply evaluated using DPPH free radical scavenging assy. Oxidative stress due to free radicals formation has been recognized in the pathophysiology of several chronic pathological complex conditions such as atherosclerosis, stroke, diabetes, Alzheimer’s disease and cancerCitation32–34. Therapeutic modality that has the tendency to counteract these agents are extensively used in combination therapy for the effective management of such conditionsCitation35,Citation36.
Table 2. Effect of compound 1 and standard drugs in DPPH free radical scavenging and antiglycation assays.
Glycation is the non-enzymatic combination of sugar molecule with protein amino acid or lipid resulting product is called advanced glycation endproducts (AGEs). The over expression of AGEs are believed to implicating in patients with diabetes and aging especially diabetes related late complications such as cataract, neuropathy, nephropathy, wound healing, Alzheimer’s diseaseCitation28,Citation37 arterial stiffness and decreased myocardial compliance, resulting from the loss of collagen elasticity. Agents that interfere with the formation of AGEs are targeting to be beneficial therapeutic tool for effective management of such conditions. Compound 1 exhibited marked activity and potency as an antiglycating agent and IC50 was noted as 355 ± 7.56 µM (). Rutin as a standard drug, showed IC50 = 294 ± 0.56 µM.
It is concluded that compound 1 can be used as antiglycating agent for an effective management of diabetes. The effect was further augmented by its potent antioxidant activity. The antioxidant potential of compound 1 provided chemical background to the antioxidant potential of crude methanolic extracts and its subsequent solvent fractions (chloroform and ethyl acetate)Citation38. However, detail studies are required to ascertain its efficacy, potency and safety for lead compound of long term clinical utility.
Acknowledgement
The authors are extremely thankful to Higher Education Commission (HEC) Pakistan for providing financial support. We are also highly grateful to the director of H.E.J. Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Karachi for providing facilities to carry out this research.
Declaration of interest
The authors of this article have no declaration of interest.
References
- Saboury AA, Atri MS, Sanati MH, Moosavi-Movahedi AA, Haghbeen K. Effects of calcium binding on the structure and stability of human growth hormone. Int J Biol Macromol 2005;36:305–309.
- Bhatt V, Negi G. Ethnomedicinal plant resources of Jaunsari tribe of Garhwal Himalaya, Uttaranchal. Ind J Trad Knowl 2006;5:331–335.
- Hamayun M. Ethnobotanical profile of Utror and Gabral valleys, district Swat, Pakistan. Ethnobotanical Leaflets 2005;9.
- Shinwari ZK. Medicinal plants research in Pakistan. J Med Plant Res 2010;4:161–176.
- Cutillo F, D’Abrosca B, DellaGreca M, Di Marino C, Golino A, Previtera L et al. Cinnamic acid amides from Chenopodium album: effects on seeds germination and plant growth. Phytochemistry 2003;64:1381–1387.
- Bennett EL, Bonner J. Isolation of plant growth inhibitors from Thamnosma montana. Am J Bot 1953;29–33.
- Harborne JB, Corner JJ. Plant polyphenols. 4. Hydroxycinnamic acid-sugar derivatives. Biochem J 1961;81:242–250.
- Gestetner B, Conn EE. The 2-hydroxylation of trans-cinnamic acid by chloroplasts from Melilotus alba Desr. Arch Biochem Biophys 1974;163:617–624.
- Pérez-Alvarez V, Bobadilla R, Muriel P. Structure–hepatoprotective activity relationship of 3,4-dihydroxycinnamic acid (caffeic acid) derivatives. J App Toxicol 2001;21:527–531.
- Natella F, Nardini M, Di Felice M, Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J Agric Food Chem 1999;47:1453–1459.
- Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem 2002;50:2161–2168.
- Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med 1992;13:435–448.
- Akao Y, Maruyama H, Matsumoto K, Ohguchi K, Nishizawa K, Sakamoto T et al. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull 2003;26:1057–1059.
- Li QF, Shi SL, Liu QR, Tang J, Song J, Liang Y. Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: nuclear matrix downregulation and cytoplasmic trafficking of nucleophosmin. Int J Biochem Cell Biol 2008;40:1918–1929.
- Fiuza SM, Gomes C, Teixeira LJ, Girão da Cruz MT, Cordeiro MN, Milhazes N et al. Phenolic acid derivatives with potential anticancer properties–a structure-activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem 2004;12:3581–3589.
- Subash Babu P, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde–a potential antidiabetic agent. Phytomedicine 2007;14:15–22.
- Adisakwattana S, Roengsamran S, Hsu WH, Yibchok-anun S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and streptozotocin-induced diabetic rats. Life Sci 2005;78:406–412.
- Adisakwattana S, Moonsan P, Yibchok-Anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J Agric Food Chem 2008;56:7838–7844.
- Khan MA, Khan H, Khan S, Mahmood T, Khan PM, Jabar A. Anti-inflammatory, analgesic and antipyretic activities of Physalis minima Linn. J Enzyme Inhib Med Chem 2009;24:632–637.
- Khan H, Saeed M, Gilani AU, Khan MA, Khan I, Ashraf N. Antinociceptive activity of aerial parts of Polygonatum verticillatum: attenuation of both peripheral and central pain mediators. Phytother Res 2011;25:1024–1030.
- Khan H, Saeed M, Muhammad N, Ghaffar R, Khan SA, Hassan S. Antimicrobial activities of rhizomes of Polygonatum verticillatum: attributed to its total flavonoidal and phenolic contents. Pak J Pharm Sci 2012;25:463–467.
- Ibrar M, Muhammad N, Barkatullah, Khan H, Jahan F, Ashraf N. Antinociceptive and anticonvulsant activities of essential oils of Zanthoxylum armatum leaves. Phytopharmacol 2012;3:191–198.
- Muhammad N, Saeed M, Khan H, Haq I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J Nat Med 2012; DOI: 10.1007/s11418-012-0636-0.
- Muhammad N, Saeed M. Biological screening of Viola betonicifolia Smith whole plant. Afri J Pharm Pharmacol 2011;5:2323–2329.
- Muhammad N, Saeed M, Barkatullah, Ibrar M, Khan H. Pharmacognostic studies of Viola betonicifolia. Afri J Pharm Pharmacol 2012;6:43–47.
- Khan H, Saeed M, Khan MA, Khan I, Ahmad M, Muhammad N, Khan A. Antimalarial and free radical scavenging activities of rhizomes of Polygonatum verticillatum supported by isolated metabolites. Med Chem Res 2011; DOI10.1007/s00044-011-9637-x:1-5.
- Khan H, Tariq SA, Khan MA, Inayat-Ur-Rehman, Ghaffar R, Saifullah. Cholinesterase and lipoxygenase inhibition of whole plant Withania somnifera. Afri J Pharm Pharmacol. 2011;5:2272–2275.
- Mahera S, Ahmad VU, Saifullah SM, Mohammad FV, Ambreen K. Steroids and triterpenoids from grey mangrove Avicennia marina. Pak J Bot 2011;43:1417–1422.
- Choudhary MI, Adhikari A, Rasheed S, Marasini BP, Hussain N, Kaleem WA. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of [alpha]-glucosidase enzyme and protein glycation. Phytochem Lett 2011;4:404–406.
- Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–3062.
- Moore CW. Proceedings of the Chemical Society, London 1911;27,119.
- Gülçin I, Elias R, Gepdiremen A, Chea A, Topal F. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25:44–53.
- Khan H, Khan MA, Muhammad N, Ashraf N, Gul F, Tariq SA. Antiinflammatory and antioxidant activity of Joshanda partially mediated through inhibition of lipoxygenase. Phytopharmacol 2012;3:19–28.
- Neelam S, Khan Z-U. Antioxidant activity of Galium aparine L. from Punjab, Pakistan. Pak J Bot 2012;44:251–253.
- McDowell A, Thompson S, Stark M, Ou ZQ, Gould KS. Antioxidant activity of puha (Sonchus oleraceusL.) as assessed by the cellular antioxidant activity (CAA) assay. Phytother Res 2011;25:1876–1882.
- Lateef M, Iqbal L, Fatima N, Siddiqui K, Afza N, Zia-ul-Haq M et al. Evaluation of antioxidant and urease inhibition activities of roots of Glycyrrhiza glabra. Pak J Pharm Sci 2012;25:99–102.
- Choudhary MI, Abbas G, Ali S, Shuja S, Khalid N, Khan KM et al. Substituted benzenediol Schiff bases as promising new anti-glycation agents. J Enzyme Inhib Med Chem 2011;26:98–103.
- Muhammad N, Saeed M. Biological screening of Viola betonicifolia Smith whole plant. Afr J Pharm Pharmacol 2011;5; 2323–2329.