Abstract
Nine novel 4-[3-(4-Dimethylamino-phenyl)-5-aryl-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamides (2a-i) were synthesized and evaluated for their anti-inflammatory and antiproliferative activities. These compounds (2a-i) showed moderate to strong anti-inflammatory activity in carrageenan rat paw oedema test. Compounds 2b, 2d and 2g showing comparable anti-inflammatory activity to that of reference drug celecoxib were evaluated for their ulcerogenic and analgesic activities. The effect of 2b, 2d and 2g on the content of NO, TNF-α and PGE2 in exudates from rat paw stimulated by carrageenan was also evaluated. The compound 2c showed considerable antitumor activities against all 60 human tumor cell lines with effective GI50 (MG-MID) value of 3.63 µM. It exhibited maximum activity against melanoma (LOX IMVI and SK-MEL-5) cancer cell lines with GI50 value less than 2 μM.
Introduction
Pyrazoline act as useful lead molecules as they show significant pharmacological properties and clinical applications. These derivatives have been reported to possess activities like anti-inflammatory,Citation1–4 antidepressant,Citation5 anticancer,Citation5,Citation6 anti-bacterial,Citation5 anti-tubercular,Citation7,Citation8 analgesic,Citation7,Citation8 anti-diabetic.Citation9 Some of the pyrazole derivatives like antipyrine, phenylbutazone, celecoxib etc. have found application in clinical medicine.
Chlacones bearing dimethylaminophenyl moiety are reported as potential inhibitors of NO (nitric oxide) and PGE2 (prostaglandin E2) production in the RAW 264.7 macrophage cell line.Citation10 More over dimethylaminophenyl bearing pyrazolines have proved to possesses significant anti-inflammatoryCitation2,Citation4 and anti-cancerous activities.Citation11
The sulfonamides constitute an important class of drugs, with several types possessing a host of biological properties including antibacterial,Citation12 anti-carbonic anhydrase,Citation13,Citation14,Citation15 diuretic,Citation13,Citation16 hypoglycaemic,Citation17 antithyroidCitation18 and anti-protease activities.Citation19–21 A large number of structurally novel sulfonamide derivatives have recently been reported to show substantial antitumor activity, both in vitro and/or in vivo. Although they have a common chemical motif of aromatic/heterocyclic sulfonamide, there are a variety of mechanisms of their antitumor action, most of them poorly understood at this moment. Some of these derivatives are currently being evaluated in clinical trials, and there is much optimism that they may lead to novel alternative anticancer drugs, devoid of the side effects of the presently available pharmacological agents.Citation22 Many selective COX-2 inhibitors belong to a group of compounds which have a central tri-substituted planar ring (five-membered heterocyclic ring) attach to pendent benzene rings and a lipophilic group. One of the phenyl rings is attached to SO2NH2. The SO2NH2 pharmacophore is believed to induce COX-2 selectivity.Citation23
Promoted with the above mentioned findings the present study aimed at gathering the two bioactive entities (chlacones containing dimethylaminophenyl moiety and benzenesulfonamide) into one compact structure and evaluating their biological activities as anti-inflammatory agent with reduced gastric toxicity.
Results and discussion
Chemistry
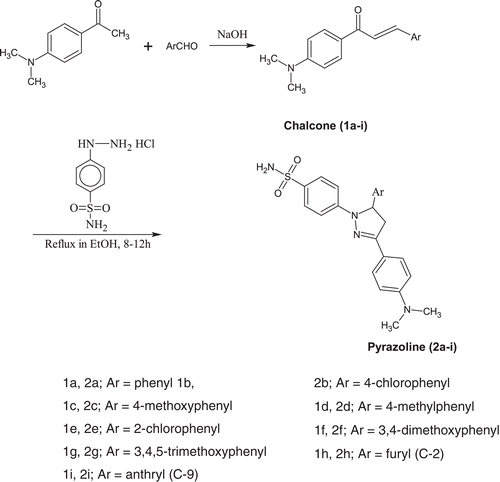
The target compounds were synthesized according to steps outlined in . The intermediates chalcones (1a-i) were obtained by Claisen-Schmidt condensation of 1-[4-(dimethylamino)phenyl]ethanone with appropriate aromatic aldehydes in presence of base (NaOH). Treatment of the intermediate chalcone with 4-hydrazinobenzenesulfonamide hydrochloride in refluxing absolute ethanol yielded corresponding 2-pyrazoline derivatives (2a-i). The structures of all novel compounds was established through spectroscopic (IR, 1H NMR, 13C NMR, MS) and elemental analysis data. The IR spectra of pyrazolines (2a-i) showed absorption bands in the region of 1587–1601 cm−1 corresponding to C=N stretching bands because of ring closure. Also, infrared spectra revealed NH2 peak at 3309–3413 cm−1 and 3216–3305 cm−1 and for SO2NH2 peak at 1311–1334 cm−1 and 1146–1155 cm−1. In the 1HNMR spectra of 2-pyrazolines, the three hydrogen atoms attached to the C-4 and C-5 carbon atoms of the heterocyclic ring gave an ABX spin system. Measured chemical shift and coupling constant values (cf. Experimental Section) prove the 2-pyrazoline structure. NH proton of the SO2NH2 group was observed at 6.89–7.03 ppm as singlet. The protons of methyl and aromatic protons were observed at expected ppms. In 13C spectra of these compounds (2a-i) the chemical shift values of carbon atoms of pyrazoline ring are C-3 (150.88–151.63 ppm), and C-5 (59.98–62.63 ppm), however the signal of C-4 of pyrazoline was found to be merged with solvent (DMSO) peak.
Pharmacology
Anti-inflammatory activity
The anti-inflammatory activity of target compounds (2a-i) was evaluated by applying carrageenan-induced hind paw oedema bioassay in ratsCitation24 using celecoxib as reference drug as it has some chemical structural resemblance with target compounds. The results are summarised in . These compounds (2a-i) showed moderate to strong anti-inflammatory activity (49.5–75.9% at 3 h and 42.2–88.8% at 5 h). The anti-inflammatory activity of 2d and 2g is almost comparable to that exhibited by standard drug celecoxib at 3 h and 5 h. Compound 2b showed 86.6% inhibition of paw oedema at 5 h which is comparable to that of celecoxib (82.2%). Introduction of methyl or chloro or methoxyl group in 5-phenyl ring increases the activity. Replacement of 5-phenyl ring with furyl ring diminishes the activity at 5 h (2a vs. 2h).
Table 1. Effect of dimethylamino-phenyl based pyrazolines on carageenan induced hind paw oedema in rats.
Ulcerogenic activity
Ulcerogenic activity of the compounds 2b, 2d and 2g was also recorded at dose of 0.15 mmole/kg and compared with reference drug celecoxibCitation25 (0.15 mmole/kg). These compounds did not show any ulceration or harmful effects on the stomach in fasted rats.
Effect of 2b, 2d and 2g on TNF-α, nitrite and PGE2 production in exudates from rat paw stimulated by carrageenan
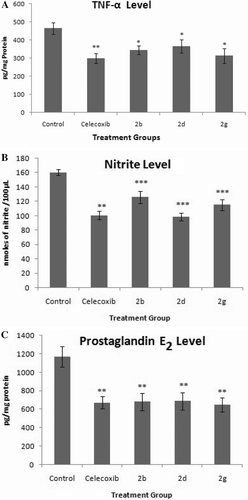
Compounds showing good anti-inflammatory activity 2b, 2d and 2g (0.05 mmole/kg) were evaluated for their action on the pro-inflammatory cytokine (TNF-α), nitrite and PGE2 levels in exudates from rat paw stimulated by carrageenan. The results are shown in – (Supplementary data) respectively. These three compounds reduced the content of NO, TNF-α and PGE2 significantly. Compound (2g) inhibited the production of TNF-α and PGE2 significantly as compared to the celecoxib.
Analgesic activity
The analgesic activity for the compounds 2b, 2d and 2g was determined by tail immersionCitation26 method at the dose of 0.05 mmole/kg bw. The result is summarized in . The result shows that compound 2d and 2g are significantly more potent than standard drug celcecoxib. However compound 2b shows activity almost equal to standard drug.
Table 2. Analgesic effect of compounds 2b, 2d and 2g by tail immersion method.
In-vitro anticancer activity
Primary in vitro one-dose (10–5 M) anticancer assay was performed using full panel of about 60 human tumor cell lines in accordance with the protocol of the Drug Evaluation Branch, National Cancer Institute (NCI), Bethesda, and described elsewhere.Citation27–32 The human tumor cell lines were derived from nine different cancer types: Leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancers. Out of the synthesized compounds (2a-i), two compounds, namely 2b and 2c were selected by the National Cancer Institute (NCI). The compounds 2b displayed some sensitivity towards cell lines K-562 (Leukemia) and PC-3 (prostrate cancer) (Supplementary Table S1). The compound 2c (NSC 754832) possessed considerable antiproliferative activity (Supplementary Table S2) and it was selected for an advanced assay against a full panel (approximately 60 cell lines) at five concentrations at 10-fold dilution (100, 10, 1, 0.1 and 0.001 μM). A 48 h continuous drug exposure protocol was used and sulforhodamine B (SRB) protein assay was used to estimate cell growth. Details of this system and the information which is encoded by the activity pattern over all cell lines have been published.Citation27–29 The anticancer activity of tested compounds is given by three parameters for each cell line: log GI50 value (GI50 = molar concentration of the compound that inhibits 50% net cell growth), log TGI value (TGI = molar concentration of the compound leading to total inhibition) and log LC50 value (LC50 = molar concentration of the compound leading to 50% net cell death). Furthermore, a mean graph midpoint (MG_MID) is calculated for each of the mentioned parameters, giving an averaged activity parameter over all cell lines. For the calculation of the MG_MID, insensitive cell lines are included with the highest concentration tested. Selectivity of the compound with respect to one or more cell lines of the screen is characterized by a high deviation (▴) of the particular cell line parameter compared to the MG_MID value.
The compound 2c showed considerable antitumor activities against the entire tested tumor cell lines and showed effective growth inhibition GI50 (MG-MID) values of 3.63 µM. The compound 2c exhibited maximum activity against melanoma (LOX IMVI and SK-MEL-5) cancer cell lines with a GI50 value of less than 2 μM. It also displayed good activity against non-small cell lung cancer (NCI-H226, NCI-H460, NCI-H522), colon (COLO 205, HCC-2998, HCT-116, HCT-15), melanoma (LOX IMVI, SK-MEL-5), renal cancer (A498, RXF 393, SN12C) and breast (BT-549) cancer cell lines with a GI50 less than 3.0 μM (Supplementary Table S3).
Conclusions
The present study describes the synthesis of a series of nine novel 4-[3-(4-Dimethylamino-phenyl)-5-aryl-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamides (2a-i) and their evaluation for anti-inflammatory and anti-poliferative activities. Among these synthesised compounds three compounds (2b, 2d and 2g) exhibited anti-inflammatory activity comparable with that of celecoxib and had superior gastro-intestinal safety profile when tested for their ulcerogenic activity. The effect of 2b, 2d and 2g on the content of NO, TNF-α and PGE2 in exudates from rat paw stimulated by carrageenan was also evaluated. Compound 2g reduced the production of TNF-α and PGE2 significantly. The analgesic activity for the compounds 2b, 2d and 2g was determined by tail immersion method. The compounds 2d and 2g showed more potent analgesic activity than standard drug celecoxib. However compound 2b shows activity almost equal to standard drug. Two compounds, namely 2b and 2c were screened for their antiproliferative activity. The compound 2c showed considerable antitumor activities against all 60 human tumor cell lines and showed effective growth inhibition GI50 (MG-MID) values of 3.63 µM.
Experimental section
Chemistry
Melting points were determined by open capillary tubes and are uncorrected. All the Fourier Transform Infra Red (FTIR) spectra were recorded on a Brukers Vector 22 spectrophotometer in film; υmax values are given in cm−1. 1H NMR spectra were recorded on a Bruker Spectrospin DPX 300 MHz/400 MHz spectrometer using deuterated DMSO as solvent and tetramethyl silane (TMS) as an internal standard. Chemical shifts are given in δ (ppm) scale and coupling constants (J values) are expressed in Hz. Mass spectra (MS) were scanned by ESI Q-TOF Water. The m/z values of the more intense peaks are mentioned. 13C NMR spectra was recorded on Bruker spectrospin DPX 400 MHz using deuterated DMSO as a solvent and tetramethyl silane (TMS) as internal standard. Purity of the compounds was checked on TLC plates (silica gel G) which were visualized by exposing to iodine vapors. Elemental analysis was carried out on CHNS Elementar (Vario EL III).
General procedure for the synthesis of chalcones (1a-i)
To a cold solution (below 10°C) of acetophenone (0.01 mole) and desired aromatic aldehyde (0.01 mole) in ethanol (20 mL) was added a chilled solution of sodium hydroxide (10 mL of 30% solution). The reaction mixture was covered with a layer of petroleum ether (60–80°C) and left at room temperature for a period of 12 h. It was poured on crushed ice (100 mL) and acidified with HCl. The product obtained was filtered, washed with water and crystallized from appropriate solvent.
General procedure for the preparation of pyrazolines (2a-i)
A solution of appropriate chalcone (1a-i) (0.01 mole) and 4-hydrazinobenzenesulfonamide hydrochloride (0.01 mole) in absolute ethanol (20–150 ml) was refluxed for 12–16 h. The reaction mixture was concentrated to a small volume and left at room temperature when 2-pyrazoline separated out, which was filtered, dried and crystallized from appropriate solvent.
4-[3-(4-Dimethylamino-phenyl)-5-phenyl-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2a)
Recrystalized from MeOH as pale yellow solid, yield 71%, m.p. 240–241°C, Rf = 0.58 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3378 cm−1 & 3272 cm−1 (NH2), 1594 cm−1 (C=N), 1328 cm−1 & 1146 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.94 [6H, s, N(CH3)2], 3.20 [1H, dd, J = 12.6 Hz, J = 21.6 Hz, H-4 trans (pyrazoline)], 3.87–3.83 [1H, m, H-4 cis (pyrazoline)], 5.52–5.49 [1H, m, H-5 (pyrazoline)], 6.72 (2H, d, J = 9.0 Hz, H-3′, H-5′), 6.95 (2H, s, SO2NH2), 6.97 (2H, d, J = 9.0 Hz, H-3′’, H-5′’), 7.31-7.20 (5H, m, H-2, H-3, H-4, H-5, H-6), 7.51 (2H, d, J = 8.7 Hz H-2′’, H-6′’), 7.58 (2H, d, J = 8.7 Hz, H-2′, H-6′); 13C NMR (75 MHz, DMSO, δ): 43.30 N(CH3)2, 61.91 (C-5 pyrazoline), 151.01 (C-3 pyrazoline). ESI-MS (m/z): 420 [M+], 419 [M+-1], 421[M++1]; CHNS Analysis: Found (Calculated): C: 65.71 (65.69), H: 5.71 (5.75), N: 13.33 (13.32), S: 7.61 (7.62); Molecular Formula: C23H24N4O2S.
4-[5-(4-Chloro-phenyl)-3-(4-dimethylamino-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2b)
Recrystalized from MeOH as off white solid, yield 61%, m.p. 228–229oC, Rf = 0.40 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3379 cm−1 & 3273 cm−1 (NH2), 1595 cm−1 (C=N), 1325 cm−1 & 1152 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.94 [6H, s, N(CH3)2], 3.09 [1H, dd, J = 4.8 Hz, J = 17.7 Hz, H-4 trans (pyrazoline)], 3.85 [1H, dd, J = 12.0 Hz, J = 17.7 Hz, H-4 cis (pyrazoline)], 5.55–5.52 [1H, m, H-5 (pyrazoline)], 6.73 (2H, d, J = 8.7 Hz, H-3′, H-5′), 6.99 (4H, s, SO2NH2 H-3′’, H-5′’), 7.23 (2H, d, J = 8.1 Hz, H-3, H-5), 7.38 (2H, d, J = 8.4 Hz, H-2, H-6), 7.82–7.54 (4H, m, H-2′, H-6′, H-2′’, H-6′’); 13C NMR (75 MHz, DMSO, δ): 43.50 N(CH3)2, 61.65 (C-5 pyrazoline), 150.88 (C-3 pyrazoline); ESI-MS (m/z): 454 [M+], 453 [M+-1], 455 [M++1]; CHNS Analysis: Found (Calculated): C: 60.79 (60.72), H: 5.06 (5.10), N: 12.33 (12.31), S: 7.04 (7.05); Molecular Formula: C23H23ClN4O2S.
4-[3-(4-Dimethylamino-phenyl)-5-(4-methoxy-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2c)
Recrystalized from MeOH as pale yellow solid, yield 67%, m.p. 185–187oC, Rf = 0.25 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3343 cm−1 & 3251 cm−1 (NH2), 1598 cm−1 (C=N), 1329 cm−1 & 1153 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.93 [6H, s, N(CH3)2], 3.03 [1H, dd, J = 17.4 Hz, J = 21.6 Hz, H-4 trans (pyrazoline)], 3.78 (3H, s, OCH3), 3.85 [1H, dd, J = 12.0 Hz, J = 17.7 Hz, H-4 cis (pyrazoline)], 5.46–5.43 [1H, m, H-5 (pyrazoline)], 6.72 (2H, d, J = 8.4 Hz, H-3′, H-5′), 6.85 (2H, d, J = 8.1Hz, H-3, H-5), 6.95 (2H, s, SO2NH2), 6.97 (2H, d, J = 9.0 Hz, H-2, H-6), 7.13 (2H, d, J = 8.4 Hz, H-3′’, H-5′’), 7.51 (2H, d, J = 8.7 Hz, H-2′’, H-6′’), 7.57 (2H, d, J = 8.4 Hz, H-2′, H-6′); 13C NMR (75 MHz, DMSO, δ): 43.80 N(CH3)2, 55.93 (OCH3 at C-3 and C-4), 62.35 (C-5 (yrazoline), 151.41 (C-3 pyrazoline); ESI-MS (m/z): 450 [M+], 449 [M+-1], 451 [M++1]; CHNS Analysis: Found (Calculated): C: 64.00 (63.98), H: 5.77 (5.82), N: 12.44 (12.44), S: 7.11 (7.12); Molecular Formula: C24H26N4O3S.
4-[3-(4-Dimethylamino-phenyl)-5-p-tolyl-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2d)
Recrystalized from MeOH as pale yellow solid, yield 53%, m.p. 237–239°C, Rf = 0.67 (chloroform: actone: triethylamine, 9: 1: 0.1), IR υmax (KBr): 3413 cm−1 & 3283 cm−1 (NH2), 1593 cm−1 (C=N), 1333 cm−1 & 1155 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.24 (3H, s, CH3), 2.96 [6H, s, N(CH3)2], 3.08 [1H,dd, J = 4.8 Hz, J = 17.4 Hz, H-4 trans (pyrazoline)], 3.88 [1H, dd, J = 12.3 Hz, J = 17.7 Hz,, H-4 cis (pyrazoline)], 5.49 [1H, dd, J = 4.8 Hz, J = 11.7 Hz, H-5 (pyrazoline)], 6.74 (2H, d, J = 9.0 Hz, H-3′, H-5′), 6.98 (2H, s, SO2NH2), 6.99 (2H, d, J = 8.4 Hz, H-3′’, H-5′’), 7.13 (4H, s, H-2, H-3, H-5, H-6 of tolyl unit appeared as singlet i.e accidentally equivalent protons), 7.54 (2H, d, J = 8.7 Hz, H-2′’, H-6′’), 7.61 (2H, d, J = 9.0Hz, H-2′, H-6′); 13C NMR (75 MHz, DMSO, δ): 20.06 (CH3 at C-4), 43.27 N(CH3)2, 61.70 (C-5 pyrazoline), 150.99 (C-3 pyrazoline); ESI-MS (m/z): 434 [M+], 433 [M+-1], 435 [M++1]; CHNS Analysis: Found (Calculated): C: 66.35 (66.33), H: 5.99 (6.03), N: 12.90 (12.89), S: 7.37 (7.38); Molecular Formula: C24H26N4O2S.
4-[5-(2-Chloro-phenyl)-3-(4-dimethylamino-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2e)
Recrystalized from acetone off white solid, yield 43%, m.p. 244–245oC, Rf = 0.38 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3402 cm−1 & 3305 cm−1 (NH2), 1601 cm−1 (C=N), 1311 cm−1 & 1147 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.93 [6H, s, N(CH3)2], 3.10–3.04 [1H, m, H-4 trans (pyrazoline)], 3.98 [1H, dd, J = 12 Hz, J = 17.4 Hz, H-4 cis (pyrazoline)], 5.71–5.68 [1H, m, H-5 (pyrazoline)], 6.71 (2H, d, J = 8.1 Hz, H-3′, H-5′), 6.89 (2H, d, J = 8.1 Hz, H-2′, H-6′), 7.59–6.99 (10 H, m, SO2NH2, H-2′’, H-6′’,H-3′’, H-5′’, H-2, H-3, H-4, H-5); 13C NMR (75 MHz, DMSO, δ): 42.37 N(CH3)2, 59.98 (C-5 pyrazoline), 151.48 (C-3 pyrazoline); ESI-MS (m/z): 454 [M+], 453 [M+-1], 455 [M++1]; CHNS Analysis: Found (Calculated): C: 60.70 (60.72), H: 5.06 (5.10), N: 12.33 (12.31), S: 7.04 (7.05); Molecular Formula: C23H23ClN4O2S.
4-[5-(3,4-Dimethoxy-phenyl)-3-(4-dimethylamino-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2f)
Recrystalized from MeOH pale yellow solid, yield 58%, m.p. 161–162oC, Rf = 0.22 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3344 cm−1 & 3265 cm−1 (NH2), 1599 cm−1 (C=N), 1334 cm−1 & 1151 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.93 [6H, s, N(CH3)2], 3.05–3.11 [1H, m, H-4 trans (pyrazoline)], 3.58–3.67 [6H, m, (OCH3)2], 3.82–3.84 [1H, m, H-4 cis (pyrazoline)], 5.41–5.43 [1H, m, H-5 (pyrazoline)], 6.63 (1H, d, J = 9.0 Hz H-5), 6.71–6.85 (2H, d, J = 8.7 Hz, H-3′, H-5′), 6.97–6.85 (6H, m, SO2 NH2, H-2, H-6, H-3′’, H-5′’), 7.52 (2H, d, J = 6.9 Hz, H-2′’, H-6′’), 7.57 (2H, d, J = 8.1 Hz, H-2′, H-6′); 13C NMR (75 MHz, DMSO, δ): 43.80 N(CH3)2, 55.93 (OCH3 at C-3 and C-4), 62.35 (C-5 pyrazoline), 151.41 (C-3 pyrazoline); ESI-MS (m/z): 480 [M+], 479 [M+-1], 481 [M++1]; CHNS Analysis: Found (Calculated): C: 62.50 (62.48), H: 5.83 (5.87), N: 11.66 (11.66), S: 6.66 (6.67); Molecular Formula: C25H28N4O4S.
4-[3-(4-Dimethylamino-phenyl)-5-(3,4,5-trimethoxy-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2g)
Recrystalized from MeOH off white solid, yield 51%, m.p. 240–241oC, Rf = 0.45 (petrol: acetone, 7: 3), IR υmax (KBr): 3309 cm−1 & 3216 cm−1 (NH2), 1596 cm−1 (C=N), 1334 cm−1 & 1154 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.96 [6H, s, N(CH3)2], 3.15 [1H, dd, J = 6.0 Hz, J = 18.0 Hz, H-4 trans (pyrazoline)], 3.61 (3H, s, OCH3 at C-4), 3.68 [6H, s, 2(OCH3) at C-3, C-5], 3.87 [1H, dd, J = 11.1 Hz, J = 16.8 Hz H-4 cis (pyrazoline)], 5.38 [1H, dd, J = 5.7 Hz, J = 11.1 Hz, H-5 (pyrazoline)], 6.56 (2H, s, H-2, H-6), 6.74 (2H, d, J = 8.7 Hz, H-3′, H-5′), 6.98 (2H, s, SO2NH2), 7.03 (2H, d, J = 8.4 Hz, H-3′’, H-5′’), 7.57 (2H, d, J = 9.3 Hz, H-2′’, H-6′’), 7.60 (2H, d, J = 9.3 Hz, H-2′, H-6′); 13C NMR (75 MHz, DMSO, δ): 43.46 N(CH3)2, 55.90 (OCH3 at C-3 and C-5), 60.01 (OCH3 at C-4), 62.63 (C-5 pyrazoline), 151.63 (C-3 pyrazoline); ESI-MS (m/z): 510 [M+], 509 [M+-1], 511 [M++1]; CHNS Analysis: Found (Calculated): C: 61.17 (61.16), H: 5.88 (5.92), N: 10.98 (10.97), S: 6.27 (6.28); Molecular Formula: C26H30N4O5S.
4-[3-(4-Dimethylamino-phenyl)-5-furan-2-yl-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2h)
Recrystalized from MeOH off white solid, yield 47%, m.p. 282–284oC, Rf = 0.68 (chloroform: acetone: triethylamine 9: 1: 0.1), IR υmax (KBr): 3346 cm−1 & 3258 cm−1 (NH2), 1592 cm−1 (C=N), 1325 cm−1 & 1147 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.94 [6H, s, N(CH3)2], 3.76 [1H, dd, J = 12.0 Hz, J = 17.4 Hz, H-4 cis (pyrazoline)], 5.66 [1H, dd, J = 4.5 Hz, J= 11.4 Hz, H-5 (pyrazoline)], 6.38 (1H, d, J = 1.5 HZ, H-3), 6.45 (1H, d, J = 2.4 Hz, H-4), 6.76 (2H, d, J = 9.0 Hz, H-3′, H-5′), 7.03 (2H, s, SO2NH2), 7.14 (2H, d, J = 9.0 Hz, H-3′’, H-5′’), 7.64-7.57 [5H, m, H-2′, H-5′, H-2′’, H-6′’, H-5 (thio group)]; ESI-MS (m/z): 410 [M+], 411 [M++1]; 13C NMR (75 MHz, DMSO, δ): 60.72 (C-5 pyrazoline), 151.53 (C-3 pyrazoline); CHNS Analysis: Found (Calculated): C: 61.46 (61.44), H: 5.36 (5.40), N: 13.60 (13.65), S: 7.80 (7.81); Molecular Formula: C21H22N4O3S.
4-[5-Anthracen-9-yl-3-(4-dimethylamino-phenyl)-4,5-dihydro-pyrazol-1-yl]-benzenesulfonamide (2i)
Recrystalized from MeOH yellow solid in 53% yield, m.p. 316–318°C, Rf = 0.32 (toluene: ethyl acetate: formic acid, 5: 4: 1), IR υmax (KBr): 3403 cm−1 & 3304 cm−1 (NH2), 1587 cm−1 (C=N), 1332 cm−1 & 1152 cm−1 (SO2N<); 1H NMR (300 MHz, DMSO, δ): 2.99 [6H, s, N(CH3)2], 3.46 [1H, dd, J = 9.6 Hz, J = 18.3 Hz, H-4 cis (pyrazoline)], 4.25 [1H, dd, J = 13.5 Hz, J = 17.7 Hz,, H-5 (pyrazoline)], 6.89–6.75 (6H, m, SO2NH2, H-3′, H-5′, H-2′’, H-6′’), 7.31 (2H, d, J = 8.7 Hz, H-3′’, H-5′’), 7.45–7.35 (2H, m, H-3, H-6), 7.64 (1H, t, H-7/H-2), 7.73 (3H, d, J = 8.4Hz, H-7/H-2, H-2′, H-6′), 7.99 (1H, d, J = 8.7 Hz, H-5/H-4), 8.10 (1H, d, J = 8.1 Hz, H-5/H-4), 8.21 (1H, d, J = 8.1 Hz, H-8/H-1), 8.67 (1H, s, H-9), 8.84 (1H, d, J = 9.0 Hz, H-8/H-1); ESI-MS (m/z): 520 [M+], 519 [M+-1], 521 [M++1]; CHNS Analysis: Found (Calculated): C: 71.53 (71.51), H: 5.38 (5.42), N: 10.76 (10.76), S: 6.15 (6.16); Molecular Formula: C31H28N4O2S.
Pharmacological activity
All the experiments were carried out in albino rats of Wistar strain (either sex) were procured from Central Animal House of Jamia Hamdard, New Delhi (Registration no. 173/CPCSEA). The experiments were performed in accordance with the guidelines for the care and use of laboratory animals, laid down by the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA), Ministry of Social Justice and Empowerment, Govt. of India, Jan. 2000. Animals described as fasted were deprived of food for at least 16–24 h but allowed free access to water. CMC (1% w/v) in distilled water was used as vehicle for dosing in all the experiments. All treatments were given orally except carrageenan.
Anti-inflammatory activity
Carrageenan induced hind paw oedema method was used for evaluating anti-inflammatory activity.Citation24 Fasted rats were divided into eleven groups of six animals each. Group I served as control and was given vehicle only (10 mL/kg), while animals of group II were given celecoxib 0.05 mmole/kg suspended in the vehicle and served as standard. Test compounds (2a-i) (0.05 mmole/kg) suspended in vehicle (10 mL/kg) were administered to respective groups. After 30 min, all animals were injected with 0.1 mL of 1% carageenan solution (prepared in normal saline) in the subplantar aponeurosis of left hind paw to induce inflammation and the volume of injected paw was measured by using plethysmometer immediately (at 0 h). The paw volume was again measured after 3 h and 5 h. The average paw volume in a group of treated rats was compared with vehicle (control group) and the percentage inhibition of oedema was calculated by using the formula:
Where Vt is the mean paw volume of the test drug treated rats and Vc is the mean paw volume of the control.
The experiment was carried out under normal laboratory conditions. The animals were handled gently to avoid too much of stress on them which could result in an increased adrenal output. A mark was made at the lateral maleous of the left hind paw so that dipping was done to the same level while measuring the paw volume.
Determination of TNF-α, nitrite and prostaglandin E2 in exudates from rat paw stimulated by carrageenan
Overnight fasted rats (16 h) were divided into five groups of six animals each. One group of rats, which served as control was given vehicle (1% CMC in water in a volume of 10 ml/kg) only. Celecoxib (0.05 mmole/kg b.w.) and test compounds (0.05 mmole/kg b.w.), suspended in vehicle (10 ml/kg) were administered orally to respective groups. They received a subplantar injection of carrageenan (0.1 ml of a 1% suspension in normal saline) into the right hind paw after 30 min of drug administration. At specified time (5 h) after the subplantar injection of carrageenan, rats were sacrificed and each paw was cut at the level of the calcaneus bone. Paws were gently centrifuged at 250 g for 20 min. in order to recover a sample of the oedematous fluid. The amount of fluid that was recovered from each paw was then passed through millipore cut-off filter (10,000 mol wt.) to remove any traces of blood cells (Millipore, Bedford, MA, USA).
Oedematous levels of pro-inflammatory cytokine TNF- α were measured by the commercially available kit (Rat TNF-α ELISA Ready-SET-Go! eBioscience, USA) following manufacturers protocol. The nitrite concentration in oedematous fluid was measured according to Griess reaction, and the calculated concentration was taken as an indicator of NO production. 100 µl of oedematous fluid was mixed with an equal volume of Griess reagent (1% sulphanilamide in 5% phosphoric acid and 0.1% naphthyl ethylenediamine dihydrochloride in water). The reaction mixture was incubated at room temperature for 10–15 min. The optical density at 550nm was measured and calculated against a sodium nitrite standard curve. The oedematous levels of PGE2 by ELISA based kit following instruction of the manufacturer (Cayman chemicals, Ann Arbor, MI, USA).
Ulcerogenic activity
Acute gastric ulcerogenic effect of the compounds 2b, 2d and 2g were evaluated in Albino Wistar rats.Citation25 Albino wistar rats (150–220g) were fasted over 24 h and where randomly allotted into five groups of six animals each. Group I served as control and was given vehicle 10 ml/kg (CMC 1% w/v in distilled water) orally. Groups II served as standard and was administered orally celecoxib (0.15 mmole/kg i.e. three times the dose given in anti-inflammatory activity) suspended in vehicle. Group III, IV and V were administered orally compounds 2b, 2d and 2g (0.15 mmole/kg) suspended in vehicle, respectively. They were sacrificed under deep anathesia after 6 h of dosing. Their stomach were removed and opened through greater curvature for examining lesions or bleedings. Compounds 2b, 2d and 2g showed no ulcers.
Analgesic activity
Analgesic activity of the compounds 2b, 2d and 2g were evaluated in Albino Wistar rats.Citation26 The albino rats weighing 150–200 g were fasted overnight and were made into five groups of six animals. Group I served as control and was given vehicle 10ml/kg (CMC 1% w/v in distilled water) orally. While the animals of group II were given celecoxib 0.05 mmole/kg suspended in the vehicle (CMC 1% w/v). Rest of three groups were given 2b, 2d and 2g (0.05 mmole/kg b.w.) suspended in the vehicle (CMC 1% w/v), respectively. 3–4 cm area of the tail was immersed in the water bath thermostatically maintained at 55°C. The withdrawal time of the tail from the hot water (in seconds) was noted as reaction time, initially as tail flick latency before the administration of any drug and then recorded at 30, 60 and 90 min after the administration of the test compounds and celecoxib. The maximum cut off time for immersion was 30 s to avoid the injury of the tissue of the tail.
Declaration of interest
The authors are very grateful to the staff members of Department of Health and Human Services, National Cancer Institute (NCI), Bethesda, Maryland, USA, for carrying out in vitro anticancer screening of the newly synthesized compounds. One of the authors, Syed Ovais is thankful to HNF for awarding a fellowship.
References
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med Chem Lett 2008;18:918–922.
- Rathish IG, Javed K, Ahmad S, Bano S, Alam MS, Pillai KK et al. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 2009;19:255–258.
- Gürsoy A, Demirayak S, Capan G, Erol K, Vural K. Synthesis and preliminary evaluation of new 5-pyrazolinone derivatives as analgesic agents. Eur J Med Chem 2000;35:359–364.
- Bashir R, Ovais S, Yaseen S, Hamid H, Alam MS, Samim M et al. Synthesis of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg Med Chem Lett 2011;21:4301–4305.
- Johnson M, Younglove B, Lee L, LeBlanc R, Holt H Jr, Hills P et al. Design, synthesis, and biological testing of pyrazoline derivatives of combretastatin-A4. Bioorg Med Chem Lett 2007;17:5897–5901.
- Rostom SA. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem 2006;14:6475–6485.
- Sahu SK, Banerjee M, Samantray A, Behera A, Azam MA. Synthesis, analgesic, anti-inflammatory and antimicrobial activities of some novel pyrazoline derivatives. Tropical journal of pharmaceutical research, 2008;7;961–968.
- Chandra T, Garg N, Lata S, Saxena KK, Kumar A. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur J Med Chem 2010;45:1772–1776.
- Ahn JH, Kim HM, Jung SH, Kang SK, Kim KR, Rhee SD et al. Synthesis and DP-IV inhibition of cyano-pyrazoline derivatives as potent anti-diabetic agents. Bioorg Med Chem Lett 2004;14:4461–4465.
- Rojas J, Domínguez JN, Charris JE, Lobo G, Payá M, Ferrándiz ML. Synthesis and inhibitory activity of dimethylamino-chalcone derivatives on the induction of nitric oxide synthase. Eur J Med Chem 2002;37:699–705.
- Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Alam MS. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur J Med Chem 2011;46:5763–5768.
- Drew J. Drug discovery: a historical perspective. Science, 2000;287:1960–1964.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Exp Opin Ther Patents 2000;10:575–600.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem Immunol Endoc Metab Agents 2001;1:61–97.
- Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A et al. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. J Med Chem 2004;47:550–557.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Boyd AE 3rd. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 1988;37:847–850.
- Thornber CW. Isosterism and molecular modification in drug design. Chem Soc Rev 1979;8:563–580.
- Ogden RC, Flexner CW. (2001). Protease Inhibitors in AIDS Therapy. New York, Basel: Marcel Dekker.
- Supuran C, Scozzafava A, Mastrolorenzo A. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Expert Opin Ther Patents 2001;11:221–259.
- Scozzafava A, Supuran CT. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 2000;43:3677–3687.
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002;2:55–75.
- Amgad G, Habeeb PN, Parveen R, Edward EK. Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of sulfonamide and methylsulfonyl pharmacophores by an azido bioisostere. J Med Chem 2001;44:3039–3042.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547.
- Daidone G, Maggio B, Raffa D. Synthesis and pharmacological study of ethyl 1-methyl-5-[2-substituted-4-oxo-3(4H)-quinazolinyl]-1H-pyrazole-4-acetates. Eur J Med Chem 1994;29:707–711.
- di Stasi LC, Costa M, Mendaçolli SL, Kirizawa M, Gomes C, Trolin G. Screening in mice of some medicinal plants used for analgesic purposes in the state of São Paulo. J Ethnopharmacol 1988;24:205–211.
- Boyd MR. Am Assoc Cancer Res. 1989;30:652–663.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 1991;83:757–766.
- Weinstein JN, Myers TG, O’Connor PM, Friend SH, Fornace AJ Jr, Kohn KW et al. An information-intensive approach to the molecular pharmacology of cancer. Science 1997;275:343–349.
- Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci 1995;57:131–141.
- Grever MR, Schepartz SA, Chabner BA. The National Cancer Institute: cancer drug discovery and development program. Semin Oncol 1992;19:622–638.
- Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109.
- Boyd MR. Status of the NCI preclinical antitumor drug discovery screen. Princ Pract Oncol 1989;3:1–12.