Abstract
It was found by virtual screening that 3-amino-1H-pyrazolo[3,4-b]quinolines could have wide protein kinase inhibitory activity. Amides of titled amines and thioureas were synthesized regioselectively. 3-Amino-7-methoxy-1H-pyrazolo[3,4-b]quinoline demonstrated in vitro significant inhibitory activity on bacterial serine/threonine protein kinases (inhibition of resistance to kanamycin in Streptomyces lividans regulated by protein kinases). The studies of Structure Activity Relationship (SAR) showed that the substitution of the NH2 group and 1-NH of pyrazole ring or aromatic ring at the position 6 decreased or removed inhibitory activity.
Introduction
Bacterial serine/threonine specific protein kinase(s) (BSTK) are important factors for cellular functions such as growth, differentiation, pathogenicity, biofilm formation and secondary metabolism. BSTK are necessary in most important steps of bacterial pathogenesisCitation1. Also, some BSTK are involved in virulence of Mycobacteria and resistance of Streptomyces to aminoglycoside antibioticsCitation2,Citation3. Since it is evident that BSTK play an indispensable role in some prokaryotic organisms, these protein kinases are considered as attractive antibacterial drug targetsCitation1,Citation4. It was also shown that protein kinase inhibitors of eukaryotic serine/threonine protein kinase(s) (ESTK) could be active against some BSTKCitation1,Citation4,Citation5.
Several years ago, we have synthesized a combinatorial library of compounds to find inhibitors of ESTKCitation5. Surprisingly, we found that some of bis-3,4-bis(indol-1-yl)maleimide analogues of known inhibitor Bis I ( and ) are active in tests on Streptomyces lividans with overexpressed aminoglycoside phosphotransferase type VIII (aphVIII) to kanamycin by inhibiting BSTK mediated aphVIII phosphorylationCitation4,Citation5. These results correlate with the aforementioned trend. In recent years some efforts have been directed toward the synthesis of combinatorial libraries of 1H-pyrazolo[3,4-b]quinoline compounds as eukaryotic protein kinase C inhibitors. It was found that congeners of pyrazolo[2,3-b]quinoline have inhibitory activity on several ESTK from several species with wide ranging activity and relatively low specificity. For example, CID 665826 (1) and related compounds reveal inhibitory activity on GSK-3, CDK and ATM kinasesCitation6. Compound 2 built on the 1H-pyrazolo[3,4-b]pyridine fragment showed inhibition on disease-relevant protein kinases DYRK1A, CDK5 and GSKCitation7. 1H-Pyrazolo[3,4-d]pyrimidines were selected in a research based on pharmacophore model for the design of EGF-R tyrosine kinase inhibitorsCitation8. These compounds were compared with ATP in the EGF-R tyrosine kinase active site by computer-aided design methods, then they were synthesized and showed inhibitory activity against both tyrosine kinases EGF-R, v-Abl and serine/threonine kinases c-Src, PKC-α and CDK1Citation8. Often in aforementioned studies it was assumed that low ESTK specificity and wide ranging inhibitory activity depends on just one active fragment – 3-amino-1H-pyrazolo[3,4-b]pyridine with one hydrogen of the NH2 group and a NH group of pyrazole ring (). There are a lot of data describing antibacterial and antifungal tests for the compounds constructed on 1H-pyrazolo[3,4-b]quinoline scaffold. All described compounds have showed sufficiently poor Minimal Inhibition Concentration (MIC) values (>32 μ/mL) in comparison with regular fluoroquinolones and antibioticsCitation9–12. We have not found if there is some link between antibacterial and BSTK inhibitory activity for congeners of 1H-pyrazolo[3,4-b]quinoline.
Table 1. 1H-pyrazolo[3,4-b]quinolin 4-7 BSTK inhibitory activity. Predicted vs. experimental activity.
Figure 1. The compounds possessed wide range of inhibitory activity on ESTK. ESTK, eukaryotic serine/threonine protein kinase(s).
In the present study we describe the synthesis of several new 1H-pyrazolo[3,4-b]quinoline amides by regioselective acylation to reveal the inhibitors of BSTK. The relationship between in vitro BSTK inhibitory activity and antibacterial (including antimycobacterial) properties of synthesized compounds is discussed.
Materials and methods
Chemistry
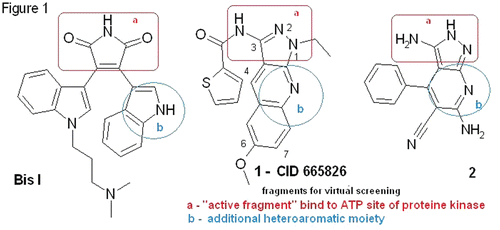
All chemicals were from Sigma-Aldrich or Acros (USA) and were used without further purification. Analytical TLC for checking of homogeneity of the compounds was made using TLC on silica gel-protected aluminum sheets (Type 60 F254, Merck) with chloroform – methanol as a mobile phase and the spots were detected by exposure to a UV-lamp at 254 nm. The structures of all synthesized compounds were confirmed by 400 MHz 1H-NMR spectra (Varian VXR-400) and high resolution ESI mass-spectrometry (microTOF-Q II, Bruker Daltonics GmbH). 1H-NMR spectra were recorded in dmso-D6. 2-Chloro-3-cyanoquinolines (3 a-d) were prepared according to the well documented methods and used as starting compoundsCitation9,Citation13,Citation14.
1 H-Pyrazolo[3,4-b]quinolines-3-amines (4a-d). General procedure. 2-Chloro-3-cyanoquinolines (3a-d) (0.01 mol) was stirred in 5 mL dry dimethylformamide (DMF) at 90–95°C and N2H4.H2O (0,075 mol) was added dropwise within 15 min, than this mixture was stirred at 95–105°C for 1.5 h. Target 1H-pyrazolo[3,4-b]quinoline 4 was filtered off after cooling of the reaction mixture at 0–5°C for 2 to 3 h, washed with methanol and dried on air, then washed with dichloromethane and dried on air.
6,7-Ethylendioxy-1H-pyrazolo[3,4-b]quinolines-3-amine (4a). Yield 89%. δ: 4.35 (4H, m, -O-CH2-CH2-O-), 5.74 (2H, s, NH2), 7.19 (1H, s, H-5), 7.38 (1H, s, H-8), 8.49 (1H, s, H-4), 11.46 (1H, s, NH-1). Calculated molecular weight (MW Calc). for C12H10N4O2 242.0804. Found in ESI-ms 243.0899 (M+H);
6,7-Methylendioxy-1H-pyrazolo[3,4-b]quinolin-3-amine (4b). Yield 87%. δ: 5.78 (2H, s, NH2), 6.20 (2H, s, -O-CH2-O-), 7.10 (1H, s, H-5), 7.34 (1H, s, H-8), 8.41 (1H, s, H-4), 11.12 (1H, s, NH-1). MW Calc. for C11H8N4O2 228.0746. Found in ESI-ms 229.0726 (M+H);
7-Methoxy-1H-pyrazolo[3,4-b]quinolin-3-amine (4c). Yield 77%. δ: 3.89 (3H, s, CH3), 5.82 (2H, s, NH2), 7.00 (1H, dd, J 4.0, 12.0, H-6), 7.16 (1H, d, J 4.0, H-8), 7.86 (1H, d, J 12.0, H-5), 8.61 (1H, s, H-4), 11.64 (s, 1H, NH-1). MW Calc. for C11H10N4O 214.0855. Found in ESI-ms 215.0813 (M+H);
6-Methoxy-1H-pyrazolo[3,4-b]quinolines-3-amine (4d). Yield 72%. %. δ: 3.88 (3H, s, CH3), 5.85 (2H, s, NH2), 7.33 (1H, d, J 4.0, H-5), 7.37 (1H, dd, J 4.0, 12.0, H-7), 7.77 (1H, d, J 12.0, H-8), 8.60 (1H, s, H-4), 11.64 (1H, s, NH-1). MW Calc. for C11H10N4O 214,0855. Found in ESI-ms 215,0870 (M+H);
N-(1H-pyrazolo[3,4-b]quinolin-3-yl)benzamides (5a-f). General procedure. Pyrazolo[3,4-b]quinoline (4a,c) (0.001 mol) was dissolved in about 7 mL of mixture DMF and triethylamine (0.01 mol). Appropriate benzoyl chloride (0.00105 mol) dissolved in 2 mL dry 1,4-dioxane was added to the reaction mixture dropwise at 10°C and the mixture was stirred at 50°C for 4 h. Ice water was added to reaction mixture and product was filtered off. Precipitate was dried on air and crystallized from DMF-methanol.
N-(7-Methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)benzamide (5a). Yield 90%. δ: 3.93 (3H, s, CH3), 7.11 (1H, dd, J 2.0, 8.0, H-6), 7.27 (1H, d, J 2.0, H-8), 7.56 (2H, t, J 8.0, H-3′,5′), 7.64 (1H, 7, J 8.0, H-4′), 8.03 (1H, d, J 8.0, H-5), 8.12 (2H, d, J 8.0, H-2′,6′), 8.90 (1H,s, H-4), 11.19 (1H, s, NH) 13.11 (1H, s, HN-C=O). MW Calc. for C18H14N4O2 318.1117. Found in ESI-ms 319.1149 (M+H);
4-Chloro-N-(7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)benzamide (5b). Yield 82%. δ: 3.94 (3H, s, CH3); 7.11 (1H, dd, J 3.0, 12.0, H-6), 7.27 (1H, d, J 3.0, H-8); 7.65 (2H, d, J 8.0 Hz, H-2’,6’), 8.03 (1H, d, J 12.0, H-5), 8.13 (2H, d, J 8.0, H-3′,5′), 8.89 (1H, s, H-4), 11.29 (1H, s, NH), 13.13 (1H, s, HN-C=O). MW Calc. for C18H13ClN4O2 352.0727. Found in ESI-ms 353.0737 (M+H) 100%, 355.0709 (M+H) 30%.
3-Chloro-N-(7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)benzamide (5c). Yield 74%. δ: 3.94 (3H, s, CH3), 7.11 (1H, dd, J 2.2, 9.2, H-6), 7.29 (1H, d, J 2.2, H-8), 7.61 (1H, t, J 8.0, H-5′), 7.71 (1H, d, J 8.0, H-6′), 8.02 (1H, d, J 9.2, H-5), 8.07 (1H, d, J 8.0, H-4′), 8.17 (1H, s, H-2′), 8.90 (1H, s, H-4), 11.27 (1H, s, NH), 13.02 (1H, s, HN-C=O). MW Calc. for C18H13ClN4O2 352.0727. Found in ESI-ms 353.0708 (M+H) 100%, 355.0773 (M+H) 30%.
3-Chloro-N-(6,7-methylendioxy-1H-pyrazolo[3,4-b]quinolin-3-yl)benzamide (5d). Yield 69%. δ: 4.38 (4H, m, -O-CH2-CH2-O-), 7.30 (1H, s, H-5), 7.54 (1H, s, H-8), 7.60 (1H, t, J 8.0, H-5′), 7.71 (1H, d, J 8.0, H-6′), 7.94 (1H, s, H-2′), 8.06 (1H, d, J 8.0, H-4′), 8.75 (1H, s, H-4), 11.32 (1H, s, NH), 13.14 (1H, s, HN-C=O). MW Calc. for C19H13ClN4O3 380.0676. Found in ESI-ms 381.0684 (M+H) 100%, 383.0663 (M+H) 30%.
N-(7-Methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)-2-(4-nitrophenyl)acetamide (5e). Yield 78%. δ: 3.92 (3H, s, CH3), 3.99 (2H, s, CH2), 7.07 (1H, dd, J 2.4, 9.2, H-6), 7.22 (1H, d, J 2.4, H-8), 7.68 (2H, d, J 8.8, H-2′,6′), 7.97 (1H, d, J 9.2, H-5), 8.23 (2H, d, J 8.8, H-3′’,5′), 8.91 (1H, s, H-4); 11.19 (1H, s, NH), 13.00 (1H, s,HN-C=O). MW Calc. for C19H15N5O4 377.1124. Found in ESI-ms 378.1185 (M+H);
1-Benzoyl-1H-pyrazolo[3,4-b]quinolin-3-amine (6 a-d). General procedure. Appropriate aryl carbonic acid (0.00105 mol) was dissolved in 2 mL 1,4-dioxane and CDI (0.0015 mol) was added, then the reaction mixture was stirred at room temperature for 1h. Pyrazolo[3,4-b]quinoline (4a,c) (0.001 mol) was added to the reaction mixture and about 5 mL of DMF, then this mixture was stirred at 60°C overnight. Ice water was added to the reaction mixture and the product was filtered off. Precipitate was dried on air and crystallized from DMF-methanol.
1-(3-Chlorobenzoyl)-7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-amine (6a). Yield 67%. δ: 3.96 (3H, s, CH3), 6.80 (2H, s, NH2), 7.27 (1H, dd, J 2.2, 9.0, H-6), 7.38 (1H, d, J 2.2, H-8), 7.54 (1H, t, J 7.9, H-5′), 7.63 (1H, d, J 7.9, H-4′), 7.79 (1H, d, J 7.9, H-6′), 7.88 (1H, s, H-2′), 8.03 (1H, d, J 9.0, H-5), 8.83 (1H, s, H-4). MW Calc. for C18H13ClN4O2 352.0727. Found in ESI-ms 353.0705 (M+H) 100%, 355.0776 (M+H) 30%.
1-Isonicotinoyl-7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-amine (6b). Yield 80%. δ: 3.96 (3H, s, CH3), 6.84 (2H, s, NH2), 7.27 (1H, dd, J 2.2, 9.2, H-6), 7.37 (1H, d, J 2.2, H-8), 7.73 (2H, d, J 5.8, H-2′,6′), 8.03 (1H, d, J 9.2, H-5), 8.74 (2H, d, J 5.8, H-3′,5′), 8.83 (1H, s, H-4). MW Calc. for C17H13N5O2 319.1069. Found in ESI-ms 320.1210 (M+H).
1-Nicotinoyl-7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-amine (6c). Yield 81%. δ: 3.96 (3H, s, CH3), 6.82 (2H, s, NH2), 7.27 (1H, dd, J 2.4, 9.2, H-6), 7.36 (1H, d, J 2.4, H-8), 7.55 (1H, dd, J 4.8, 7.8, H-5’), 8.03 (1H, d, J 9.2, H-8), 8.23 (1H, d, J 7.8, H-6′), 8.33 (1H, d, J 4.8, H-4′), 8.83 (1H, s, H-4), 9.01 (1H, s, H-2′). MW Calc. for C17H13N5O2 319.1069. Found in ESI-ms 320.1213 (M+H).
1-(5-Nitro-2-furoyl)-6,7-methylendioxy-pyrazolo[3,4-b]quinolin-3-amine (6d). Yield 28%. δ: 4.42 (4H, m, -O-CH2-CH2-O-), 7.00 (2H, s, NH2), 7.46 (1H, s, H-8), 7.56 (1H, s, H-5), 7.86 (1H, d, J 3.8, H-3′), 8.03 (1H, d, J 3.8, H-4′), 8.72 (1H, s, H-4). MW Calc. for C17H11N5O6 381.0709. Found in ESI-ms 382.0702 (M+H).
N-(1H-Pyrazolo[3,4-b]quinolin-3-yl)thiourea (7a,b). General procedure. Amine 4a (0.001 mol) was dissolved in 5 mL DMF and appropriate isothiocyanate (0.00105 mol) was added to the reaction mixture dropwise. This mixture was stirred at 90°C for 1 h. Product was filtered off, washed with methanol and dried on air.
N-ethyl-N′-(7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)thiourea (7a). Yield quantitative δ:1.23 (3H, t, J 7.2, CH3), 3.64 (2H, q, J 6.0, 7.2, CH2), 3.93 (3H, s, CH3); 7.11 (1H, d, J 9.6, H-6), 7.24 (1H, s, H-8), 7.91 (1H, d, J 9.6, H-5), 9.21 (1H, s, H-4), 10.11 (1H, t, J 6.0, S=C-NH-), 11.21 (1H, s, NH), 12.91 (1H, s, -NH-C=S). MW Calc. for C14H15N5OS 301.0997. Found in ESI-ms 302.1127 (M+H).
N-(7-methoxy-1H-pyrazolo[3,4-b]quinolin-3-yl)-N′-phenylthiourea (7b). Yield quantitative. δ: 3.90 (3H, s, CH3); 7.04 (1H, d, J 10.0, H-6), 7.14 (1H, s, H-8), 7.65 (5H, m, -Ph), 7.80 (1H, d, J 10.0, H-5), 9.00 (1H, s, H-4), 10.01 (1H, s, S=C-NH-), 11.00 (1H, s, NH), 12.85 (1H, s, -NH-C=S). MW Calc. for C18H15N5OS 349.0997. Found in ESI-ms 350.1068 (M+H).
Biological methods.
A strain of St. lividans harboring pSU23 plasmid carrying the aphVIII gene (St. lividans aphVIII+ strain) was used as a test culture to analyze the inhibitors of BSTK. The gene product, aminoglycoside phosphotransferase aphVIII, phosphorylates and inactivates kanamycin, thereby rendering bacteria resistant to this antibiotic. The activity of aphVIII is dependent on phosphorylation by a BSTK. The kinase inhibitory activity of new compounds was investigated by the paper disk method. Paper disks (7 mm in diameter) containing kanamycin (5 μg/disk) and various amounts of tested compounds were applied on the plates with logarithmically growing St. lividans aphVIII+ and incubated at 28°C for 20 h. The halo diameters formed after exposure of bacteria with the combination of kanamycin and potential inhibitors were compared with the respective zone for kanamycin alone or the combinations of kanamycin and known inhibitor of serine/threonine protein kinases, Bis I. To rule out the cytotoxicity of novel compound as a factor that might increase the diameter of the zone of lysis, we used subtoxic concentrations of tested compounds. The subtoxic concentrations were approximately two times lower than the minimal toxic concentrations, i.e. minimal doses that inhibited growth of test bacteria. At subtoxic concentrations no inhibition of bacterial growth was observed. The detailed methods are given in previous publicationCitation2,Citation5.
MIC evaluation
The antibacterial activity of the synthesized compounds 4–7 was evaluated in vitro against panel of microbes by well documented bioassays MIC (minimal inhibition concentration in μg/mL). The detailed methods are given in previous publicationCitation9–12. The antimycobacterial activity was evaluated by the agar diffusion test with Mycobacterium smegmatis as described previouslyCitation2,Citation5.
Results and discussion
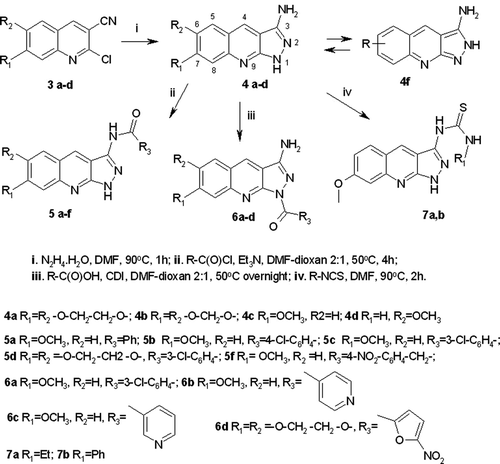
Since there is no information to provide reliable SAR that could be a basis for the directed synthesis of 3-amino-1H-pyrazolo[3,4-b]quinolines BSTK inhibitors, we implemented virtual screening by PASS-onlineCitation15. This Internet resource provides probability of wide ranging pharmacological activities for individual compounds. Software PASS-online are based on probabilistic methodology of experimental activity for chemical fragmentsCitation15. In our opinion, a wide range of inhibitory activity of the discussed compounds could be provided by the presence of two active fragments a and b (). We kept these two fragments in each virtual query to search for a positive probability of “protein kinase inhibitor” (with low specificity and wide ranging activity against several classes of protein kinases). Then we have selected several compounds for the synthesis ().
We have synthesized a set of quinolines 3a–d with electron-donating substituents, 3-amino-1H-pyrazolo [3,4-b]quinolines 4a–d, amides 5 a–e, 6a–d and thioureas 7a,b to reveal the influence of the substitution both in aromatic and pyrazole rings on inhibitory activity (). For these purposes we improved the conditions of the synthetic procedures and regioselective acylation for the 3-NH2 and 1-NH groups of 4. We found that acylation of 4 by aryl carboxylic acid chlorides in the presence of triethylamine or pyridine led to 3-N-acylamides 5 which is in agreement with the previously published dataCitation11. Interestingly, when 4 had bulky aromatic fragment in the 4-position (compound 2), the substitution by aryl acyl moiety was regioselectively directed to the 9-N positionCitation16. The reaction of aryl carboxylic acids, CDI and amine 4 led regioselectively to the 1-N-acyl derivatives of 6. Castro reagents (BOP or PyBOP) as acylating agents led to the mixtures of amides 5 and 6 in aprotic polar solvents (). In these solvents isothiocyonates reacted quantitatively and regioselectively with 3-NH2 group of amines 4 to give thioureas 7 (). Regioselectivity of the acylation reaction was confirmed by NMR of amides 5 and 6: 3-NH2 group in 4 had two hydrogen atoms signal at 5.80 ppm, whereas amide 5 had one hydrogen atom signal at 13.00 ppm. Likewise, N-H at the 1-position of 4 had signal at 11.00 ppm, but amide 6 had only the signal of two hydrogen atoms of 3-NH2 group at 5.80 ppm. Since nitrogen of pyrazole ring is stronger nucleophile than nitrogen of 3-NH2 group, each of aminopyrazoles 4 exists as a single tautamer ()Citation17,Citation18. Another factor that influences regioselectivity is that the acyl chloride is stronger electrophile than the acylimidazole formed in the reaction with CDI. It demonstrates that the acylation of 3-NH2 group goes under kinetic control, and the acylation of 1-NH in the pyrazole ring runs under thermodynamic control.
The in vitro inhibitory activity () of compounds 4–7 against BSTK was determined by a method based on disappearance of resistance to kanamycin of St. lividance as there are several BSTK expressed in this strainCitation4. In order to increase similarity with CID 665826 according to the PASS-online prediction, amines 4 were acylated with aryl carboxylic acids. In another series of compounds the 3-NH2 groups of 4 were substituted by thiourea fragments. In both cases the bioassay did not show any activity. Only amines 4 have demonstrated some inhibitory activity against BSTK. Among all amines, amine 4c with 7-MeO group has manifested significant activity in the comparison with the known BSTK inhibitor Bis I. 6-MeO group in 4d and substitution at the 6,7-positions of 4a and b led to the decrease of activity. Perhaps, the difference in the substitution in aromatic ring of 4 may influence on binding in BSTK active site (). These data suggest that the activity of amines 4 depends on both the presence of unaltered active fragments a and b () and the position of substitution in aromatic ring. This could explain a wide range of inhibitory activity on BSTK in our bioassay.
shows the comparison of predicted and experimental results. Since PASS-online data for broad “protein kinase inhibitory activity” were collected for ESTK inhibitors but we have used these data for BSTK inhibitors, we obtained acceptable correlation with R2 = 0.31 only for amines 4.
All compounds 4–7 were tested in vitro on the panel of Gr+ or Gr− bacteria. All of it had no antibacterial activity with MIC >64 μg/mL and the lack of inhibition at concentration 1000 nM/disk in antimycobacterial test. Only 6d demonstrated both some inhibitory activity on BSTK and antibacterial (including antimycobacterial) activity against Gr− bacteria () what can be explained by the presence of 5-nitrofuryl antibacterial moiety. The lack of antibacterial activity of 4c can be explained by different specificities BSTK of St. lividans and test microorganisms in our in vitro experiments.
Table 2. Antibacterial properties as a MIC μg/mL.
Conclusion
It was found by virtual screening with PASS-online that 3-amino-1H-pyrazolo[3,4-b]quinolines could have broad protein kinase inhibitory activity. For synthetic purposes we have elaborated reliable and simple regioselective methods of acylation to synthesize the library of pyrazolo[3,4-b]quinolines congeners for testing inhibitory activity on BSTK. It was demonstrated that additionally to the described inhibition of ESTK, compounds 4 with unsubstituted 3-NH2 and 1-NH groups of pyrazole ring had inhibitory activity against BSTK, amine 4c with 7-OMe group being most active. Amides 5, 6 and thioureas 7 demonstrated lack of this activity. It suggests that there is no common pharmacophore among 3-amino-1H-pyrazolo[2,3-b]quinolines amides or thioureas with inhibitory activity on BSTK and antibacterial properties.
Acknowledgment
We gratefully acknowledge the excellent technical assistance of Dr. Korolev A.M. for ESI – mass spectrometry and Dr. Luzikov Y.N. for NMR spectra; both are from Gause institute of new antibiotics.
Declaration of interest
Authors report no conflicts of interest.
References
- Kurosu M, Begari E. Bacterial protein kinase inhibitors. Drug Development Res 2010;71 168–187.
- Bekker OB, Elizarov SM, Alekseeva MT, Liubimova IK, Danilenko VN. [Ca2+-dependent modulation of antibiotic resistance in Streptomyces lividans 66 and Streptomyces coelicolor A3(2)]. Mikrobiologiia 2008;77:630–638.
- Petrícková K, Petrícek M. Eukaryotic-type protein kinases in Streptomyces coelicolor: variations on a common theme. Microbiology (Reading, Engl) 2003;149:1609–1621.
- Danilenko VN, Osolodkin DI, Lakatosh SA, Preobrazhenskaya MN, Shtil AA. Bacterial eukaryotic type serine-threonine protein kinases: from structural biology to targeted anti-infective drug design. Curr Top Med Chem 2011;11:1352–1369.
- Danilenko VN, Simonov AY, Lakatosh SA, Kubbutat MH, Totzke F, Schächtele C et al. Search for inhibitors of bacterial and human protein kinases among derivatives of diazepines[1,4] annelated with maleimide and indole cycles. J Med Chem 2008;51:7731–7736.
- Electronic Resources. Available at: http://pubchem.ncbi.nlm.nih.gov/assay. Accessed March 2012.
- Chioua M, Samadi A, Soriano E, Lozach O, Meijer L, Marco-Contelles J. Synthesis and biological evaluation of 3,6-diamino-1H-pyrazolo[3,4-b]pyridine derivatives as protein kinase inhibitors. Bioorg Med Chem Lett 2009;19:4566–4569.
- Traxler P, Bold G, Frei J, Lang M, Lydon N, Mett H et al. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J Med Chem 1997;40:3601–3616.
- Parekh N, Maheria K, Patel P, Rathod M. Study on antibacterial activity for multidrug resistance stain by using phenyl pyrazolones substituted 3-amino-1H-pyrazolon[3,4-b]quinoline derivative in vitro condition. Inter J Pharm Tech Res 2011;3:540–548.
- Selvi ST, Nadaraj V, Mohan S, Sasi R, Hema M. Solvent free microwave synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinolines. Bioorg Med Chem 2006;14:3896–3903.
- Amin MAS, Ismail MM, Barakat SES, Abdul-Rahman AAA, Bayomi AH, El-Gamal KMA. Synthesis and antimicrobial activity of some new quinoline and 1H-pyrazolo[3,4-b]quinoline derivatives. Bull Pharm Sci Assiut Univ 2004; 27:237–245.
- el-Sayed OA, Aboul-Enein HY. Synthesis and antimicrobial activity of novel pyrazolo[3,4-b]quinoline derivatives. Arch Pharm (Weinheim) 2001;334:117–120.
- Abdel-Rahman AE, Bakhite EA, Abdel-Moneam MI, Mohamed TA. Synthesis and antibacterial activities of some new thieno[2,3-b]quinolines. Phosphorus, Sulfur and Silicon 1992;73:219–227.
- Afghan A, Baradarani MM, Jouleb JA. Efficient syntheses of 1,3-unsubstituted 1H-pyrazolo[3,4-b]quinolines. ARKIVOC 2009:20–30.
- Geronikaki A, Druzhilovsky D, Zakharov A, Poroikov V. Computer-aided prediction for medicinal chemistry via the Internet. SAR QSAR Environ Res 2008;19:27–38.
- Chaczatrian K, Chaczatrian G, Danel A, Tomasik P. The synthesis of 4-aryl-1H-pyrazolo[3,4-b]quinolines by cyclization of 4-arylidenepyrazolin-5-ones with anilines. ARKIVOC 2001:63–69.
- Elnagdi MH, Abdel-Galil FM, Riad BY, Elgemei GEH. Recent development in chemistry of 3(5)-aminopyrazoles. Heterocycles 1983; 20:2437.
- Stadlbauer W, Hojas G. Synthesis of 4-azido-3-diazo-3H-3H-pyrazolo[3,4-b]quinoline from 3-amino-4-hydrazino-1H-pyrazolo[3,4-b]quinoline. J Chem Soc Perkin Trans I 2000:3085–3087.