Abstract
Protein Tyrosine Phosphatase 1B (PTP1B) is a major negative regulator of insulin signaling pathways. Finding selective PTP1B inhibitors from natural sources has been widely recognized as a potential drug target for the treatment of diabetes mellitus and obesity. In the present study, we evaluated the inhibitory activity of cinnamic acid derivatives against PTP1B in vitro. Among 14 cinnamic acid derivatives and related compounds, the most potent inhibitor PTP1Bs were o-hydroxycinnamic acid and p-hydroxycinnamic acid, which had IC50 values of 137.67 ± 13.37 and 181.60 ± 9.34 µM, respectively. The kinetics analysis revealed that PTP1B was inhibited by o-hydroxycinnamic acid and p-hydroxycinnamic acid in a non-competitive manner. o-Hydroxycinnamic acid (25 μM) and p-hydroxycinnamic acid (25 μM), in combination with sodium orthovanadate (0.0125 μM), demonstrated a synergistic effect to inhibit PTP1B activity. In conclusion, the findings provide a new insight into naturally occurring PTP1B inhibitors that could be useful for treatment of diabetes and obesity.
Introduction
Protein tyrosine phosphatase 1B (PTP1B) is a negative regulator of insulin signaling pathways by rapid dephosphorylating at phosphotyrosine residues of the activated insulin receptor kinaseCitation1. The consequence of its action inactivates the insulin receptor and thereby terminates the insulin signaling. Current scientific evidence clearly indicates that PTP1B activity is directly associated with the insulin resistance and type 2 diabetesCitation2. Interestingly, it has been demonstrated that the inhibition of PTP1B can enhance insulin signaling, primarily through prolonged activation of the insulin receptor, resulting in increased insulin sensitivity of skeletal muscle and adipose tissueCitation3. Nowadays, PTP1B inhibitors have emerged as an attractive novel target, in particular for the treatment of type 2 diabetes and other associated metabolic syndromesCitation2. Recent studies have shown that administration of PTP1B inhibitors can enhance insulin sensitivity and improve glucose tolerance in diabetic ratsCitation4,Citation5.
The phytochemical compounds from dietary plants and fruits are tremendous sources of lead compounds in the search for new types of inhibitors of PTP1B. In particular, cinnamic acid derivatives refer to one of the most numerous and widely distributed groups of phytochemicals in plant-based foodsCitation6. Recently, cinnamic acid derivatives have been reported to possess various pharmacological properties including antioxidativeCitation7, hepatoprotectiveCitation8, and antityrosinaseCitation9, as well as antidiabetic activitiesCitation10. Several mechanisms have been proposed for the antidiabetic effect of cinnamic acid derivatives, such as the inhibition of α-glucosidaseCitation11,Citation12, the stimulatory insulin secretionCitation13,Citation14, and the increased glucose uptake in skeletal muscleCitation15,Citation16. Furthermore, cinnamic acid derivatives have been reported to inhibit fructose-mediated protein glycation in vitroCitation17. Although there have been many studies demonstrating the antidiabetic mechanisms of cinnamic acid derivatives, the inhibition of PTP1B is yet to be investigated. Therefore, it would be interesting to examine the inhibitory effect of cinnamic acid derivatives on PTP1B activity. In the present study, 14 cinnamic acid derivatives and related compounds were investigated for inhibition of PTP1B in vitro. Furthermore, the study was conducted to evaluate the types of kinetic inhibition on PTP1B. Finally, the combined effects of cinnamic acid derivatives and sodium othorvanadate (SOV), a PTP1B inhibitor, were also studied.
Materials and methods
Chemicals
Protein tyrosine phosphatase 1B (human recombinant) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). p-Nitrophenol phosphate (p-NPP), cinnamaldehyde, cinnamyl alcohol, caffeic acid (3,4-dihydroxycinnamic acid), 3,4-dimethoxycinnamic acid, and methyl cinnamate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cinnamic acid, o-hydroxycinnamic acid, m-hydroxycinnamic acid, and p-hydroxycinnamic acid were purchased from Fluka (St. Louis, MO, USA.). o-Methoxycinnamic acid, m-methoxycinnamic acid, and p-methoxycinnamic acid were purchased from ACROS (Pittsburgh, PA, USA). Ferulic acid (3-methoxy-4-hydroxycinnamic acid) and isoferulic acid (4-methoxy-3-hydroxycinnamic acid) were purchased from Chromadex (Laguna Hills, CA, USA). Sodium orthovanadate was obtained from Merck (Darmstadt, Germany). All other chemicals used were of analytical grade.
Assay for the PTP1B activity
The assessment of the PTP1B activity was slightly modified based on a previous methodCitation18. PTP1B was diluted before the experiment to 1.2 µg/mL in Tris buffer, pH 7.6 (10 mM Tris, 1.0 mM EDTA, 3.0 mM DTT, 0.01% w/v NaN3). The tested compounds were dissolved in DMSO. The enzyme reaction buffer was Tris buffer, pH 7.5 (50 mM Tris, 0.15 M NaCl, 3 mM DTT). A typical 10 μL of tested compounds was added to the enzyme solution (50 μL), and the resulting mixture was preincubated at 37°C for 30 min. The enzyme reaction was initiated by the addition of 8 mM p-NPP (40 μL). The concentration of p-nitrophenol from the reaction mixtures was recorded at 405 nm, 15-min intervals for 120 min. SOV was used as a positive control in this study.
Measurements of the kinetics constant
In order to investigate the type of inhibition, the enzyme kinetic analysis was performed according to the above reaction. Maintaining the quantity of PTP1B constant at 1.2 µg/mL of PTP1B, the test compounds were measured in final concentrations of p-NPP (0.8-6.4 mM). The types of inhibition were calculated on the base of Lineweaver–Burk by reciprocally plotted data (substrate concentration on horizontal axis and velocity on vertical axis).
The combined effect of tested compounds and sodium orthovanadate
SOV was combined with or without the tested compounds. The reaction was performed according to the above assay. Results were expressed as the percentage inhibition of the corresponding control values.
Data analysis
Data was expressed as mean ± SEM (n = 3). The IC50 values were determined from plots of log concentration of inhibitor concentration versus percentage inhibition curves. The types of inhibition and constants were calculated on the base of Lineweaver-Burk and its replot of slopes as the non-linear regression. Statistical analysis was performed by Student’s t-test (P < 0.01).
Results and discussion
As shown in , we investigated 14 promising compounds of cinnamic acid and its derivatives and related compounds for PTP1B inhibition. The result in shows the percentage inhibition of the compounds at a concentration of 100 µM. In course of screening for PTP1B inhibitors, all compounds were found to inhibit PTP1B activity by approximately 12.50–39.40%. Cinnamic acid showed a percentage inhibition of 16.0 ± 0.01, whereas the percentage inhibition of cinnamyl alcohol, cinnamaldehyde and methyl cinnamate inhibited PTP1B activity ranged from 12.5 to 18.0%. The findings suggest that replacing the carboxylic acid group of cinnamic acid with alcohol, aldehyde, and methyl ester groups had no effect to increase the percentage of PTP1B inhibition.
Figure 2. The percentage PTP1B inhibition of cinnamic acid derivatives and related compounds (100 µM). Data are expressed as mean ± SEM, n = 3.
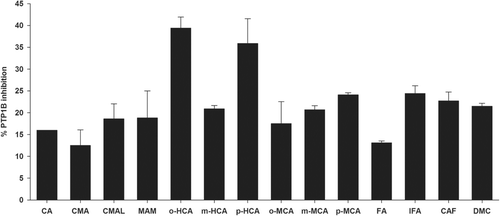
The addition of a hydroxyl residue to the cinnamic acid at the ortho- or para-positions resulted in a significant increase in the percentage of inhibition. We found that o-hydroxycinnamic acid (39.4 ± 2.52%) and p-hydroxycinnamic acid (35.9 ± 5.65%) displayed the highest percentage of PTP1B inhibition among all tested compounds. However, the presence of a hydroxyl residue at the meta-position showed a slight increase in the percentage inhibition (20.9 ± 0.76%). Replacing a hydroxyl residue, substituted in the cinnamic acid by a methoxyl residue, markedly decreased the PTP1B inhibitory activity. The findings revealed that the presence of a hydroxy substituent at the ortho- or para- position was more active than that of a methoxy substituent.
Interestingly, the introduction of two moieties in cinnamic acid markedly exerted a higher percentage of inhibition than cinnamic acid (21.5–24.4%), except when the substituent was at the meta-methoxy and para-hydroxy positions (ferulic acid). Considering the previous studies regarding the structure–activity relationship of cinnamic acid derivatives, it indicates that ferulic acid is the most potent intestinal α-glucosidase inhibitor and insulin secreting agent among cinnamic acid derivativesCitation11,Citation12. This is surprising, given the differences from the present study that ferulic acid slightly inhibited PTP1b activity. The interaction phenomena between cinnamic acid derivatives and PTP1B remain unknown. We assume that cinnamic acid derivatives may form hydrogen bonds with the polar groups of amino acid residues in the active site of PTP1B by covalent and/or non-covalent interaction. The differences in chemical structure of cinnamic acid derivatives may result in the difference in ability to bind the active site. The explanation for this hypothesis requires clarification through further investigation, using computer modeling to evaluate the binding activity of these compounds on the enzyme.
Based on the screening results, we further investigated the IC50 values of o-hydroxycinnamic acid and p-hydroxycinnamic acid. The results showed that they inhibited the hydrolysis of the p-NPP catalyzed by PTP1B in a dose-dependent manner (data not shown) with IC50 values of 137.74 ± 13.20 and 168.40 ± 17.69 µM, respectively. A known PTP1B inhibitor, SOV was employed as a positive control that had the IC50 value of 25.39 ± 0.65 nM. It was suggested that o-hydroxycinnamic acid and p-hydroxycinnamic acid were less potent than SOV on the PTP1B inhibition. Hydroxycinnamic acids are commonly found in fruits and in different parts of plantsCitation19. The highest content of hydroxycinnamic acids is commonly found in fruits such as blueberries, kiwis, plums, cherries, and apples, which contain 0.5–2 g hydroxycinnamic acids/kg fresh fruitsCitation20. It has been shown in a recent investigation that daily intake of hydroxycinnamic acids alone is estimated at 211 mg/day and can reach up to 800 mg/dayCitation21,Citation22. It is expected that consumption of plant-enriched hydroxycinnamic acids may reduce the risk of diabetes and its complications. Recent studies have reported the mechanisms underlying the antidiabetic action of hydroxycinnamic acids on the inhibition of α-glucosidaseCitation11, the stimulating pancreatic insulin releaseCitation12, the activation of glucose uptakeCitation23, the stimulating adiponectin releaseCitation24, and the inhibition of protein glycationCitation16. Considering the data obtained from this investigation, we provide a novel mechanism for the antidiabetic activity of cinnamic acid derivatives via the inhibition of PTP1B. It is possible that hydroxycinnamic acids may enhance the plasma glucose-lowering effect in diabetic animals associated with the PTP1B inhibitory activity.
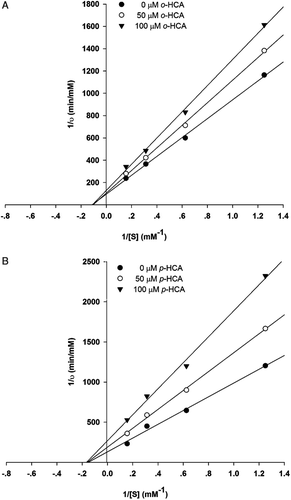
To further explore the inhibitory characteristics of cinnamic acid derivatives, the kinetic assay was performed using Lineweaver-Burk double reciprocal plots. The inhibitory mechanisms on PTP1B by o-hydroxycinnamic acid and p-hydroxycinnamic acid are shown in . A Lineweaver-Burk plot of o-hydroxycinnamic acid and p-hydroxycinnamic acid generated straight lines that had the same intersection on the X-axis, indicating that their inhibitions were of a non-competitive type. It has been reported that the identification of non-competitive inhibitors targeting the allosteric site in PTP1B may present in the alternative approaches to the development of selective PTP1B inhibitorsCitation2,Citation25–27. The results of the present study suggest that o-hydroxycinnamic acid and p-hydroxycinnamic acid are non-competitive inhibitors that can be developed into highly selective inhibitors of PTP1B.
Figure 3. Lineweaver-Burk plots for inhibitory activity of (A) o-hydroxycinnamic and (B) p-hydroxycinnamic on PTP1B.
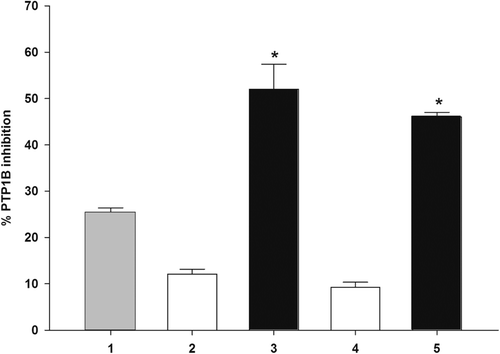
It was of interest to establish whether cinnamic acid derivatives and SOV might interact synergistically or additively on PTP1B. The assay was then performed in the solutions containing SOV alone or in mixture with cinnamic acid derivatives. The combined effects of compound o-hydroxycinnamic acid and p-hydroxycinnamic acid with SOV on PTP1B inhibition are shown in . With the addition of o-hydroxycinnamic acid and p-hydroxycinnamic acid to the assay system with SOV (0.012 µM), the percentage inhibition was significantly increased when compared with SOV or the compounds alone. The percentage inhibition of mixtures was greater than the summing effect of SOV and those compounds. The findings indicate that o-hydroxycinnamic acid and p-hydroxycinnamic acid, together with SOV, synergistically inhibits the PTP1B activity. SOV exhibits a wide variety of insulin-like effects both in vitro and in vivoCitation28. It has been recently reported that SOV inhibits protein tyrosine phosphatase activity and this in turn enhances the activity of protein tyrosine kinases, thus resulting in an enhancement of insulin sensitivity and a prolongation of insulin biological responseCitation29,Citation30. However, it has shown some short- and long-term toxicity problems in diabetic animals, such as the loss of body weight, dehydration, hematological changes, biochemical alterations, and nephrotoxicityCitation31,Citation32. At this point, the researchers trying to exploit its toxicity are attempting to develop the complex-forming capability of vanadium compounds with organic compoundsCitation33. Moreover, there have been several attempts to reduce the toxicity without compromising their biological effects by reducing the dose of vanadate and combining the treatment with herbal medicinesCitation34,Citation35. Our findings suggest that o-hydroxycinnamic acid and p-hydroxycinnamic acid may enable a lower dose of SOV to be used, leading to diminishment of the adverse effect. It is advantageous to apply SOV and hydroxycinnamic acids as a mixture for an inhibitory effect, as the coadministration can reduce the effective doses of these compounds for the same inhibitory effect on PTP1B activity.
Figure 4. The percentages of enzyme inhibition of SOV and its combination with o-hydroxycinnamic and p-hydroxycinnamic on PTP1B. (1): 0.012 µM SOV;(2): 25 μM o-hydroxycinnamic;(3): 25 μM o-hydroxycinnamic + 0.012 µM SOV;(4): 25 μM p-hydroxycinnamic; (5): 25 μM p-hydroxycinnamic + 0.012 µM SOV. Data are expressed as mean ±S.E.M., n =3. * P < 0.01, compared to SOV alone.
Although cinnamic acid derivatives have been investigated regarding the potential mechanisms for glucose-lowering effect, the present study also demonstrates a new mechanism of action via the inhibition of PTP1B activity. On the basis of the above studies, cinnamic acid derivatives can be developed as a PTP1B inhibitor for the treatment of diabetes.
Acknowledgments
We wish to thank the Faculty of Allied Health Sciences, Chulalongkorn University, for financial supporting this study. The authors would also like to thank The Medical Food Research and Development Center and The Research Group of Herbal Medicine for Prevention and Therapeutic of Metabolic diseases, which have been financially and institutionally supported by Chulalongkorn University.
Declaration of interest
The authors report no conflicts of interest.
References
- Drake PG, Posner BI. Insulin receptor-associated protein tyrosine phosphatase(s): role in insulin action. Mol Cell Biochem 1998;182:79–89.
- Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today 2007;12:373–381.
- Wu X, Hardy VE, Joseph JI, Jabbour S, Mahadev K, Zhu L et al. Protein-tyrosine phosphatase activity in human adipocytes is strongly correlated with insulin-stimulated glucose uptake and is a target of insulin-induced oxidative inhibition. Metab Clin Exp 2003;52:705–712.
- Ma YM, Tao RY, Liu Q, Li J, Tian JY, Zhang XL et al. PTP1B inhibitor improves both insulin resistance and lipid abnormalities in vivo and in vitro. Mol Cell Biochem 2011;357:65–72.
- Mohammad A, Wang J, McNeill JH. Bis(maltolato)oxovanadium(IV) inhibits the activity of PTP1B in Zucker rat skeletal muscle in vivo. Mol Cell Biochem 2002;229:125–128.
- Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 2010;11:1365–1402.
- Natella F, Nardini M, Di Felice M, Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J Agric Food Chem 1999;47:1453–1459.
- Lee EJ, Kim SR, Kim J, Kim YC. Hepatoprotective phenylpropanoids from Scrophularia buergeriana roots against CCl(4)-induced toxicity: action mechanism and structure-activity relationship. Planta Med 2002;68:407–411.
- Lee HS. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem 2002;50:1400–1403.
- Liu IM, Hsu FL, Chen CF, Cheng JT. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats. Br J Pharmacol 2000;129:631–636.
- Adisakwattana S, Sookkongwaree K, Roengsumran S, Petsom A, Ngamrojnavanich N, Chavasiri W et al. Structure-activity relationships of trans-cinnamic acid derivatives on alpha-glucosidase inhibition. Bioorg Med Chem Lett 2004;14:2893–2896.
- Adisakwattana S, Chantarasinlapin P, Thammarat H, Yibchok-Anun S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J Enzyme Inhib Med Chem 2009;24:1194–1200.
- Adisakwattana S, Moonsan P, Yibchok-Anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J Agric Food Chem 2008;56:7838–7844.
- Yibchok-anun S, Adisakwattana S, Moonsan P, Hsu WH. Insulin-secretagogue activity of p-methoxycinnamic acid in rats, perfused rat pancreas and pancreatic beta-cell line. Basic Clin Pharmacol Toxicol 2008;102:476–482.
- Chang HK, Hsu FL, Liu IM, Cheng JT. Stimulatory effect of cinnamic acid analogues on alpha1A-adrenoceptors in-vitro. J Pharm Pharmacol 2003;55:833–837.
- Huang DW, Shen SC, Wu JS. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J Agric Food Chem 2009;57:7687–7692.
- Adisakwattana S, Sompong W, Meeprom A, Ngamukote S, Yibchok-Anun S. Cinnamic Acid and its derivatives inhibit fructose-mediated protein glycation. Int J Mol Sci 2012;13:1778–1789.
- Lub ben T, Clampit J, Stashko M, Revillyan JT, Jirousek MR. In vitro enzymatic assays of protein tyrosine phosphatase 1B. In: Enna SJ, Willams M, eds. Current Protocols in Pharmacology. Chichester: Wiley; 2001. P3.8.1–3.8.13.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–747.
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol 2010;48:937–943.
- Radtke J, Linseisen J, Wolfram G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z Ernahrungswiss 1998;37:190–197.
- Radtke J, Linseisen J, Wolfram G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z Ernahrungswiss 1998;37:190–197.
- Clifford MN. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J Sci Food Agric 1999;320:362–372.
- Ohara K, Uchida A, Nagasaka R, Ushio H, Ohshima T. The effects of hydroxycinnamic acid derivatives on adiponectin secretion. Phytomedicine 2009;16:130–137.
- Prabhakar PK, Doble M. Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3T3-L1 adipocytes. J Agric Food Chem 2011;59:9835–9844.
- Wiesmann C, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, Fahr BJ, Zhong M, Taylor L, Randal M, McDowell RS, Hansen SK. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol 2004;11:730–737.
- Seo C, Sohn JH, Ahn JS, Yim JH, Lee HK, Oh H. Protein tyrosine phosphatase 1B inhibitory effects of depsidone and pseudodepsidone metabolites from the Antarctic lichen Stereocaulon alpinum. Bioorg Med Chem Lett 2009;19:2801–2803.
- Sun T, Wang Q, Yu Z, Zhang Y, Guo Y, Chen K, Shen X, Jiang H. Hyrtiosal, a PTP1B inhibitor from the marine sponge Hyrtios erectus, shows extensive cellular effects on PI3K/AKT activation, glucose transport, and TGFbeta/Smad2 signaling. Chembiochem 2007;8:187–193.
- Fantus IG, Deragon G, Lai R, Tang S. Modulation of insulin action by vanadate: evidence of a role for phosphotyrosine phosphatase activity to alter cellular signaling. Mol Cell Biochem 1995;153:103–112.
- Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 1997;272:84–851.
- Thompson KH, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C. Vanadium treatment of type 2 diabetes: a view to the future. J Inorg Biochem 2009;103:554–558.
- Ramasarma T. Vanadium complexes with insulin-mimetic actions-A second line of protection against diabetes. Indian J Clin Biochem 1996;11:92–107.
- Domingo JL. Vanadium and Tungsten derivatives as antidiabetic agents: a review of their toxic effects. Biol Trace Elem Res 2002;88:97–112.
- Barrio DA, Etcheverry SB. Potential use of vanadium compounds in therapeutics. Curr Med Chem 2010;17:3632–3642.
- Yadav UC, Moorthy K, Baquer NZ. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem 2005;268(1-2):111–20.
- Yadav UC, Moorthy K, Baquer NZ. Effects of sodium-orthovanadate and Trigonella foenum-graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J Biosci 2004;29:81–91.