Abstract
In this study, some new 2-(4-substituted piperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide derivatives were synthesized. The synthesized compounds were screened for their anticholinesterase activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes by in vitro Ellman’s method. The structural elucidation of the compounds was performed by using IR, 1H-NMR, 13C-NMR and FAB+-MS spectral data and elemental analyses results. Biological assays revealed that at 0.1 µM concentration, the most active compounds against AChE were 5n, 5o and 5p that indicated 96.44, 99.83 and 89.70% inhibition rates, respectively. Besides, IC50 value of the compound 5o was determined as 0.011 µM, whereas IC50 value of standard drug donepezil was 0.054 µM. The synthesized compounds did not show any notable inhibitory activity against BChE.
Introduction
Acetylcholine (ACh) acts as an excitatory neurotransmitter for voluntary muscles in the somatic nervous system and as a preganglionic and a postganglionic transmitter in the parasympathetic nervous system of vertebrates and invertebratesCitation1,Citation2. Acetylcholinesterase (AChE) is a terminator enzyme of nerve impulse transmission at the cholinergic synapses by quick hydrolysis of ACh to choline and acetate. Inhibition of AChE evolves a strategy for the treatment of several diseases as Alzheimer’s disease (AD), senile dementia, ataxia, myasthenia gravis and Parkinson’s diseaseCitation3. AD is one form of senile dementia which occurs due to various neuropathological conditions such as senile plaques and neurofibrillary tangles. It is the most common dementias that affects half of the population aged 85 yearsCitation4,Citation5 and seventh main cause of life lost affecting 5.3 million people over the world. In AD, growing numbers of nerve cells degenerate and die along with loss in synapse through which information flows from and to the brain. As a result cognitive impairment and dementia occurCitation6. The neuropathology of AD is generally characterized by the presence of numerous amyloid β-peptide (Aβ) plaques, neurofibrillary tangles (NFT), and degeneration or atrophy of the basal forebrain cholinergic neurons. The loss of basal forebrain cholinergic cells results in an important reduction in ACh level, which plays an important role in the cognitive impairment associated with ADCitation7.
Disruption of cholinergic transmission in AD was proved in many clinical and neuropathological studiesCitation8,Citation9. As well as deficit of ACh, the loss of presynaptic M2 muscarinic and nicotinic receptors has also been foundCitation10. There are also evidences of an interaction between AChE and Aβ, which is participated in plaques and plays an essential role in AD pathophysiology. AChE constitutes a stable complex with senile plaque components and may even enhance the aggregation of Aβ peptides and amyloid formation. The neurotoxicity of amyloid components may be risen up by the presence of AChECitation11,Citation12. In contrast to the overall decrease of AChE in AD brains, at least in its later stages, the local concentration of AChE around the plaques increases as these lesions occurCitation13. In addition to hydrolysing ACh, AChE may be also involved in other functions such as cell proliferation, differentiation, and responses to various damaging factors including stress and amyloid formationCitation11,Citation14. Hence, for the most part, inhibitors of AChE, enhancing the ACh concentration in the brain, have been introduced to the market for treating mild-to-moderate AD.
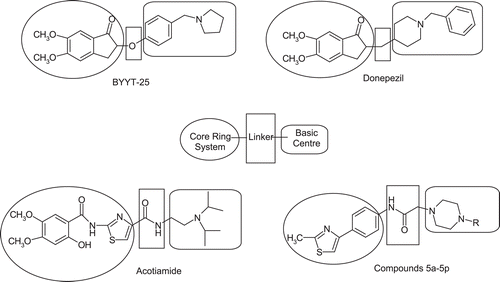
Consistent with reported studies, cholinesterase inhibitors contain a basic centre, a core ring system and a linker such as –O–, CH2, CONH, CONH(CH2)n etc. between core ring and basic centresCitation15–17. For example, chemical structures of AChE inhibitors donepezil and BYYT-25 fit well to such definition. These agents contain an indan as a core ring, methylene or oxygen as a linker and piperidine or pyrrolydine cyclic amines as a basic centre. In the light of above structural motif, we designed a new series of thiazole–piperazine derivatives (). The reason for the choice of thiazole and piperazine moieties was their AChE inhibitory potency. Namely, thiazole based biomolecule thiamine (vitamin B1) and its analogues were described as AChE inhibitorsCitation18. Acotiamide, new thiazole based drug that enhances ACh release in the enteric nervous system via muscarinic receptor antagonism and AChE inhibition was discoveredCitation19. Besides, in previous studies, cholinesterase activity of some thiazoleCitation20–22 and piperazineCitation23–25 derivatives was reported. As a result, we synthesized thiazole-piperazine derivatives so as to investigate their cholinesterase inhibitory potency, which will enable to study in vivo pharmacological activity against AD on animal models.
Materials and methods
Chemistry
All chemicals were purchased from Sigma-Aldrich Chemical Co. All melting points (m.p.) were determined by Electrothermal 9100 digital melting point apparatus and were uncorrected. Spectroscopic data were recorded with the following instruments: 1H-NMR, Bruker 400 MHz spectrometer; 13C-NMR, Bruker 100 MHz spectrometer; MS-FAB, VG Quattro Mass spectrometer and elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyser. Some characteristics of the compounds are given in .
Table 1. Some physicochemical characteristics of the synthesized compounds.
Synthesis of the compounds
N-[4-(2-Bromoacetyl)phenyl]acetamide (1)
4′-aminoacetophenone (0.05 mol, 6.75 g) and triethylamine (0.06 mol, 8.34 mL) were dissolved in THF (100 mL) with a constant stirring at 0–5°C. Acetyl chloride (0.06 mol, 4.78 mL) was added dropwise to this solution gradually. The reaction mixture thus obtained was further agitated for 1 h at room temperature. After evaporation of solvent the residue was filtered and washed with water and dried. Then the obtained N-(4-acetylphenyl)acetamide (0.04, 7.08 g mol) was brominated in 30 mL acetic acid with the presence of 0.05 mol (2.58 mL) bromine and 0.5 mL HBr to give N-[4-(2-bromoacetyl)phenyl]acetamide (1) in 86% yield.
N-[4-(2-Methyl-4-thiazolyl)phenyl]acetamide (2)
N-[4-(2-bromoacetyl)phenyl]acetamide (0.03 mol, 7.68 g) (1) and thioacetamide (0.03 mol, 2.25g) were stirred in ethanol at room temperature for 48 h. The precipitated solid was filtered, dried and recrystallized from ethanol to afford title compound in 78% yield.
4-(2-Methyl-4-thiazolyl)aniline (3)
N-[4-(2-methyl-4-thiazolyl)phenyl]acetamide (0.025 mol, 5.8 g) (2) was refluxed in ethanol (15 mL) with 1 N HCl solution (10 mL). The reaction was monitored by TLC. When the reaction was completed, the mixture was poured into ice water, neutralized with 10% NaOH solution and then filtered to give 4-(2-Methyl-4-thiazolyl)aniline (3) in 92% yield.
2-Chloro-N-[4-(2-methyl-4-thiazolyl)phenyl]acetamide (4)
Chloroacethyl chloride (0.025 mol, 2 mL) was added dropwise over 15 min to a magnetically stirred solution of the 4-(2-Methyl-4-thiazolyl)aniline (0.022 mol, 4.18 g) (3) and triethylamine (0.025 mol, 3.48 mL) in dry THF (15 mL). The reaction was monitored by TLC. After the reaction was completed, the solvent was evaporated under reduced pressure. Water was added to wash the resulting solid, the mixture was filtered, dried and recrystallized from ethanol to afford compound 4 in 83% yield.
General procedure for 2-(4-substituted piperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide derivatives (5a–5p)
A mixture of compound 4 (0.001 mol, 0.266 g), appropriate piperazine derivative (0.0011 mol) and K2CO3 (0.001 mol, 0.138 g) in acetone (15 mL) was refluxed for 2 h. After cooling, the solvent was evaporated until dryness. The residue was treated with 25 mL of water. Solidified product was filtered, washed with water and recrystallized from ethanol to give the 5a–5p.
2-(4-Methylpiperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5a)
IR (KBr) νmax(cm−1): 3282 (amide N–H), 3042 (aromatic C–H), 2978 (aliphatic C–H), 1679 (amide C=O), 1584–1411 (C=C and C=N), 1309-1018 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.14 (3H, s, N-CH3), 2.35-2.48 (8H, m, piperazine C-H), 2.67 (3H, s, C–CH3), 3.09 (2H, s, CO–CH2), 7.66 (2H, d, J = 9.2 Hz, Ar-H), 7.77 (1H, s, thiazole C5-H), 7.84 (2H, d, J = 8.8 Hz, Ar-H), 9.74 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.59 (CH3), 46.43 (CH3), 53.39 (2CH2), 55.23 (2CH2), 62.49 (CH2), 113.15 (CH), 120.13 (2CH), 127.01 (2CH), 130.15 (C), 138.92 (C), 154.27 (C), 166.02 (C), 168.98 (C).
For C17H22N4OS calculated: 61.79% C, 6.71% H, 16.95% N; found: 61.75% C, 6.70% H, 16.91% N.
MS (FAB) [M + 1]+: m/z 331.
2-(4-Ethylpiperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)pheny])acetamide (5b)
IR (KBr) νmax(cm−1): 3280 (amide N–H), 3054 (aromatic C–H), 2940 (aliphatic C–H), 1680 (amide C=O), 1594-1401 (C=C and C=N), 1280–1019 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 0.99 (3H, t, J = 7.2, 7.2 Hz, CH2–CH3), 2.32 (2H, q, J = 7.2, 14.2 Hz, CH2–CH3), 2.42-2.53 (8H, m, piperazine C-H), 2.71 (3H, s, C–CH3), 3.12 (2H, s, CO–CH2), 7.69 (2H, d, J = 8.4 Hz, Ar–H), 7.81 (1H, s, thiazole C5-H), 7.86 (2H, d, J = 8.8 Hz, Ar–H), 9.76 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 16.81 (CH3), 19.59 (CH3), 28.02 (CH2), 53.86 (2CH2), 55.47 (2CH2), 63.06 (CH2), 113.23 (CH), 120.47 (2CH), 127.08 (2CH), 130.76 (C), 139.02 (C), 154.47 (C), 166.16 (C), 169.00 (C).
For C18H24N4OS calculated: 62.76% C, 7.02% H, 16.26% N; found: 62.74% C, 7.01% H, 16.25% N.
MS (FAB) [M + 1]+: m/z 345.
2-(4-Cyclohexylpiperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)pheny])acetamide (5c)
IR (KBr) νmax(cm−1): 3288 (amide N–H), 3056 (aromatic C–H), 2991 (aliphatic C–H), 1675 (amide C=O), 1568–1407 (C=C and C=N), 1311–1020 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 1.16–1.24 (5H, m, cyclohexyl C–H), 1.59–1.86 (5H, m, cyclohexyl C–H), 2.22–2.26 (1H, m, cyclohexyl C–H), 2.51–2.62 (8H, m, piperazine C–H), 2.73 (3H, s, C–CH3), 3.09 (2H, s, CO–CH2), 7.22 (1H, s, thiazole C5-H), 7.59 (2H, d, J = 8.4 Hz, Ar–H), 7.81 (2H, d, J = 8.8 Hz, Ar–H), 9.18 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.52 (CH3), 26.04 (2CH2), 26.49 (CH2), 29.20 (2CH2), 49.33 (2CH2), 54.23 (2CH2), 62.24 (CH2), 63.62 (CH), 111.81 (CH), 119.72 (2CH), 127.16 (2CH), 130.77 (C), 137.61 (C), 154.82 (C), 166.08 (C), 168.72 (C).
For C22H30N4OS calculated: 66.30% C, 7.59% H, 14.06% N; found: 66.32% C, 7.52% H, 14.02% N.
MS (FAB) [M + 1]+: m/z 399.
2-(4-Phenylpiperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5d)
IR (KBr) νmax(cm−1): 3284 (amide N–H), 3053 (aromatic C–H), 2958 (aliphatic C–H), 1676 (amide C=O), 1596–1412 (C=C and C=N), 1302–1025 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.67–2.69 (4H, m, piperazine C–H), 2.71 (3H, s, C–CH3), 3.20–3.22 (6H, m, piperazine C–H and CO–CH2), 6.78 (1H, t, J = 7.2, 7.2, Ar–H), 6.94 (2H, d, J = 7.6 Hz, Ar–H), 7.21 (2H, t, J = 8, 8 Hz, Ar–H), 7.71 (2H, d, J = 8.8 Hz, Ar–H), 7.81 (1H, s, thiazole C5-H), 7.88 (2H, d, J = 8.8 Hz, Ar–H), 9.85 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.60 (CH3), 53.52 (2CH2), 53.65 (2CH2), 62.48 (CH2), 113.18 (CH), 116.12 (2CH), 119.02 (CH), 120.78 (2CH), 127.49 (2CH), 129.14 (2CH), 130.15 (C), 138.92 (C), 151.28 (C), 154.27 (C), 166.22 (C), 167.95 (C).
For C22H24N4OS calculated: 67.32% C, 6.16% H, 14.27% N; found: 67.30% C, 6.14% H, 14.26% N.
MS (FAB) [M + 1]+: m/z 393.
2-[4-(4-Methylphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5e)
IR (KBr) νmax(cm−1): 3285 (amide N–H), 3012 (aromatic C–H), 2963 (aliphatic C–H), 1678 (amide C=O), 1595–1413 (C=C and C=N), 1294–1026 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.18 (3H, s, C–CH3), 2.64–2.66 (4H, m, piperazine C–H), 2.69 (3H, s, C–CH3), 3.12–3.14 (4H, m, piperazine C–H), 3.18 (2H, s, CO–CH2), 6.83 (2H, d, J = 8.8 Hz, Ar–H), 7.00 (2H, d, J = 8 Hz, Ar–H), 7.69 (2H, d, J = 8, 4 Hz, Ar–H), 7.80 (1H, s, thiazole C5-H), 7.86 (2H, d, J = 8.8 Hz, Ar–H), 9.82 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.60 (CH3), 21.33 (CH3), 53.47 (2CH2), 55.84 (2CH2), 62.73 (CH2), 113.39 (CH), 120.56 (2CH), 121.56 (2CH), 127.73 (2CH), 130.73 (C), 132.48 (2CH), 138.92 (C), 139.46 (C), 141.63 (C), 154.54 (C), 166.79 (C), 168.78 (C).
For C23H26N4OS calculated: 67.95% C, 6.45% H, 13.78% N; found: 67.93% C, 6.47% H, 13.74% N.
MS (FAB) [M + 1]+: m/z 407.
2-[4-(4-Methoxyphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5f)
IR (KBr) νmax(cm−1): 3282 (amide N–H), 3050 (aromatic C–H), 2943 (aliphatic C–H), 1679 (amide C=O), 1585–1414 (C=C and C=N), 1305–1018 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.76 (3H, s, C–CH3), 2.80 (4H, t, J = 4.8, 4.8 Hz, piperazine C–H), 3.17 (4H, t, J = 4.8, 4.8 Hz, piperazine C–H), 3.21 (2H, s, CO–CH2), 3.78 (3H, s, O–CH3), 6.86 (2H, d, J = 8.4 Hz, Ar–H), 6.92 (2H, d, J = 8.8 Hz, Ar–H), 7.25 (1H, s, thiazole C5-H), 7.64 (2H, d, J = 8, 8 Hz, Ar–H), 7.85 (2H, d, J = 8.4 Hz, Ar–H), 9.20 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.32 (CH3), 50.91 (2CH2), 53.66 (2CH2), 55.56 (CH3), 62.02 (CH2), 111.61 (CH), 114.51 (2CH), 118.35 (2CH), 119.48 (2CH), 126.98 (2CH), 130.67 (C), 137.32 (C), 145 (C), 154.08 (C), 154.60 (C), 165.85 (C), 168.14 (C).
For C23H26N4O2S calculated: 65.38% C, 6.20% H, 13.26% N; found: 65.34% C, 6.23% H, 13.24% N.
MS (FAB) [M + 1]+: m/z 423.
2-[4-(4-Chlorophenyl) piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5g)
IR (KBr) νmax(cm−1): 3279 (amide N–H), 3052 (aromatic C–H), 2947 (aliphatic C–H), 1673 (amide C=O), 1579–1445 (C=C and C=N), 1289–1030 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.67 (4H, t, J = 4.4, 4.8 Hz, piperazine C–H), 2.71 (3H, s, C–CH3), 3.21 (4H, t, J = 4.4, 4.4 Hz, piperazine C–H), 3.35 (2H, s, CO–CH2), 6.95 (2H, d, J = 8.8 Hz, Ar–H), 7.23 (2H, d, J = 8.8 Hz, Ar–H), 7.72 (2H, t, J = 9.2 Hz, Ar–H), 7.81 (1H, s, thiazole C5-H), 7.88 (2H, d, J = 9.2 Hz, Ar–H), 9.85 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.59 (CH3), 52.47 (2CH2), 54.88 (2CH2), 62.73 (CH2), 113.77 (CH), 120.14 (2CH), 121.58 (2CH), 127.56 (2CH), 128,49 (C), 130.23 (C), 132.69 (2CH), 138.86 (C), 152.63 (C), 154.16 (C), 166.45 (C), 168.89 (C).
For C22H23ClN4OS calculated: 61.89% C, 5.43% H, 13.12% N; found: 61.84% C, 5.41% H, 13.00% N.
MS (FAB) [M + 1]+: m/z 427.5.
2-[4-(4-Florophenyl)piperazine-1-yl]-4-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5h)
IR (KBr) νmax(cm−1): 3277 (amide N–H), 3044 (aromatic C–H), 2973 (aliphatic C–H), 1680 (amide C=O), 1589–1410 (C=C and C=N), 1296–1012 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.68 (4H, t, J = 4.4, 4.8 Hz, piperazine C–H), 2.71 (3H, s, C–CH3), 3.16 (4H, t, J = 4.4, 4.4 Hz, piperazine C–H), 3.21 (2H, s, CO–CH2), 6.92–7.07 (4H, m, Ar–H), 7.71 (2H, d, J = 9.2 Hz, Ar–H), 7.82 (1H, s, thiazole C5-H), 7.88 (2H, d, J = 8.8 Hz, Ar–H), 9.85 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.54 (CH3), 52.47 (2CH2), 54.49 (2CH2), 62.79 (CH2), 113.25 (CH), 115.62 (2CH), 117.83 (2CH), 120.88 (2CH), 127.83 (2CH), 130.45 (C), 138.86 (C), 147.80 (C), 154.16 (C), 158.32 (C), 166.45 (C), 168.89 (C).
For C22H23FN4OS calculated: 64.37% C, 5.65% H, 13.65% N; found: 64.40% C, 5.61% H, 13.62% N.
MS (FAB) [M + 1]+: m/z 411.
2-[4-(4-Nitrophenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)pheny]acetamide (5i)
IR (KBr) νmax(cm−1): 3278 (amide N–H), 3020 (aromatic C–H), 2943 (aliphatic C–H), 1677 (amide C=O), 1598–1477 (C=C and C=N), 1311–1045 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.68 (4H, t, J = 4.4, 4.8 Hz, piperazine C–H), 2.71 (3H, s, C–CH3), 3.34 (2H, s, CO–CH2), 3.55 (4H, t, J = 5.2, 4.4 Hz, piperazine C–H), 7.04 (2H, d, J = 8.8 Hz, Ar–H), 7.71 (2H, d, J = 8.8 Hz, Ar–H), 7.81 (1H, s, thiazole C5-H), 7.88 (2H, d, J = 8.4 Hz, Ar–H), 8.06 (2H, d, J = 9.2 Hz, Ar–H), 9.88 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.61 (CH3), 53.96 (2CH2), 55.88 (2CH2), 62.49 (CH2), 112.14 (2CH), 113.29 (CH), 120.83 (2CH), 127.25 (2CH), 127.93 (2CH), 130.89 (C), 138.92 (C), 139.52 (C), 154.21 (C), 156.19 (C), 166.56 (C), 168.78 (C).
For C22H23N5O3S calculated: 60.39% C, 5.30% H, 16.01% N; found: 60.36% C, 5.32% H, 16.03% N.
MS (FAB) [M + 1]+: m/z 438.
2-[4-(3-Methylphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5j)
IR (KBr) νmax(cm−1): 3282 (amide N–H), 3041 (aromatic C–H), 2963 (aliphatic C–H), 1683 (amide C=O), 1589–1427 (C=C and C=N), 1311–1040 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.33 (3H, s, C–CH3), 2.76 (3H, s, C–CH3), 2.80 (4H, t, J = 4.8, 4.8 Hz, piperazine C–H), 3.21 (2H, s, CO–CH2), 3.26 (4H, t, J = 4.8, 4.8 Hz, piperazine C–H), 6.71–6.77 (3H, m, Ar–H), 7.17 (1H, t, J = 7.6, 7.6 Hz, Ar–H), 7.25 (1H, s, thiazole C5-H), 7.63 (2H, t, J = 9.2 Hz, Ar–H), 7.85 (2H, d, J = 8.4 Hz, Ar–H), 9.20 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.33 (CH3), 21.77 (CH3), 49.59 (2CH2), 53.58 (2CH2), 62.04 (CH2), 111.65 (CH), 113.40 (CH), 117.13 (CH), 119.47 (2CH), 121.09 (CH), 127.01 (2CH), 129.04 (C), 130.69 (C), 137.29 (CH) 138.92 (C), 151.05 (C), 154.60 (C), 165.85 (C), 168.10 (C).
For C23H26N4OS calculated: 67.95% C, 6.45% H, 13.78% N; found: 67.91% C, 6.48% H, 13.72% N.
MS (FAB) [M + 1]+: m/z 407.
2-[4-(3-Methoxyphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5k)
IR (KBr) νmax(cm−1): 3281 (amide N–H), 3043 (aromatic C–H), 2967 (aliphatic C–H), 1681 (amide C=O), 1578-1419 (C=C and C=N), 1289–1013 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.66 (4H, t, J = 4.8, 4.8, piperazine C–H), 2.71 (3H, s, C–CH3), 3.20–3.22 (4H, m, piperazine C–H), 3.32 (2H, s, CO–CH2), 3.71 (3H, s, O–CH3), 6.35–6.54 (3H, m, Ar–H), 7.11 (1H, t, J = 8, 8.2 Hz) 7.71 (2H, d, J = 9.2 Hz, Ar–H), 7.81 (1H, s, thiazole C5-H), 7.87 (2H, d, J = 8.8 Hz, Ar–H), 9.84 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.53 (CH3), 53.42 (2CH2), 55.64 (2CH2), 55.90 (CH3), 62.88 (CH2), 103.50 (CH), 106.12 (CH), 109.45 (CH), 113.79 (CH), 120.46 (2CH), 127.09 (2CH), 130.14 (CH), 130.18 (C), 138.89 (C), 150.11 (C), 154.17 (C), 160.15 (C), 166.78 (C), 168.49 (C).
For C23H26N4O2S calculated: 65.38% C, 6.20% H, 13.26% N; found: 65.37% C, 6.21% H, 13.25% N.
MS (FAB) [M + 1]+: m/z 423.
2-[4-(2-Methylphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)pheny]acetamide (5l)
IR (KBr) νmax(cm−1): 3280 (amide N–H), 3046 (aromatic C–H), 2974 (aliphatic C–H), 1679 (amide C=O), 1599–1402 (C=C and C=N), 1299–1004 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.31 (3H, s, C–CH3), 2.76 (3H, s, C–CH3), 2.80 (4H, t, J = 4.8, 4.8 Hz, piperazine C–H), 3.01 (4H, t, J = 4.4, 4 Hz, piperazine C–H), 3.23 (2H, s, CO–CH2), 6.99-7.07 (2H, m, Ar–H), 7.19 (2H, t, J = 6, 6 Hz, Ar–H), 7.25 (1H, s, thiazole C5-H), 7.65 (2H, d, J = 8 Hz, Ar–H), 7.86 (2H, d, J = 8 Hz, Ar–H), 9.26 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 18.08 (CH3), 19.54 (CH3), 52.20 (2CH2), 54.30 (2CH2), 62.34 (CH2), 111.87 (CH), 119.258 (2CH), 119.78 (CH), 123.72 (CH), 126.84 (CH), 127.24 (2CH), 130.89 (C), 131.41 (CH), 132.94 (C), 137.50 (CH), 151.27 (C), 154.83 (C), 166.14 (C), 168.61 (C).
For C23H26N4OS calculated: 67.95% C, 6.45% H, 13.78% N; found: 67.94% C, 6.49% H, 13.72% N.
MS (FAB) [M + 1]+: m/z 407.
2-[4-(2-Methoxyphenyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5m)
IR (KBr) νmax(cm−1): 3281 (amide N–H), 3054 (aromatic C–H), 2976 (aliphatic C–H), 1680 (amide C=O), 1596–1414 (C=C and C=N), 1301–1019 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.66–2.70 (4H, m, piperazine C–H), 2.71 (3H, s, C–CH3), 3.04 (4H, brs, piperazine C–H), 3.32 (2H, s, CO–CH2), 3.77 (3H, s, O–CH3), 6.85–6.95 (4H, m, Ar–H), 7.72 (2H, d, J = 8.4 Hz, Ar-H), 7.86 (1H, s, thiazole C5-H), 7.88 (2H, d, J = 8 Hz, Ar–H), 9.85 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.66 (CH3), 53.79 (2CH2), 55.90 (2CH2), 56.45 (CH3), 62.02 (CH2), 112.54 (CH), 113.56 (CH), 118.75 (CH), 120.79 (2CH), 122.64 (CH), 124.86 (CH), 127.89 (2CH), 130.79 (C), 139.52 (C), 142.14 (C), 151.46 (C), 154.17 (C), 166.52 (C), 168.99 (C).
For C23H26N4O2S calculated: 65.38% C, 6.20% H, 13.26% N; found: 65.36% C, 6.21% H, 13.26% N.
MS (FAB) [M + 1]+: m/z 423.
2-[4-(2-Pyridyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5n)
IR (KBr) νmax(cm−1): 3278 (amide N–H), 3044 (aromatic C–H), 2978 (aliphatic C–H), 1678 (amide C=O), 1595–1407 (C=C and C=N), 1307–1005 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.73–2.75 (4H, m, piperazine C–H), 2.76 (3H, s, C–CH3), 3.21 (2H, s, CO–CH2), 3.63 (4H, t, J = 5.2, 4.8 Hz piperazine C–H), 6.65–6.68 (2H, m, Ar–H), 7.26 (1H, s, thiazole C5-H), 7.50 (1H, t, J = 7.6, 4.8 Hz, Ar–H), 7.63 (2H, d, J = 8.4 Hz, Ar–H), 7.85 (2H, d, J = 8.8 Hz, Ar–H), 8.21 (1H, d, J = 5.2 Hz, Ar–H), 9.85 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.32 (CH3), 45.52 (2CH2), 53.34 (2CH2), 62.13 (CH2), 107.26 (CH), 111.62 (CH), 113.81 (CH), 127.01 (2CH), 130.71 (C), 137.28 (CH), 137.57 (C), 148.01 (CH), 154.59 (C), 159.32 (C), 165.85 (C), 168.98 (C).
For C21H23N5OS calculated: 64.10% C, 5.89% H, 17.80% N; found: 64.14% C, 5.87% H, 17.83% N.
MS (FAB) [M + 1]+: m/z 394.
2-[4-(2-Benzyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5o)
IR (KBr) νmax(cm−1): 3276 (amide N–H), 3023 (aromatic C–H), 2986 (aliphatic C–H), 1676 (amide C=O), 1598–1421 (C=C and C=N), 1301–1027 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.50–2.65 (8H, brs, piperazine C–H), 2.77 (3H, s, C–CH3), 3.14 (2H, s, CO–CH2), 3.55 (2H, s, N–CH2–C), 7.25–7.34 (6H, m, thiazole C5-H and Ar–H), 7.62 (2H, d, J = 9.2 Hz, Ar–H), 7.85 (2H, d, J = 9.2 Hz, Ar–H), 9.21 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.59 (CH3), 53.48 (2CH2), 53.80 (2CH2), 62.22 (CH2), 63.17 (CH2), 111.82 (CH), 119.72 (2CH), 127.21 (2CH), 127.43 (CH), 128.54 (2CH), 129.37 2CH), 130.81 (C), 137.59 (CH), 138.14 (C), 154.86 (C), 166.11 (C), 168.67 (C).
For C23H26N4OS calculated: 67.95% C, 6.45% H, 13.78% N; found: 67.93% C, 6.41% H, 13.74% N.
MS (FAB) [M + 1]+: m/z 407.
2-[4-(2-Furoyl)piperazine-1-yl]-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide (5p)
IR (KBr) νmax(cm−1): 3288 (amide N–H), 3056 (aromatic C–H), 2969 (aliphatic C–H), 1679 (amide C=O), 1599–1427 (C=C and C=N), 1280–1020 (C–N and C–O).
1H NMR (400 MHz, DMSO-d6): 2.71 (4H, t, J = 4.4, 4.8 Hz, piperazine C–H), 2.77 (3H, s, C–CH3), 3.21 (2H, s, CO–CH2), 3.92 (4H, brs, piperazine C–H), 6.49–6.50 (1H, m, furan C4-H), 7.05 (1H, d, J = 3.6 Hz, furan C3-H), 7.27 (1H, s, thiazole C5-H), 7.49 (1H, s, furan C5-H), 7.63 (2H, d, J = 8.4 Hz, Ar–H), 7.87 (2H, d, J = 8.8 Hz, Ar–H), 9.06 (1H, s, N–H).
13C NMR (100 MHz, DMSO-d6): 19.56 (CH3), 53.83 (2CH2), 55.31 (2CH2), 62.30 (CH2), 113.66 (CH), 111.93 (CH), 117.13 (CH), 119.81 (2CH), 127.27 (2CH), 131.09 (C), 137.34 (C), 144.05 (CH), 148.01 (C), 154.27 (C), 159.34 (C), 166.02 (C), 168.98 (C).
For C21H22N4O3S calculated: 61.44% C, 5.40% H, 13.65% N; found: 61.40% C, 5.43% H, 13.68% N.
MS (FAB) [M + 1]+: m/z 411.
AChE/BChE Inhibition
All compounds were subjected to a slightly modified method of Ellman’s testCitation26 in order to evaluate their potency to inhibit AChE and BuChE. The spectrophotometric method is based on the reaction of released thiocholine to give a coloured product with a chromogenic reagent 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB). AChE, (E.C.3.1.1.7 from Electric Eel, 500 units), BChE, (E.C. 3.1.1.8, from horse serum, 1000 units) and donepezil hydrochloride were purchased from Sigma–Aldrich (Steinheim, Germany). Potassium dihydrogen phosphate, DTNB, potassium hydroxide, sodium hydrogen carbonate, gelatine, acetylthiocholine iodide (ATC) and butrylthiocholine iodide (BTC) were obtained from Fluka (Buchs, Switzerland). Spectrophotometric measurements were performed on a 1700 Shimadzu UV-1700 UV–Vis spectrophotometer.
Cholinesterase activity of the compounds (5a–5p) was measured in 100 mM phosphate buffer (pH 8.0) at 25°C, using ATC and BTC (75 mM) as substrates. In both cases, DTNB (10 mM) was used in order to observe absorbance changes at 412 nm. Donepezil hydrochloride was used as a positive controlCitation27.
Enzymatic assay
Enzyme solutions were prepared in gelatine solution (1%), at a concentration of 2.5 units/mL. AChE or BChE solution (50 µL) and compound solution (50 µL), which is prepared in 2% DMSO at a concentration range of 10−1–10−6 mM, were added to 3.0 mL phosphate buffer (pH 8 ± 0.1) and incubated at 25°C for 5 min. The reaction was started by adding (DTNB) (50 µL) and ATC (10 µL) to the enzyme-inhibitor mixture. The production of the yellow anion was recorded for 10 min at 412 nm. As a control, an identical solution of the enzyme without the inhibitor was processed following the same protocol. The blank reading contained 3.0 mL buffer, 50 μL 2% DMSO, 50 μL DTNB and 10 μL substrate. All processes were assayed in triplicate. The inhibition rate (%) was calculated by the following equation:
where AI is the absorbance in the presence of the inhibitor, AC is the absorbance of the control and AB is the absorbance of blank reading. Both of the values were corrected with blank-reading value. SPSS for Windows 15.0 was used for statistical analysis. Data was expressed as Mean ± SD.
Results and discussion
Chemistry
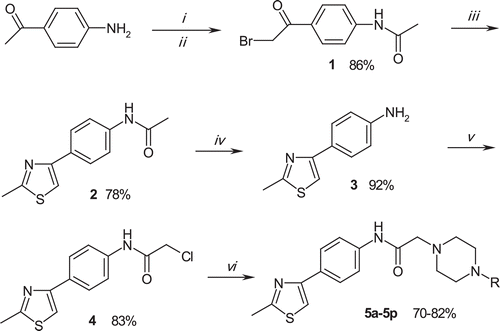
In the present study, some 2-(4-substituted piperazin-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide derivatives (5a–5p) were synthesized. Target compounds were obtained at five steps. Initially, 4-aminoacetophenone was acetylated with acetyl chloride to obtain N-(4-acetylphenyl)acetamide. Thus, amine group was protected in further ring closure step. N-(4-acetylphenyl)acetamide was brominated to afford N-[4-(2-bromoacetyl)phenyl]acetamide (1). Reaction of compound 1 with thioacetamide gave the N-[4-(2-methyl-4-thiazolyl)phenyl]acetamide (2) , which was deacetylated in 10% HCl solution to 4-(2-Methyl-4-thiazolyl)aniline (3) in the next reaction step. 2-Chloro-N-[4-(2-methyl-4-thiazolyl)phenyl]acetamide (4) was obtained via acetylation of compound 3 with chloroacetyl chloride. At the final reaction step, corresponding N-substituted piperazines were reacted with compound 4 to achieve 2-(4-substituted piperazine-1-yl)-N-[4-(2-methylthiazol-4-yl)phenyl]acetamide derivatives (5a–5p). Synthetic protocol for the compounds is outlined in . Some characteristics of the synthesized compounds are presented in .
Scheme 1. Synthesis of the compounds (5a–5p). Reagents: (i) acetyl chloride, TEA, THF, 0–5°C; (ii) Br2, AcOH; (iii) thioacetamide, EtOH, r.t.; (iv) 1 N HCl, EtOH, reflux; (v) chloroacetyl chloride, TEA, THF, r.t.; (vi) appropriate 4-substituted piperazine, K2CO3, acetone, reflux.
Structure elucidations of the final compounds were performed with IR, 1H NMR and FAB-MS spectroscopic methods and elemental analysis. Characteristic stretching absorption of C=O groups were observed at 1683–1673 cm−1. The stretching absorption at about 3288–3276, 1599–1402 and 1311–1004 cm−1 were recorded for N–H bonds, C=C and C=N double bonds, and C–O and C–N bonds, respectively. In the 1H NMR spectra, all of the aromatic and aliphatic protons were observed at estimated areas. N–H and CH protons of the acetamide moiety gave peaks at about δ 9.88–9.06 and 3.35–3.09 ppm as singlet, respectively. The C5-H proton of the thiazole was observed as a singlet at δ 7.86–7.22. Methyl protons on the thiazole ring gave a singlet at δ 2.77–2.18 ppm. Protons of piperazine ring were resonated at δ 3.92–2.35 largely as two different triplets. Two doublets belonging to aromatic protons of 1,4-disubstituted phenyl ring were observed at δ 7.88–7.59 ppm. In the 13C NMR spectra, the signal of characteristic carbonyl carbon was seen at about δ 166 ppm. Methylene (CH2) carbon, which was bonded to carbonyl was seen at about δ 62.02–63.06 ppm. Methyl carbon on the thiazole ring was appeared at about δ 19.32–19.66 ppm. The carbons of the piperazine were seen at δ 45.52-55.84 ppm as two different peaks. M + 1 peaks in MS spectra were in agreement with the calculated molecular weight of the target compounds. Elemental analysis results for C, H and N elements were satisfactory within ± 0.4 % calculated values of the compounds.
Enzymatic activity
Synthesized compounds were assayed by Ellman’s method so as to investigate their inhibitory activity against AChE and BChE. Results are given in . Test compounds were found to be inactive on BChE. On the other hand, they inhibited AChE to different extents. IC50 values of the compounds 5d–5i, 5k, and 5l could not be calculated due to their poor inhibition potency, which did not exceed the 50% even at the highest concentration (100 µM). However, the compounds 5a–5c, and 5m showed IC50 values in the range of 6.34–8.42 µM. Besides, the compounds 5n, 5o, and 5p indicated significant AChE inhibitory activity, which is comparable with that of donepezil. The compound 5n, containing 2-pyridyl moiety at 4th position of piperazine ring, displayed similar inhibitory potency with the reference drug. Furthermore, IC50 (0.11 µM) of 4-benzylpiperazine fragment bearing compound 5o was five-fold lower than that of donepezil.
Table 2. Percentage AChE and BChE inhibition of the compounds and IC50 values.
Observed results prompted us to explore a relationship between AChE inhibitory activity and chemical structures of the compounds. The compounds 5d–5m, which carry N-(substituted-phenyl)piperazines as variable side groups showed poorest AChE inhibitory potency in the series. In these compounds, substitution of phenyl ring on different positions with methyl, methoxy, chloro or nitro groups did not influence the enzymatic activity. The reason of ineffectiveness of the compounds 5d–5m may be related with electron withdrawing character of the phenyl ring, which decreases electron density on the piperazine moiety. In a previous study, electron donating effect was reported as the most important factor on the benzyl benzene ring, suggesting a role in regulating the protonation equilibrium at the benzylic nitrogen of the piperazine skeleton [24]. This report may be useful for explanation of inhibition potency differences of the compounds (5a-5p) against AChE. Contrary the phenyl substituent, aliphatic side groups at 4th position of piperazine as methyl, ethyl, cyclohexyl and benzyl enhance the AChE inhibitory activity to different extents. In addition, N-substitution of piperazine with heteroaryls as 2-pridyl or 2-furoyl also increases the biological activity. However, similar to phenyl ring, heteroaryl moieties decrease the electron density of piperazine ring, but there is a significant difference between the inhibitory activity of the compounds 5d-5m and 5n and 5p, which contain N-phenyl substituted piperazine derivatives and N-heteroaryl substituted piperazine derivatives, respectively. Thus, some other factors to electron donating or withdrawing characteristics of substituents need to be sought. In this case, polarizability and hydrogen bonding ability distances between phenyl and heteroaryl side groups may be suggested for the reason of enzymatic activity differences. Due to increasing polarizability and hydrogen bonding capability, interaction between AChE may be higher for N-heteroaryl substituted piperazine containing compounds 5n and 5p than the N-phenyl substituted piperazine bearing compounds (5d-5m). As a result of higher interaction, inhibitory activity enhancement may be occurred for the compounds 5n and 5p.
Declaration of interest
The authors report no declaration of interest.
References
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta 2006;366:1–13.
- Fulton MH, Key PB. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 2001;20:37–45.
- Rahman AU, Choudhary MI. Bioactive natural products as a potential source of new pharmacophores a theory of memory. Pure Appl Chem 2001;73:555–560.
- Olson RE, Thompson LA. Secretase inhibitors as therapeutics for Alzheimer’s disease. Annu Rep Med Chem 2000;35:31–40.
- Mudher A, Lovestone S. Alzheimer’s disease-do tauists and baptists finally shake hands? Trends Neurosci 2002;25:22–26.
- Brühlmann C, Ooms F, Carrupt PA, Testa B, Catto M, Leonetti F et al. Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J Med Chem 2001;44:3195–3198.
- Castro A, Martinez A. Peripheral and dual binding site acetylcholinesterase inhibitors: implications in treatment of Alzheimer’s disease. Mini Rev Med Chem 2001;1:267–272.
- Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982;217:408–414.
- Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science 1983;219:1184–1190.
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976;2:1403.
- Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003;39:75–83.
- Talesa VN. Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev 2001;122:1961–1969.
- Tago H, McGeer PL, McGeer EG. Acetylcholinesterase fibers and the development of senile plaques. Brain Res 1987;406:363–369.
- Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem 1999;264:672–686.
- Huang W, Yu H, Sheng R, Li J, Hu Y. Identification of pharmacophore model, synthesis and biological evaluation of N-phenyl-1-arylamide and N-phenylbenzenesulfonamide derivatives as BACE 1 inhibitors. Bioorg Med Chem 2008;16:10190–10197.
- Sheng R, Lin X, Li J, Jiang Y, Shang Z, Hu Y. Design, synthesis, and evaluation of 2-phenoxy-indan-1-one derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem Lett 2005;15:3834–3837.
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 2005;4:107–120.
- Alspach JD, Ingraham LL. Inhibition of acetylcholinesterase by thiamine. A structure-function study. J Med Chem 1977;20:161–164.
- Tack J, Janssen P. Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin Investig Drugs 2011;20:701–712.
- Nagel AA, Liston DR, Jung S, Mahar M, Vincent LA, Chapin D et al. Design and synthesis of 1-heteroaryl-3-(1-benzyl-4-piperidinyl)propan-1-one derivatives as potent, selective acetylcholinesterase inhibitors. J Med Chem 1995;38:1084–1089.
- Ali MA, Ismail R, Choon TS, Kumar RS, Osman H, Arumugam N, Almansour AI, Elumalai K, Singh A. AChE inhibitor: A regio- and stereo-selective 1,3-dipolar cycloaddition for the synthesis of novel substituted 5,6-dimethoxy spiro[5.3′]-oxindole-spiro-[6.3′’]-2,3-dihydro-1H-inden-1′’-one-7-(substituted aryl)-tetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole. Bioorg Med Chem Lett 2012;22:508–511.
- Sabb LA. 2-arylamidothiazole derivatives with CNS activity. US Patent, No: 5712270. Application Number:08/739559 Publication Date:01/27/1998.
- Klochkova IN, Semenova NN, Safonova AA, Noritsina MV. Search for potential cholinesterase inhibitors among substituted pyrrolidines and piperazines. Pharmaceut Chem J 1999;33:12–14.
- Sadashiva CT, Narendra Sharath Chandra JN, Ponnappa KC, Veerabasappa Gowda T, Rangappa KS. Synthesis and efficacy of 1-[bis(4-fluorophenyl)-methyl]piperazine derivatives for acetylcholinesterase inhibition, as a stimulant of central cholinergic neurotransmission in Alzheimer’s disease. Bioorg Med Chem Lett 2006;16:3932–3936.
- Moore N, Stensbol TB. 1-[2-(2,4-Dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-ht3 and 5-ht1a activity for the treatment of pain or residual symptoms in depression relating to sleep and cognition. Patent application number: 20110009422
- Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 2000;52:895–902.
- Ellman GL, courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.