Abstract
A new series of quinoline analogs have been synthesized and found active against P. falciparum in vitro and P. yoelli in vivo. Compounds 8, 10 and 11 exhibited superior in vitro activity compared to chloroquine. Selected compounds 8, 10 and 11 exhibited significant suppression of parasitaemia in vivo assay. These analogs form a complex with hematin and inhibit the β-hematin formation, suggesting that this class of compounds act on a heme polymerization target. Further this study confirms that quinoline ring nitrogen is essential for both transportation of the molecule across the membrane as well as for tight binding to hematin.
Introduction
Malaria remains one of the greatest global health challenges despite intensive efforts to control the disease. It is endemic in many developing countries and leads to the death of 1–2 million people yearly. The rapid spread of multidrug resistance, especially toward chloroquine (CQ), the main drug in use for many years, is a cause for major concern. It clearly highlights the urgent need of novel chemotherapeutic agents for the treatment of malaria. Intensive drug discovery efforts have been aimed at developing new antimalarial drugs or modifying existing agentsCitation1,Citation2.
Recent literature evidences show that alteration of chain length (shortening) of CQ and its derivatives is active against CQ-resistant parasite strains, it clearly indicates that the resistance mechanism does not involve any change in the target of this class of drugs but involves a compound specific resistanceCitation3,Citation4. The molecular biology studies suggested that the CQ and closely related 4-aminoquinoline compounds enter the food vacuole and inhibit the parasite growth by forming a complex with hematin (Fe(III)FPIX), thereby inhibiting the hemozoin formation. The hematin exerts a toxic effect on the parasiteCitation5–7. In this continuation several research groups have developed numerous 4-aminoquinoline derivatives, which are significantly more potent than CQ against P. falciparum in in vitro studiesCitation8–11.
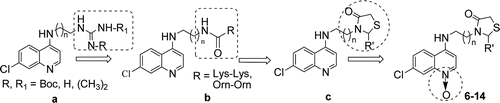
In our ongoing drug discovery program for malaria chemotherapeutic agent, we have carried out modification on 4-aminoquinoline lateral side chain with guanyl and tetramethyl guanyl moieties () leads to increase the pKa2 value and the resulting analogs showed in vitro and in vivo antimalarial activity against NF-54 strain P. falciparum and N-67 strain of P. yoelii, respectivelyCitation12. Continuation of our efforts for the identification of new molecules by selectively modifying the pendant amino group of 4-aminoquinoline terminal side chain with amide bond of (-CONH-) cationic amino acid of lysine and ornithine conjugates () showed that promising in vitro antimalarial activityCitation13. Also we have demonstrated that 4-aminoquinoline analogues with altered chain length having thiazolidin-4-ones () and thiazolidine exhibit potential activity against P. falciparum NF-54 strain in vitro and N-67 strain of P. yoelii in vivo and some of these compounds were indeed more effective than CQCitation14,Citation15.
Figure 1. Some lead molecules of 4-aminoquinoline derived antimalarials developed from this laboratory.
As an obvious extension of this work we turned our attention on the role of quinoline nitrogen atom (pKa1) with doppelganger objective in mind namely, whether basicity of this nitrogen is important for the transportation of 4-aminoquinoline scaffold across the membrane or to form association complex with hematin or both. Apart from these in literature very few studies have shown modification on quinoline ring nitrogenCitation16. To address this issue a new series of compounds having Nω-oxide in the quinoline ring () were synthesized. The modification carried out in the present study may affect the basicity and lipophilic nature of molecules, which could have a cascading effect on the antimalarial activity. Such a modification drastically decreases the basicity (pKa1) yet allows complexation with hematin through metal-oxygen type interaction. These results are described in this communication.
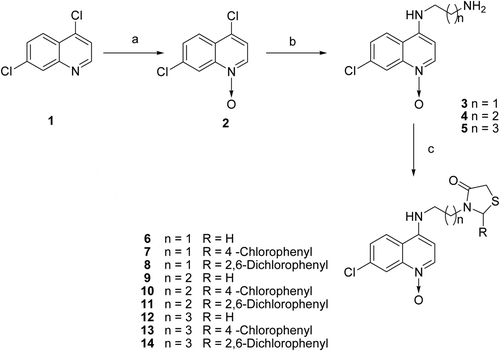
Scheme 1. Synthesis of quinoline Nω oxide derived thiazolidin-4-ones. Reagents and conditions: (a) Glacial Acetic acid/ H2O2, 70–80ºC for 12 h (b) Diamino alkane, ethanol reflux for 6–8 h; (c) RCHO, Mercapto acetic acid, Toluene reflux 18–24 h.
Experimental
Meting points (mp) were determined on a Complab melting point apparatus and are uncorrected. IR spectra (cm−1) were recorded on Perkin-Elmer 621 spectrometer using the KBr disc technique. The 1H-NMR spectra were recorded on a DPX-200 MHz Bruker FT-NMR spectrometer using CDCl3 and DMSO-d6 as solvent. Tetramethylsilane (δ 0.0 ppm) was used as an internal standard. Fast Atom Bombardment Mass Spectra (FAB-MS) were obtained on Jeol (Japan)/SX-102 spectrometer using glycerol or m-nitrobenzyl alcohol as matrix. Elemental analysis was performed on a Perkin-Elmer 2400 C, H, N analyzer and values were within the acceptable limits of the calculated values. The progress of the reaction was monitored on readymade silica gel plates (Merck) using chloroform-methanol (9:1) as a solvent system. Iodine was used as developing agent or by spraying with Dragendorff’s reagent. Chromatographic purification was performed over silica gel (100–200 mesh). All chemicals and reagents were obtained from Aldrich (USA), Lancaster (UK) or Spectrochem Pvt. Ltd (India) and were used without further purification.
General synthetic procedure for N1-(7-chloro-1-oxidoquinolin-4-yl)diaminoalkane (3–5)
A mixture of 4,7-dichloroquinoline-1-oxide 2 (2.5 g, 12.5 mmol) and appropriate diaminoalkane (25 mmol) was refluxed in absolute ethanol for 6–8 h with continues stirring. The reaction mixture was cooled to room temperature and concentrated under vacuum; the residue was taken up in dichloromethane. The organic layer was successively washed with 5% aq. NaHCO3 followed by water wash and then finally with brine. The organic layer was dried over anhydrous Na2SO4 and solvent was removed under reduced pressure and the residue was precipitated by addition of 80:20 hexane: chloroform.
General synthetic procedure for 3-[(7-chloro-1-oxidoquinolin-4-ylamino)alkyl]-2-(unsubstituted/substituted)-1,3-thiazolidin-4-one (6–14)
The appropriate amine (1.0 mmol), aldehyde (2.0 mmol) and mercaptoacetic acid (3.0 mmol) in 20 mL dry toluene was heated to reflux for 20–22 h. The reaction mixture was cooled to room temperature and concentrated to dryness under reduced pressure and the residue was taken up in chloroform and washed with 5% aq. sodium hydrogen carbonate and then finally with brine solution. The organic phase was then dried over sodium sulfate, the filtrate was concentrated to dryness under reduced pressure and the crude product was purified by column chromatography on silica gel using chloroform-methanol.
Biological and biophysical studies
Measurement of in vitro antimalarial activity
The in vitro antimalarial assay was carried out in 96-well microtitre platesCitation17–19. The cultures of P. falciparum NF 54 strain are routinely maintained in medium RPMI 1640 supplemented with 25 mM HEPES, 1% D-glucose, 0.23% sodium bicarbonate and 10% heat inactivated human serumCitation20. The asynchronous parasites of P. falciparum were synchronized after 5% D-sorbitol treatment to obtain only the ring stage parasitized cells. For carrying out the assay, the initial ring stage parasitaemia of 0.8 to 1.5% at 3% haematocrit in a total volume of 200 µL of medium RPMI-1640 was uniformly maintained. The test compound in 20 µL volume concentrations ranging between 0.5 and 50 µg/mL in duplicate well were incubated with parasitized cell preparation at 37ºC in a candle jar. After 36–40-h incubation, the blood smears from each well prepared and stained with giemsa stainCitation19,Citation20. The slides were microscopically observed to record maturation of ring stage parasites into trophozoites and schizonts in presence of different concentrations. The test concentrations, which inhibited the complete maturation into schizonts, were recorded as the minimum inhibitory concentration (MIC). CQ was used as the standard reference drug.
In vivo antimalarial efficacy test
The in vivo drug response was evaluated in Swiss mice infected with P. yoelii (N-67 strain)Citation20,Citation21. The mice (22 ± 2 g) were inoculated with 1 × 106 parasitised RBC on day 0 and treatment was administered to a group of five mice from day 0 to 3, once daily. The aqueous suspension of compounds was prepared with a few drops of Tween 80. The efficacy of test compounds was evaluated at 30.0 mg/kg/day and required daily dose was administered in 0.2 mL volume via intraperitoneal route. Parasitaemia levels were recorded from thin blood smears between days 4 and 28Citation20,Citation21. The mean value determined for a group of five mice was used to calculate the percent suppression of parasitaemia with respect to the untreated control group. Mice treated with CQ served as positive controls.
Determination of haematin-4 aminoquinoline derivatives association constant
Association constant for haematin-4-aminoquinoline derivatives complex formation were determined by spectrometric titration procedure in aqueous dimethyl sulfoxide (DMSO) at pH 7.5Citation22. In this assay condition, haematin is strictly in monomeric state and interpretation of results is not complicated by the need to consider haematin disaggregation process. Association constant calculated in this technique is a good reflection of the interaction would occur in the acidic food vacuole. The pH 7.5 improves the stability of haematin solutions and quality of data.
In vitro inhibition of β-haematin polymerization
The ability of the 4-aminoquinoline derivatives to inhibit β-haematin polymerization was induced by 1-oleoyl-rac-glycerol using UV spectrophotometer and measurements were carried out at 405 nmCitation23. The triplicate values obtained from the assay are expressed as percent inhibition relative to haemozoin formation in a drug free control. The 50% inhibitory concentration (IC50) values for the compounds were obtained from the sigmoidal dose–response curves using non-linear regression curve fitting analyses with GraphPad Prism version 3.00 softwareCitation24. Each IC50 value is the result of at least three separate experiments performed in duplicate.
Results and discussion
Chemistry
The desired target compounds (6–14) were synthesized as outlined in . 4,7-Dichloroquinoline Nω oxide (2) was synthesized by the reported procedure in which 4,7-dichloroquinoline (1) was refluxed at 70–80°C with glacial acetic acid/hydrogen peroxide (H2O2) solution for 18 hCitation16. The amino components (3–5) used in the present study were prepared by aromatic nucleophilic substitution on 4,7-dichloroquinoline Nω oxide with excess of diaminoalkane in ethanol reflux. The desired thiazolidin-4-one compounds (6–14) were obtained from appropriate amines, substituted aldehyde and mercapto acetic acid in toluene reflux protocol. Both analytical and spectral data of the synthesized compounds are in agreement with the structures of the synthesized compounds.
Pharmacology
All the compounds were evaluated for their antimalarial activity against the NF-54 strain of P. falciparum in in vitro for the determination of minimum inhibitory concentration (MIC) values according to reported protocol, and the data is presented in . Among all the 12 compounds tested, 3 compounds showed MIC range between 0.27 and 0.52 μM, 9 compounds showed MIC range between 2.30 and 4.34 μM. The difference in the MIC values can be attributed to the number of carbon atoms in the side chain and C-2 substitutions on the thiazolidin-4-one ring system. This result suggests that the modification at quinoline nitrogen atom is tolerated for antimalarial activity in vitro. Among the amino component (3–5), compounds having two carbon atoms (3) in the side chain are less active compared to which have three (4) or four carbon atoms (5) in the side chain. These findings are in consonance with the earlier data from this laboratoryCitation12,Citation14. In amino component (3–5) introduction of thiazolidin-4-one ring system resulted molecules having interesting antimalarial activity.
Table 1. Biological and biophysical data of the compounds (3–14).
The compounds having thiazolidin-4-ones ring system (6, 9, 12) on lateral side chain leads to increases the antimalarial activity. The compound having substitution of 4-chlorophenyl (7, MIC = 2.30 μM) on C-2 position of thiazolidin-4-one ring system leads to increased antimalarial activity in comparison to unsubstituted compound (6, MIC = 3.18 μM). The compound having 4-chlorophenyl substitution on C-2 position of thiazolidin-4-one ring system (10, MIC = 0.28 μM) leads to substantial increase in the antimalarial activity in comparison to the CQ (MIC = 0.39 μM). However introduction of 2,6-dichlorophenyl substitution on thiazolidin-4-one ring system (11) (MIC = 0.52 μM) leads to reduced the antimalarial activity twofold in comparison to 4-chlorophenyl substituted compound (10). Conversely, introduction of 2,6-dichlorophenyl substitution of C-2 position of thiazolidin-4-one ring system (8) (MIC = 0.27 μM) leads to substantial increase in activity as compared to CQ. The compounds having substitution of 4-chlorophenyl (13) (MIC = 4.04 μM) and 2,6-dichlorophenyl (14) (MIC = 4.32 μM) on C-2 position of thiazolidin-4-one ring system leads to decreased antimalarial activity.
Compounds with most significant activity in vitro (8, 10 and 11) were selected for in vivo activity against P. yoelli (N-67 strain) in Swiss mice (). The mice were treated with compounds (30 mg/kg) intraperitoneally, once daily for four consecutive days, and their survival time and parasitaemia on day four were compared with those of control mice receiving saline (). These compounds showed significant activity against P. yoelli infections in mice. The compounds 8, 10, 11 suppressed 56.76, 59.49, 48.23% parasitaemia on day 4 compared to 100% suppression displayed by CQ. The mean survival time (MST) is also in accordance with inhibition data. The compounds (8, 10, 11) have shown lesser Log P value than CQ (). The Log P values of these compounds are decreased due to Nω oxide modification in quinoline ring system. If lipophilic nature of molecules affects (decrease), it may decrease the cell permeability. It clearly highlights the importance of lipophilicity in the antimalarial activity of the synthesized compounds.
Table 2. In vivo antimalarial activity data of the compounds (8, 10, 11) against N-67 strain of P. yoelii in Swice mice.
To understand mode of action of these derivatives, all the compounds were evaluated for the association complex formation with haematin (Log K) and ability to inhibit β-haematin formation (IC50). The ability of the compounds synthesized in the present study to form association complex with hematin was investigated by UV spectrophotometer and it may be inferred from the data shown in . All the compounds bind with an association constant in the range 4.29–5.25 with hematin and form a complex. It is appropriate to mention that higher Log K value (i.e. >5.0) indicate tight binding of theses analogs to haematin. The results suggest that the principle interaction might be involving metal type interaction of the quinoline ring Nω oxide with the porphyrin ring system of haematinCitation25–27. Furthermore the most active compounds 8, 10, 11 have shown lesser Log K values in comparison to CQ (Log K = 5.52). The leeser Log K value is due to Nω oxide modification in quinoline ring system.
The ability of these compounds to inhibit hemozoin formation was studied to shed some light on the mode of action. In addition to that all the compounds inhibited the β-hematin formation in a concentration-dependent manner. The most active compounds 8, 10, 11 have shown the inhibitory concentration values of β-haematin formation are in the range of 0.35–0.43 μM. It clearly suggests that the Nω oxide derivative of thiazolidin-4-one analogs inhibit the β-haematin formation. Further biophysical study results suggest that this class of compounds act on haem polymerization target. It may be inferred from the above data that these compounds binds to haematin monomer or haematin µ-oxo dimers and inhibit the β-haematin formation by blocking the growing face of crystal by a capping effectCitation28–30.
The modification carried out in the present study may affect the pKa values of quinoline nitrogen atom (). Therefore, we have calculated the pKa1 of these compounds using PallasCitation31. The data presented in clearly indicate that the pKa1 value of quinoline ring nitrogen atom also affected (decreased), due to Nω oxide formation. In addition to that the Log P value of these compounds () also decreased. Because of the decreased pKa1 value, less Log P value and weaker haematin association constant indicate that there is loss of in vivo activity of these compounds. The present study suggests that Nω oxide modification on quinoline ring affect the antimalarial activity of this class of compounds. The acquired home message emerging from this study is that quinoline ring nitrogen is essential for both transportation of the molecule across the membrane as well as for tight binding to hematin.
Conclusion
In summary, a new series of 4-aminoquinoline derivatives has been synthesized and evaluated for the in vitro antimalarial activity against NF-54 strain of P. falciparum. Some of the compounds exhibited superior in vitro activity compared to chloroquine and exhibited significant suppression in the in vivo assay. All the compounds exhibit modest antiplasmodial activity in vitro and a few are comparable to CQ. The biophysical studies suggest that the mechanism of action is similar to that of CQ, all the compounds form an association complex with haematin and thereby inhibit β-haematin formation. Further this study suggests that 4-aminoquinoline scaffold is essential for activity and modification on quinoline ring nitrogen atom leads to affect in vivo antimalarial activity of this class of compounds.
Acknowledgements
The authors thank the Director, CDRI for the support and the SAIF for the spectral data. One of the authors (V.R.S.) thanks the CSIR, New Delhi for Senior Research Fellowship. CDRI communication no. 93/2012/SBK.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wiesner J, Ortmann R, Jomaa H, Schlitzer M. New antimalarial drugs. Angew Chem Int Ed Engl 2003;42:5274–5293.
- O’Neill PM, Ward SA, Berry NG, Jeyadevan JP, Biagini GA, Asadollaly E et al. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr Top Med Chem 2006;6:479–507.
- Stocks PA, Raynes KJ, Bray PG, Park BK, O’Neill PM, Ward SA. Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J Med Chem 2002;45:4975–4983.
- Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A, Masciadri R et al. 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother 1996;40:1846–1854.
- Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 1995;374:269–271.
- Egan TJ, Marques HM. The Role of Haem in the Activity of Chloroquine and Related Antimalarial Drugs. Coord Chem Rev 1999;190–192:493–517.
- Pandey AV, Bisht H, Babbarwal VK, Srivastava J, Pandey KC, Chauhan VS. Mechanism of malarial haem detoxification inhibition by chloroquine. Biochem J 2001;355:333–338.
- Ray S, Madrid PB, Catz P, LeValley SE, Furniss MJ, Rausch LL et al. Development of a new generation of 4-aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models. J Med Chem 2010;53:3685–3695.
- Iwaniuk DP, Whetmore ED, Rosa N, Ekoue-Kovi K, Alumasa J, de Dios AC et al. Synthesis and antimalarial activity of new chloroquine analogues carrying a multifunctional linear side chain. Bioorg Med Chem 2009;17:6560–6566.
- Omodeo-Salè F, Cortelezzi L, Basilico N, Casagrande M, Sparatore A, Taramelli D. Novel antimalarial aminoquinolines: heme binding and effects on normal or Plasmodium falciparum-parasitized human erythrocytes. Antimicrob Agents Chemother 2009;53:4339–4344.
- Yearick K, Ekoue-Kovi K, Iwaniuk DP, Natarajan JK, Alumasa J, de Dios AC et al. Overcoming drug resistance to heme-targeted antimalarials by systematic side chain variation of 7-chloro-4-aminoquinolines. J Med Chem 2008;51:1995–1998.
- Solomon VR, Haq W, Smilkstein M, Srivastava K, Rajakumar S, Puri SK et al. Synthesis and antimalarial activity of novel side chain modified antimalarial agents derived from 4-aminoquinoline. Med Chem 2008;4:446–456.
- Solomon VR, Puri SK, Srivastava K, Katti SB. Design and synthesis of new antimalarial agents from 4-aminoquinoline. Bioorg Med Chem 2005;13:2157–2165.
- Solomon VR, Haq W, Srivastava K, Puri SK, Katti SB. Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J Med Chem 2007;50:394–398.
- Solomon VR, Haq W, Puri SK, Srivastava K, Katti SB. Design, Synthesis of 4-aminoquinoline derived thiazolidines and their antimalarial activity and heme polymerization inhibition studies. J Enzyme Inhib Med Chem 2012; Manuscript accepted for publication. Please cite doi:10.3109/14756366.2012.666537.
- Elsager EF, Gold EH, Tendick FH, Werbel LM, Worth DF. Amodiaquine N-oxides and other 7-chloro-4-aminoquinoline n-oxides. J Heterocycl Chem 1964;1:6–12.
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976;193:673–675.
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979;65:418–420.
- Srivastava K, Puri SK. Plasmodium falciparum: modified medium composition supports continuous cultivation with foetal bovine serum. Exp Parasitol 2004;108:74–75.
- Puri SK, Singh N. Azithromycin: antimalarial profile against blood- and sporozoite-induced infections in mice and monkeys. Exp Parasitol 2000;94:8–14.
- Diggens SM, Gregory K. Comparative response of various rodent malarias to chemotherapy. Trans R Soc Trop Med Hyg 1969;63:7.
- Egan TJ, Mavuso WW, Ross DC, Marques HM. Thermodynamic factors controlling the interaction of quinoline antimalarial drugs with ferriprotoporphyrin IX. J Inorg Biochem 1997;68:137–145.
- Tripathi AK, Khan SI, Walker LA, Tekwani BL. Spectrophotometric determination of de novo hemozoin/beta-hematin formation in an in vitro assay. Anal Biochem 2004;325:85–91.
- GraphPad Prism, version 4.0b, GraphPad Software Inc., 10855 Sorrento Valley Rd. #203, San Diego, CA 92121, 2007.
- Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J. Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of beta-hematin formation, and antiplasmodial activity. J Med Chem 2000;43:283–291.
- Kaschula CH, Egan TJ, Hunter R, Basilico N, Parapini S, Taramelli D et al. Structure-activity relationships in 4-aminoquinoline antiplasmodials. The role of the group at the 7-position. J Med Chem 2002;45:3531–3539.
- Egan TJ. Hemozoin (malaria pigement): A Unique Crystalline Drug Target. Targets 2003;3:115–124.
- Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem Pharmacol 1998;55:727–736.
- Vippagunta SR, Dorn A, Matile H, Bhattacharjee AK, Karle JM, Ellis WY et al. Structural specificity of chloroquine-hematin binding related to inhibition of hematin polymerization and parasite growth. J Med Chem 1999;42:4630–4639.
- Vippagunta SR, Dorn A, Ridley RG, Vennerstrom JL. Characterization of chloroquine-hematin mu-oxo dimer binding by isothermal titration calorimetry. Biochim Biophys Acta 2000;1475:133–140.
- Pallas 3.0, Compu-Drug Chemistry, Ltd., San Francisco, CA, 1984.
