Abstract
Novel pyridine, thiophene, thiazole, chromene and benzochromene derivatives bearing a N,N-dimethylbenzenesulfonamide moiety 6–20 were synthesized. The target compounds were obtained through employing a series of heterocyclization reactions utilizing the key intermediate hydrazide hydrazone derivative 3. The structures of the newly synthesized compounds were confirmed by elemental analyses, IR, 1H-NMR and 13C-NMR spectral data. All the newly synthesized compounds were evaluated for their in vitro antiproliferative activity against the human breast cancer cell line MCF-7. Biological screening results showed that sulfonamides 6, 9, 11, 16 and 17 with IC50 values 21.81, 25.50, 20.60, 25.83 and 31.20 μM, respectively, possessed higher antiproliferative activity compared to doxorubicin, IC50 value 32.00 μM, as position control. Molecular docking study was also performed to assess the binding mode of the synthesized sulfonamides with their potential biomolecular target, carbonic anhydrase IX (CA IX), which is usually highly expressed in some types of cancer cells.
Introduction
According to WHO population-based data, cancer is a leading cause of mortality worldwide accounting for almost 13% of all deathsCitation1. Among all types of cancer, lung, breast, colorectal, stomach and prostate cancers are the underlying causes for the majority of cancer deathsCitation1. Hitherto, chemotherapy remains one of the therapeutic strategies adopted worldwide for the management of cancer either alone or in conjunction with surgery and/or radiotherapy. Current in clinical use anticancer agents suffer from a number of drawbacks correlated to drugs’ associated side effects and/or tumors’ multi-drug resistanceCitation2,Citation3. Hence, it obviously is still of interest to search for new bioactive molecules having anticancer activity.
From the chemical and structural point of view, literature survey showed that sulfonamideCitation4, hydrazoneCitation5 and sulfoneCitation6 bearing molecules play an important role for the anticancer activity. In addition, various compounds with a heterocyclic backbone scaffold demonstrated promising anticancer activity. For example, a number of pyridine derivatives were claimed to possess interesting anticancer activitiesCitation7. On the other hand, thiophenesCitation8 and thiazolesCitation9 have been reported to possess interesting biological and pharmacological activities where several derivatives of this ring are used as anticancer agents. Moreover, it is established that chromene and benzochromene derivatives can exhibit a wide spectrum of biological activities, especially anticancer activitiesCitation10.
Sulfonamides anticancer activity has been in many instances attributed to inhibition of carbonic anhydrase enzymesCitation4. Carbonic anhydrases (CA, EC 4.2.1.1) embody a family of Zn based metalloenzymes that catalyzes the conversion between carbon dioxide and bicarbonate with generation of protons. The carbonic anhydrase isozyme IX (CA IX) is reported to be associated with tumorogenesis being highly overexpressed in hypoxic tumors with limited expression in normal tissuesCitation11. CA IX inhibitors have been shown to display promising anticancer activity in addition to having fewer side effects compared to other anticancer drugs. Many research endeavors have reported sulfonamide bearing molecules as promising anticancer agents acting through inhibition of carbonic anhydrase IXCitation11.
As part of our ongoing research program directed towards developing new varieties of heterocyclic ring systems with potential anticancer activity especially those bearing sulfonamide groupsCitation4,Citation8–10, we herein report the design and synthesis of novel N,N-dimethylbenzenesulfonamide derivatives with various heterocyclic backbone scaffolds. Also, the results for the in vitro antiproliferative screening of the newly synthesized compounds against the breast cancer cell line MCF-7 are presented. Finally, docking data with CA IX as a potential molecular target for these molecules is reported as well.
Materials and methods
Chemistry
Melting points (°C, uncorrected) were determined in open capillaries on a Gallen Kemp melting point apparatus (Sanyo Gallen Kemp, Southborough, UK). Pre-coated silica gel plates (silica gel 0.25 mm, 60 G F 254; Merck, Darmstadt, Germany) were used for thin-layer chromatography, dichloromethane/methanol (9.5:0.5 mL) mixture was used as a developing solvent system. IR spectra were recorded employing KBr discs and using an IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). NMR spectra in (DMSO-d6) were recorded on Bruker Ac-500 ultra shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 500 MHz, using TMS as internal standard. Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All compounds were within ±0.4 % of the theoretical values.
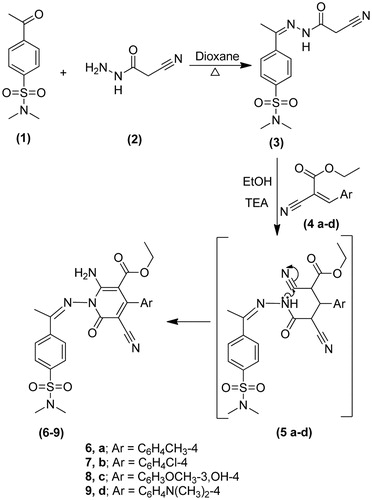
4-(1-(2-(2-Cyanoacetyl)hydrazono)ethyl)-N,N-dimethylbenzenesulfonamide (3): A solution of 2-cyanoacetohydrazide 2 (1.0 g, 0.01 mol) and 4-acetyl-N,N-dimethylbenzenesulfonamide 1 (2.3 g, 0.01 mol) in dioxane (20 mL) was refluxed for 2h. the reaction mixture was poured onto ice/water and the solid product formed was collected by filtration and recrystallized from ethanol to give 3. Yield, 89%; m.p. 150.3 °C; IR (KBr, cm−1): 3390 (NH), 3066 (CH arom.), 2965, 2854 (CH aliph.), 2222 (C≡N), 1669 (C=O), 1620 (C=N), 1394, 1166 (SO2). 1H-NMR (DMSO-d6) δ: 0.9 [s, 3H, CH3], 2.3 [s, 6H, N(CH3)2], 4.1 [s, 2H, CH2], 7.0–7.9 [m, 4H, Ar-H], 8.2 [s, 1H, NH, D2O exchangeable]. 13C-NMR (DMSO-d6): 12.9, 28.3, 36.8 (2), 117.4, 128.6 (2), 129.8 (2), 134.9, 144.7, 166.8, 171.1. Anal. Calcd For C13H16N4O3S (308.36): C, 50.64; H, 5.23; N, 18.17. Found: C, 50.37; H, 5.56; N, 18.48.
General procedure for the preparation of: Ethyl 2-amino-5-cyano-1-(1-(4-(N,N-dimethylsulfamoyl)phenyl)ethylidene-amino)-6-oxo-4-substituted-1,6-dihydropyridine-3-carboxylate (6–9): A mixture of 3 (3.08 g, 0.01 mol) and ethyl α-cyanocinnamate derivatives (4a–d) (0.01 mol) in dioxane (30 mL) containing triethylamine (1 mL) was refluxed for 5 h. The reaction mixture was poured onto ice/water and the formed solid was recrystallized from dioxane to give 6–9, respectively.
Ethyl 2-amino-5-cyano-1-(1-(4-(N,N-dimethylsulfamoyl)phenyl)ethylidene-amino)-6-oxo-4-p-tolyl-1,6-dihydropyridine-3-carboxylate (6): Yield, 78%; m.p. 188.5 °C; IR (KBr, cm−1): 3346, 3280 (NH2), 3076 (CH arom.), 2970, 2836 (CH aliph.), 2216 (C≡N), 1730, 1681 (2C=O), 1610 (C=N), 1396, 1161 (SO2). 1H-NMR (DMSO-d6) δ: 1.1 [s, 3H, CH3], 1.2 [t, 3H, J = 6.0 Hz, CH3 ethyl], 2.2 [s, 3H, CH3 tolyl], 2.6 [s, 6H, N(CH3)2], 4.3 [q, 2H, J = 6.0 Hz, CH2 ethyl], 7.3–8.0 [m, 10H, Ar-H + NH2]. Citation13C-NMR (DMSO-d6): 12.9, 14.6, 25.6, 37.5 (2), 62.7, 94.1, 115.3, 116.8, 125.8 (2), 126.0 (2), 128.4 (2), 129.7, 130.9 (2), 135.0, 135.7, 143.6, 158.1, 163.6, 165.3, 168.8, 169.1. Anal. Calcd For C26H27N5O5S (521.59): C, 59.87; H, 5.22; N, 13.43 Found: C, 59.59; H, 5.46; N, 13.10.
Ethyl 2-amino-4-(4-chlorophenyl)-5-cyano-1-(1-(4-(N,N-dimethylsulfamoyl)phenyl)-ethylideneamino)-6-oxo-1,6-dihydropyridine-3-carboxylate (7): Yield, 68%; m.p. 194.1 °C; IR (KBr, cm−1): 3420, 3380 (NH2), 3090 (CH arom.), 2960, 2840 (CH aliph.), 2204 (C≡N), 1740, 1676 (2C=O), 1626 (C=N), 1394, 1161 (SO2), 707 (C-Cl). 1H-NMR (DMSO-d6) δ: 1.1 [s, 3H, CH3], 1.2 [t, 3H, J = 5.5 Hz, CH3 ethyl], 2.6 [s, 6H, N(CH3)2], 4.2 [q, 2H, J = 5.5 Hz, CH2 ethyl], 7.2–8.0 [m, 10H, Ar-H + NH2]. Citation13C-NMR (DMSO-d6): 14.0, 14.8, 37.5 (2), 62.4, 89.6, 115.6, 116.4, 126.0 (2), 127.6 (2), 128.6 (2), 129.7 (2), 131.3, 134.0, 139.8, 143.1, 154.3, 165.7, 167.9, 169.8, 179.9. Anal. Calcd For C25H24ClN5O5S (542.01): C, 55.40; H, 4.46; N, 12.92. Found: C, 55.09; H, 4.71; N, 12.59.
Ethyl 2-amino-5-cyano-1-(1-(4-(N,N-dimethylsulfamoyl)phenyl)ethylidene-amino)-4-(4-hydroxy-3-methoxyphenyl)-6-oxo-1,6-dihydropyridine-3-carboxylate (8): Yield, 77%; m.p. 115.1 °C; IR (KBr, cm−1): 3490 (OH), 3360, 3290 (NH2), 3066 (CH arom.), 2976, 2886 (CH aliph.), 2218 (C≡N), 1742, 1680 (2C=O), 1620 (C=N), 1340, 1163 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 1.3 [t, 3H, J = 6.0 Hz, CH3 ethyl], 2.6 [s, 6H, N(CH3)2], 3.8 [s, 3H, OCH3], 4.3 [q, 2H, J = 6.0 Hz, CH2 ethyl], 6.6 [s, 2H, NH2, D2O exchangeable], 7.3–8.0 [m, 7H, Ar-H], 10.9 [s, 1H, OH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 14.0, 18.5, 37.5 (2), 56.0, 61.9, 89.6, 112.3, 114.0, 115.9, 116.6, 122.7, 127.0, 127.6 (2), 129.8 (2), 135.6, 143.4, 147.7, 152.7, 154.9, 162.6, 164.6, 167.2, 169.8. Anal. Calcd For C26H27N5O7S (553.59): C, 56.41; H, 4.92; N, 12.65. Found: C, 56.76; H, 4.60; N, 12.33.
Ethyl 2-amino-5-cyano-4-(4-(dimethylamino)phenyl)-1-(1-(4-(N,N-dimethyl-sulfamoyl)phenyl)ethylideneamino)-6-oxo-1,6-dihydropyridine-3-carboxylate (9): Yield, 59%; m.p. 135.3 °C; IR (KBr, cm−1): 3290, 3210 (NH2), 3056 (CH arom.), 2960, 2950 (CH aliph.), 2208 (C≡N), 1705, 1690 (2C=O), 1612 (C=N), 1382, 1166 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 1.2 [t, 3H, J = 6.0 Hz, CH3 ethyl], 2.6 [s, 6H, N(CH3)2], 3.0 [s, 6H, N(CH3)2Ph], 4.2 [q, 2H, J = 6.0 Hz, CH2 ethyl], 6.7 [s, 2H, NH2, D2O exchangeable], 7.0–8.1 [m, 8H, Ar-H]. Citation13C-NMR (DMSO-d6): 13.8, 14.0, 37.5 (2), 40.0 (2), 61.8, 91.9, 115.8 (2), 116.1, 117.5, 118.2, 126.9 (2), 127.5 (2), 134.9 (2), 141.8, 147.3, 151.1, 153.6, 154.0, 159.7, 163.4, 166.0. Anal. Calcd For C27H30N6O55S (550.63): C, 58.89; H, 5.49; N, 15.26. Found: C, 58.66; H, 5.13; N, 15.50.
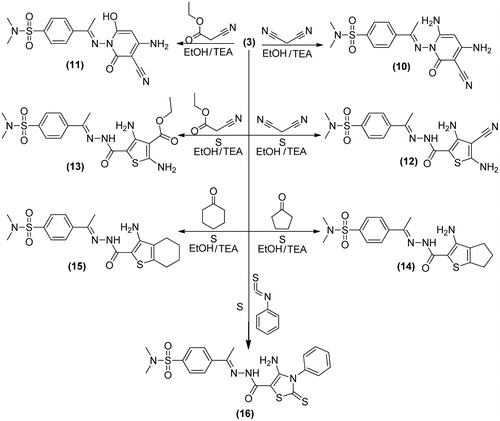
General procedure for the preparation of: 4-(1-(4,6-Diamino-3-cyano-2-oxopyridin-1(2H)-ylimino)ethyl)-N,N-dimethylbenzenesulfonamide (10) and 4-(1-(4-amino-3-cyano-6-hydroxy-2-oxopyridin-1(2H)-ylimino)ethyl)-N,N-dimethylbenzenesulfonamide (11): Equimolecular amounts of compound 3 (3.08 g, 0.01 mol) and either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.13 g, 0.01 mol) in dioxane (20 mL) containing triethylamine (1 mL) was heated under reflux for 7 h. The reaction mixture was left to cool and evaporated under vacuum the remaining product was triturated with ethanol and the formed solid product was recrystallized from ethanol to give 10, 11, respectively.
10; Yield, 68%; m.p. >300 °C; IR (KBr, cm−1): 3460, 3334, 3248, 3209 (2NH2), 3100 (CH arom.), 2960, 2863 (CH aliph.), 2212 (C≡N), 1653 (C=O), 1571 (C=N), 1328, 1161 (SO2). 1H-NMR (DMSO-d6) δ: 1.1 [s, 3H, CH3], 2.6 [s, 6H, N(CH3)2], 4.1 [s, 5H, 2NH2 + CH Pyridine], 7.3–7.9 [m, 4H, Ar-H]. Citation13C-NMR (DMSO-d6): 14.8, 37.5 (2), 70.9, 81.3, 116.3, 126.3 (2), 128.7 (2), 139.0, 144.8, 147.3, 158.1, 158.9, 161.4. Anal. Calcd For C16H18N6O3S (374.42): C, 51.33; H, 4.85; N, 22.45. Found: C, 51.62; H, 4.49; N, 22.11.
11; Yield, 72%; m.p. 222.7 °C; IR (KBr, cm−1): 3400 (OH), 3197, 3111 (NH2), 3092 (CH arom.), 2939, 2826 (CH aliph.), 2264 (C≡N), 1689 (C=O), 1624 (C=N), 1379, 1163 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 2.3 [s, 6H, N(CH3)2], 3.9 [s, 1H, CH pyridine], 4.2 [s, 2H, NH2 D2O exchangeable], 7.7–8.0 [d, 4H, Ar-H, AB system, J = 7.1 Hz], 11.2 [s, 1H, OH, D2O exchangeable]. Anal. Calcd For C16H17N5O4S (375.40): C, 51.19; H, 4.56; N, 18.66. Found: C, 51.09; H, 4.50; N, 18.32.
General procedure for the preparation of: 4-(1-(2-(3,5-Diamino-4-cyanothiophene-2-carbonyl)hydrazono)ethyl)-N,N-dimethylbenzenesulfonamide (12) and ethyl 2,4-diamino-5-(2-(1-(4-(N,N-dimethylsulfamoyl)phenyl)ethylidene)hydrazinecarbonyl)-thiophene-3-carboxylate (13): To a solution of 3 (3.08 g, 0.01 mol) in ethanol (30 mL) containing triethylamine (1 mL) either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.13 g, 0.01 mol) together with elemental sulfur (0.32 g, 0.01 mol) were added. The whole reaction mixture was refluxed for 2h. Then poured onto ice water and the obtained solid was recrystallized from dioxane to give 12 and 13, respectively.
12; Yield, 63%; m.p. 295.5 °C; IR (KBr, cm−1): 3406, 3317, 3207 (NH, 2NH2), 2942, 2827 (CH aliph.), 2206 (C≡N), 1651 (C=O), 1618 (C=N), 1396, 1155 (SO2). 1H-NMR (DMSO-d6) δ: 1.1 [s, 3H, CH3], 2.6 [s, 6H, N(CH3)2], 6.5 [s, 2H, NH2, D2O exchangeable], 6.7–7.9 [m, 6H, Ar-H + NH2], 8.1 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 18.4, 37.5 (2), 76.7, 116.2, 127.9 (2), 128.1 (2), 135.4, 136.1, 146.8, 152.0, 155.8, 163.8, 166.4. Anal. Calcd For C16H18N6O3S2 (406.48): C, 47.28; H, 4.46; N, 20.67. Found: C, 47.61; H, 4.15; N, 20.38.
13; Yield, 59%; m.p. 114.6 °C; IR (KBr, cm−1): 3412, 3390, 3260 (NH, 2NH2), 3076 (CH arom.), 2966, 2836 (CH aliph.), 1730, 1662 (2C=O), 1618 (C=N), 1366, 1162 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 1.2 [t, 3H, J = 5.5 Hz, CH3 ethyl], 2.4 [s, 6H, N(CH3)2], 4.4 [q, 2H, J = 5.5 Hz, CH2 ethyl], 4.6 [s, 4H, 2NH2, D2O exchangeable], 7.0–7.4 [m, 4H, Ar-H], 10.1 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 14.4, 15.6, 37.8 (2), 62.7, 125.9 (2), 129.0 (2), 132.7, 139.1, 139.7, 139.8, 147.0, 162.8, 170.4, 193.2, 213.0. Anal. Calcd For C18H23N5O5S2 (453.54): C, 47.67; H, 5.11; N, 15.44. Found: C, 47.38; H, 5.40; N, 15.18.
General procedure for the preparation of: 4-(1-(2-(3-Amino-5,6-dihydro-4H-cyclopenta[b]thiophene-2-carbonyl)hydrazono)ethyl)-N,N-dimethylbenzenesulfonamide (14) and 4-(1-(2-(3-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-2-carbonyl)hydrazono)-ethyl)-N,N-dimethylbenzenesulfonamide (15): To a solution of compound 3 (3.08 g, 0.01 mol) in absolute ethanol (30 mL) containing triethylamine (1 mL) either cyclopentanone or cyclohexanone (0.01 mol) together with elemental sulfur (0.23 g, 0.01 mol) were added. The reaction mixture was refluxed for 2 h then poured onto ice/water and the obtained solid was recrystallized from dioxane to give 14 or 15, respectively.
14; Yield, 66%; m.p. 178.2 °C; IR (KBr, cm−1): 3390, 3370, 3210 (NH, NH2), 3010 (CH arom.), 2978, 2846 (CH aliph.), 1694 (C=O), 1396, 1161 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 2.0–2.4 [m, 2H, 3CH2 cyclopentane], 2.4 [s, 6H, N(CH3)2], 2.5–2.7 [m, 4H, 3CH2 cyclo], 4.3 [s, 2H, NH2, D2O exchangeable], 7.6–8.0 [m, 4H, Ar-H], 9.0 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 14.3, 21.0, 28.1, 33.8, 38.9 (2), 125.9, 127.6 (2), 129.0 (2), 136.2, 138.7, 140.6, 144.5, 149.1, 169.3, 202.7. Anal. Calcd For C18H22N4O3S2 (406.52): C, 53.18; H, 5.45; N, 13.78. Found: C, 53.51; H, 5.17; N, 13.50.
15; Yield, 73%; m.p. 152.0 °C; IR (KBr, cm−1): 3350, 3309 (NH, NH2), 3086 (CH arom.), 2929, 2816 (CH aliph.), 1701 (C=O), 1627 (C=N), 1394, 1161 (SO2). 1H-NMR (DMSO-d6) δ: 1.1 [s, 3H, CH3], 1.5–2.2 [m, 8H, 4CH2 cyclohexane], 2.4 [s, 6H, N(CH3)2], 6.9 [s, 2H, NH2, D2O exchangeable], 7.3–8.0 [m, 4H, Ar-H], 8.2 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 15.4, 18.6, 24.2 (2), 26.4, 37.5 (2), 125.7, 128.3 (2), 130.1 (2), 135.7, 138.9, 140.4, 141.9, 148.8, 172.1, 210.8. Anal. Calcd For C19H24N4O3S2 (420.55): C, 54.26; H, 5.75; N, 13.32. Found: C, 54.46; H, 5.98; N, 13.63.
4-(1-(2-(4-Amino-3-phenyl-2-thioxo-2,3-dihydrothiazole-5-carbonyl)hydrazono)-ethyl)-N,N-dimethylbenzenesulfonamide (16): A mixture of 3 (3.08 g, 0.01 mol) elemental sulfur (0.32 g, 0.01 mol) and phenylisothiocyanate (1.30 g, 0.01 mol) in absolute ethanol (20 mL) was refluxed for 5 h. The reaction mixture was cooled and the obtained solid was recrystallized from acetic acid to give 16; Yield, 68%; m.p. 69.5 °C; IR (KBr, cm−1): 3213, 3196, 3161 (NH, NH2), 3100 (CH arom.), 2960, 2842 (CH aliph.), 1697 (C=O), 1597 (C=N), 1375, 1161 (SO2), 1201 (C=S). 1H-NMR (DMSO-d6) δ: 1.3 [s, 3H, CH3], 2.5 [s, 6H, N(CH3)2], 4.5 [s, 2H, NH2, D2O exchangeable], 7.0–7.9 [m, 9H, Ar-H], 11.0 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 13.9, 38.9 (2), 67.1, 121.6, 122.9 (2), 124.5 (2), 128.4 (2), 128.7 (2), 136.1, 138.7, 146.0, 160.8, 167.9, 187.2, 198.2. Anal. Calcd For C20H21N5O3S3 (475.61): C, 50.51; H, 4.45; N, 14.73. Found: C, 50.27; H, 4.14; N, 14.44.
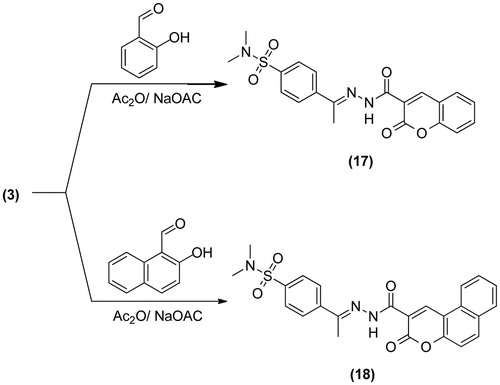
General procedure for the preparation of: N,N-Dimethyl-4-(1-(2-(2-oxo-2H-chromene-3-carbonyl)hydrazono)ethyl)-benzenesulfonamide (17) and N,N-dimethyl-4-(1-(2-(3-oxo-3H-benzo[f]-chromene-2-carbonyl)hydrazono)ethyl)-benzenesulfonamide (18): Equimolecular mixture of 3 (3.08 g, 0.01 mol) and either salicaldehyde (1.22 g, 0.01 mol) or 2-hydroxy-1-naphthaldehyde (1.72 g, 0.01 mol) in dioxane (20 mL) containing piperidine (0.5 mL) was refluxed for 3h. The reaction mixture was poured onto ice/water and the obtained solid was recrystallized from dioxane containing few drops of HCl to give 17 or 18, respectively.
17; Yield, 91%; m.p. 165.1 °C; IR (KBr, cm−1): 3370 (NH), 3056 (CH arom.), 2935, 2866 (CH aliph.), 1680, 1672 (2C=O), 1608 (C=N), 1388, 1182 (SO2). 1H-NMR (DMSO-d6) δ: 0.9 [s, 3H, CH3], 2.4 [s, 6H, N(CH3)2], 7.0–8.0 [m, 8H, Ar-H], 9.0 [s, 1H, CH chromene], 10.4 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 15.3, 37.5 (2), 116.1, 120.4, 121.7, 123.6, 125.9, 126.4 (2), 127.8, 129.6 (2), 134.9, 135.8, 145.6, 153.2, 161.3, 165.7, 175.6. Anal. Calcd For C20H19N3O5S (413.45): C, 58.10; H, 4.63; N, 10.16. Found: C, 58.38; H, 4.33; N, 10.51.
18; Yield, 93%; m.p. 257.1 °C; IR (KBr, cm−1): 3309 (NH), 3066 (CH arom.), 2940, 2862 (CH aliph.), 1683, 1676 (2C=O), 1570 (C=N), 1398, 1157 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 2.3 [s, 6H, N(CH3)2], 7.4–8.0 [m, 10H, Ar-H], 9.2 [s, 1H, CH chromene], 13.8 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 14.6, 37.5 (2), 113.2, 117.1, 119.0, 121.3, 122.8, 126.0 (2), 127.2, 128.4 (2), 129.7 (2), 130.6, 131.2, 135.0, 136.8, 144.1, 151.9, 158.8, 169.4, 174.2. Anal. Calcd For C24H21N3O5S (463.51): C, 62.19; H, 4.57; N, 9.07. Found: C, 62.55; H, 4.21; N, 9.39.
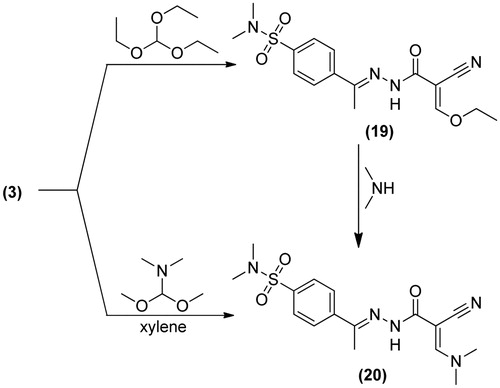
4-(1-(2-(2-Cyano-3-ethoxyacryloyl)hydrazono)ethyl)-N,N-dimethylbenzene-sulfonamide (19): A mixture of 3 (3.08 g, 0.01 mol) and triethylorthoformate (5 mL) in acetic anhydride (5 mL), was refluxed for 5h. The obtained solid was recrystallized from ethanol to give 19. Yield, 81%; m.p. 181.2 °C; IR (KBr, cm−1): 3310 (NH), 3065 (CH arom.), 2984, 2846 (CH aliph.), 2226 (C≡N), 1683 (C=O), 1595 (C=N), 1340, 1165 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 1.1 [t, 3H, J = 6.0 Hz, CH3 ethyl], 2.3 [s, 6H, N(CH3)2], 4.2 [q, 2H, J = 6.0 Hz, CH2 ethyl], 7.5–8.1 [m, 5H, Ar-H + CH], 11.1 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 13.8, 14.9, 37.4 (2), 62.9, 80.1, 117.1, 129.0 (2), 135.5 (2), 139.8, 147.4, 156.2, 167.4, 197.3. Anal. Calcd For C16H20N4O4S (364.42): C, 52.73; H, 5.53; N, 15.37. Found: C, 52.48; H, 5.31; N, 15.09.
4-(1-(2-(2-Cyano-3-(dimethylamino)acryloyl)hydrazono)ethyl)-N,N-dimethylbenzene-sulfonamide (20): Method (A): A mixture of 3 (3.08 g, 0.01 mol) and dimethylformamide-dimethylacetal (1.2 g, 0.01 mol) in dry xylene was refluxed for 8h. The obtained solid was recrystallized from ethanol to give 20. Method (B): A mixture of compound 19 (3.63 g, 0.01 mol), and dimethylamine (0.45 g, 0.01 mol) in ethanol (30 mL), was refluxed for 4h. The solid obtained was recrystallized from ethanol to give 20 (m.p., mixed m.p. and TLC). Yield, 66%; m.p. 220.8 °C; IR (KBr, cm−1): 3197 (NH), 3100 (CH arom.), 2939, 2872 (CH aliph.), 2222 (C≡N), 1685 (C=O), 1618 (C=N), 1398, 1163 (SO2). 1H-NMR (DMSO-d6) δ: 1.0 [s, 3H, CH3], 2.3 [s, 6H, N(CH3)2SO2], 2.8 [s, 6H, N(CH3)2], 7.7 [s, 1H, CH], 7.8–7.9 [d, 4H, J = 7.6 Hz, Ar-H,], 11.2 [s, 1H, NH, D2O exchangeable]. Citation13C-NMR (DMSO-d6): 13.8, 37.5 (2), 40.1 (2), 99.1, 116.1, 127.5 (2), 134.9 (2), 141.9, 147.4, 151.2, 159.7, 166.0. Anal. Calcd For C16H21N5O3S (363.43): C, 52.88; H, 5.82; N, 19.27. Found: C, 52.59; H, 5.50; N, 19.01.
In vitro antiproliferative screening
The cytotoxic activity was measured in vitro for the newly synthesized compounds using the SulfoRhodamine-B stain (SRB) assay using the method of Skehan et al. Citation12. The in vitro anticancer screening was done at the Pharmacology Unit, the National Cancer Institute, Cairo University. Cells were plated in 96-multiwell microtiter plate (104 cells/well) for 24 h before treatment with the compound(s) to allow attachment of cell to the wall of the plate. Test compounds were dissolved in DMSO and diluted with saline to the appropriate volume. Different concentrations of the compound under test (10, 25, 50 and 100 µM) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the compound(s) for 48 h at 37 °C and in atmosphere of 5% CO2. After 48 h, cells were fixed, washed and stained for 30 min. with 0.4% (W/V) with SRB dissolved in 1% acetic acid. Excess unbound dye was removed by four washes with 1% acetic acid and attached stain was recovered with Tris-EDTA buffer. Color intensity was measured in an enzyme-linked immunosorbent assay ELISA reader. The relation between surviving fraction and drug concentration was plotted to get the survival curve for breast tumor cell line MCF-7 after the specified time. The molar concentration required for 50% inhibition of cell viability (IC50) was calculated and the results are given in ().
Table 1. In vitro anticancer screening of the newly synthesized compounds against human breast cancer cell line (MCF-7).
Docking study
The molecular model of the new sulfonamide derivatives was built in their dissociated form, using standard bond lengths and angles, with the MOE software suite 10.2008. Following geometry optimization, a systematic conformational search was carried out to an RMS gradient of 0.01 Å with energy minimization of the resultant conformations employing the ConfSearch module implemented in MOE. All molecular mechanics computations were performed with the Merck Force Field (MMFF94s). The experimental crystallographic structures of CA IX complex was retrieved from the Protein DataBank (PDB ID: 4BCW). Missing hydrogens were added to the enzyme and partial charges were calculated. After removing the co-crystallized inhibitor, validation followed by docking of the compounds was carried out using MOE software suite 10.2008. The target protein was kept rigid, while the ligands were left free to explore the conformational space inside the enzyme cavity; 200 separate docking simulations were run using default parameters and the conformations were chosen based on the combination of S score data, E conformation and appropriate fitting with the relevant amino acids in binding pocket.
Results and discussion
Chemistry
outline the synthetic pathway used to obtain the sulfonamide derivatives 3–20. The starting material 4-(1-(2-(2-cyanoacetyl)hydrazono)ethyl)-N,N-dimethylbenzenesulfonamide 3 was prepared via the condensation reaction between 2-cyanoacetohydrazide 2 and 4-acetyl-N,N-dimethylbenzenesulfonamide 1. The structure of compound 3 was proved on the basis of analytical and spectral data. Thus, 1H-NMR spectrum revealed the presence of a singlet at δ 0.9 ppm corresponding to CH3 group, a singlet at 2.3 ppm due to N(CH3)2 group and a singlet at 4.1 ppm attributed to CH2 group. Moreover, the Citation13C-NMR data exhibited the presence of δ 12.9 (CH3), 28.3 (CH2). Further elucidation for the structure of 3 was obtained through studying its chemical reactivity through some chemical reagents. Thus, the reaction of compound 3 with ethyl α-cyanocinnamate derivatives 4a–d, yielded the dihydropyridine derivatives 6–9, respectively. The reaction took place through the intermediate formation of 5a–d. Structures 6–9 were based on analytical and spectral data. IR spectra of compounds, 6–9 revealed the presence of bands for (NH2), CH aliphatic, (C≡N), and two (C=O) groups. While 1H-NMR spectra of compounds 6–9 revealed the presence of a triplet and a quartet attributed to the ester group. The reaction of 3 with either malononitrile or ethyl cyanoacetate furnished the pyridin-2-one derivatives 10 and 11, respectively. Analytical and spectral data are consistent with the proposed structures. The reaction of 3 with either malononitrile or ethyl cyanoacetate and elemental sulfur in the presence of triethylamine gave the thiophene derivative 12 and 13, respectively. The reaction goes in parallel to the reported Gewald's thiophene synthesisCitation13. Structures of 12 and 13 were confirmed on the basis of elemental analyses and spectral data. IR spectrum of 12 revealed the presence of bands at 2206 cm−1 for (C≡N), 1651 cm−1 for (C=O) and 1396, 1155 cm−1 (SO2). 1H-NMR spectrum of 12 reveled signals at 6.5 ppm attributed to (NH2), and 8.1 ppm due to (NH) group. IR spectrum of 13 showed the absence of (C≡N) band and presence of two bands at 1730, 1662 cm−1 for (C=O) groups. 1H-NMR spectrum of 13 exhibited a triplet at 1.2 ppm for the (CH3) of ethyl group and a quartet at 4.4 ppm for the (CH2) of ethyl group. Similarly, the reaction of 3 with cyclopentanone or cyclohexanone and elemental sulfur yielded the corresponding thiophene derivatives 14 and 15, respectively. Formation of 14 and 15 took place according to the similar reported reaction of cyclohexanone with methylene reagents and elemental sulfurCitation14. IR spectra of compounds 14 and 15 revealed the absence of (C≡N) band and presence of bands for (NH2), (C=O) and (SO2) groups. 1H-NMR spectra of 14 and 15 showed signals of 4.3, 6.9 ppm attributed to their NH2 groups. On the other hand, the reaction of 3 with elemental sulfur and phenyl isothiocyanate furnished the corresponding thiazole derivative 16. Formation of the latter product took place in accordance with the reported Hanzesch reactionCitation15. Structure of compound 16 was based on analytical and spectral data. IR spectrum of 16 showed bands at 3213, 3196, 3161 cm−1 for (NH, NH2), 1697 cm−1 for (C=O), and 1201 cm−1 for (C=S) groups. 1H-NMR spectrum of 16 revealed singlet at 4.5 ppm assigned to the (NH2) group. Interaction of 3 with salicylaldehyde or 2-hydroxy-1-naphthaldehyde gave the corresponding chromene 17 and benzochromene derivative 18, respectively. The reaction goes in analogy with the reported literatureCitation16,Citation17. IR spectra of 17 and 18 exhibited the absence of a (C≡N) band and presence of bands for two carbonyl groups. 1H-NMR spectra of 17 and 18 revealed signals at 9.0 and 9.2 ppm attributed to their chromene (CH) protons. Finally, treatment of 3 with triethyl orthoformate or dimethylformamide-dimethylacetal (DMF-DMA) afforded the corresponding acryloylhydrazono derivatives 19 and 20, respectively. 1H-NMR spectrum of 19 showed a triplet at 1.1 ppm due to the (CH3) and a quartet at 4.2 ppm attributed to the (CH2) of the ethyl group. 1H-NMR spectrum of 20 revealed a singlet at 2.3 ppm attributed to N(CH3)2SO2 and the appearance another singlet at 2.8 ppm attributed to the new N(CH3)2 group.
In vitro anticancer activity and SAR findings
All the newly synthesized compounds were evaluated for their in vitro cytotoxic activity against human breast cancer cell line MCF-7. The relationship between surviving fraction and drug concentration was plotted to obtain survival curve from which the response parameter IC50 value, corresponding to the concentration required for 50% inhibition of cell viability, was calculated and data are presented in . Doxorubicin (CAS 23214-92-8) was used as a positive control in this study for comparative purposes (IC50 32 μM).
Regarding the pyridone derivatives which were synthesized with two patterns of substitution, the first series of which are the analogs 6–9 having a 4-substituted phenyl group as well as a 2-amino and 3-carboxylate ethyl ester functions; while the second set of derivatives 10 and 11 had a 4-amino group, either an amino or hydroxyl group at the 2 position and a free 3 position.
SAR findings for the first series represented by derivatives 6–9 showed that the presence of an electron donating group on the phenyl ring is much favored over an electron withdrawing group which is detrimental to the cytotoxic efficacy as evidenced through the IC50 values of the p-Cl derivative (IC50 60.10 μM) compared to that of the p-Me, m-OMe, p-OH and the p-N(CH3)2 analogs displaying IC50 values 21.81, 36.4 and 25.5 μM, respectively. It also can be inferred from the cytotoxicity data that para substitution with a small or bulky electron donating group, as in case of derivatives 6 and 9, IC50 21.81 and 25.5 μM respectively, is well tolerated giving higher potency than the meta, para disubstituted derivative 8 (IC50 36.4 μM) and even than that of the reference drug doxorubicin (IC50 32 μM).
With respect to pyridones 10 and 11 possessing 2-amino group (IC50 49.3 μM) and 2-hydroxyl group (IC50 20.60 μM), respectively, screening results showed that replacement of the OH group for the NH2 group increases the potency by more than double giving in fact the most potent derivative in this study.
With reference to the screening results of the thiophene derivatives 12–15, it is shown that substitution at the 4-position with a cyano group gives a slightly more potent derivative 12 (IC50 44.2 μM) than the 4-ethyl carboxylate ester counterpart 13 (IC50 51.2 μM). On the other hand, fusion of the thiophene ring with a cyclopentene or cyclohexene ring as in the case of the cyclic analogs 14 (IC50 38.46 μM) and 15 (IC50 38.9 μM), respectively, markedly increases activity not only conferring that the second ring is tolerated but in fact is favored.
Furthermore, the thiazole derivative 16 (IC50 25.83 μM) displayed higher cytotoxic efficacy than the reference drug doxorubicin (IC50 32 μM) and an enhanced antiproliferative power when compared to the bioisosteric series of thiophene derivatives.
As for the chromene and benzochromene derivatives 17 (IC50 31.20 μM) and 18 (IC50 37.9 μM) while the first was almost equipotent with doxorubicin the second was obviously less potent than the reference drug. This infers that the extra fused benzene ring in 18 interfers with the biological activity either due to steric effect or increased lipophilicity or both.
Finally, in vitro antiproliferative screening results for the ethoxyacryloyl derivative 19 (IC50 44.2 μM) and its dimethylaminoacryloyl analog 20 (IC50 34.4 μM) showed that the diemthylamino derivative displayed higher potency against MCF-7.
Molecular modeling and docking
Carbonic anhydrases (CA, EC 4.2.1.1) represent a family of Zn based metalloenzymes that catalyzes the interconversion between carbon dioxide and bicarbonate with generation of protons. The carbonic anhydrase isozyme IX (CA IX) is reported to be associated with tumorogenesis being highly overexpresed in hypoxic tumors and restrictedly expressed in normal tissues, thus inhibition of which has proven beneficial in the field of selective cancer therapy. It has also been well reported that sulfonamide bearing molecules act as promising anticancer agents through inhibition of carbonic anhydrase IXCitation11.
Crystallographic data revealed that CA IX, which is a membrane-associated α-CA, is comprised of a dimer each monomer of which consists of 10-stranded antiparallel-sheets forming the core of the molecule with an intramolecular disulfide bond between Cys23 and Cys20311,18. CA IX active site contains a Zn(II) in coordination with His94, 96, 119 and a water molecule. The essential binding pocket amino acids are the proton shuttle residue (His64) in addition to those residues involved in binding of inhibitorsCitation11,Citation18. Thus, Leu91, Val121, Val131, Leu135, Leu141, Val143, Leu198 and Pro202 define the hydrophobic region of the active site, whereas Arg58, Arg60, Asn62, His64, Ser65, Gln67, Thr69 and Gln92 identify the hydrophilic one.
In an attempt to rationalize the cytotoxic activity profile exhibited by the synthesized compounds, a molecular modeling study was carried out. A conformational search using an implicit solvent model was accomplished for the prepared compounds; this was followed by refinement of the geometry of local minima through a quantum-mechanical (QM) method. Subsequently, flexible docking of the compounds was performed in the crystallographic structure of the CA IX co-crystallized with a sulfonamide native ligand obtained from the Protein DataBank (PDB ID: 4BCW)Citation18 to assess the plausible ability of the new sulfonamide derivatives to bind in the active pocket of CA IX as a potential molecular target. The poses obtained for our sulfonamides were found to bind in a co-crystallized ligand-like fashion with CA IX ().
Figure 1. Docking validation for the re-docked ligand (magenta) compared to the co-crystallized ligand (green) in the active site of CA IX (rmsd = 0.6715 Å, S = −14.44 Kcal mol−1).
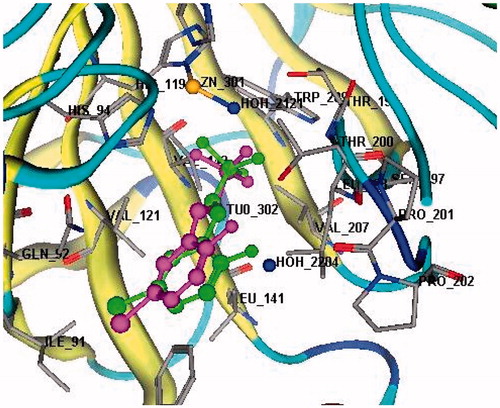
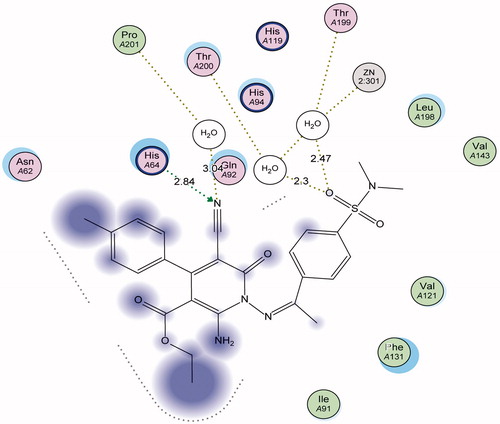
Figure 2. 3D Docking of sulfonamide derivative 6 (magenta) (S = −12.92 Kcal mol−1) compared to the co-crystallized ligand (green) in the active site of CA IX (hydrogen bonds are represented by red dashed lines).
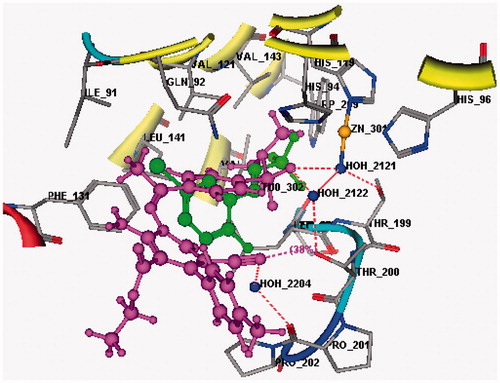
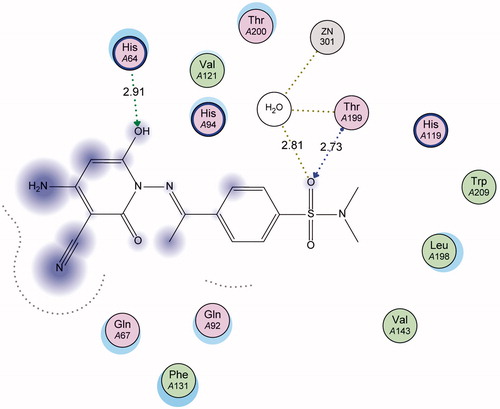
Figure 4. 3D Docking of sulfonamide derivative 11 (magenta) (S = −11.99 Kcal mol−1) compared to the co-crystallized ligand (green) in the active site of CA IX (hydrogen bonds are represented by red dashed lines).
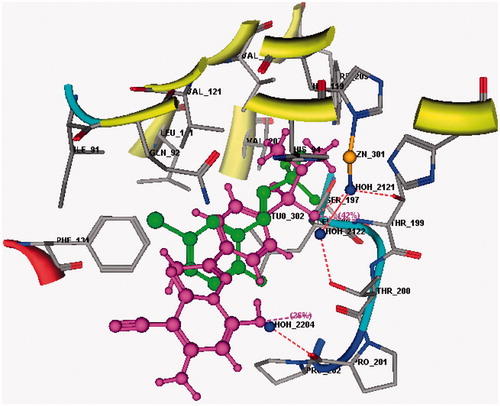
Initially, docking validation was performed to assert the ability of the docking protocol to recognize the active site and reproduce the docking results. displays the superposition of the co-crystallized ligand and the re-docked ligand which was found to bind at the same position and with the same manner (rmsd = 0.6715 Å, S = −14.44 Kcal mol−1).
Docking of all the synthesized compounds was performed and herein the findings obtained for the 2 most active compounds in this study, sulfonamide derivatives 6 ( and ) and 11 ( and ), is presented.
3D ligand interaction of sulfonamide derivative 6 () shows that the compound binds in the same site as the co-crystallized ligand with a binding energy reported by an S score of −12.92 Kcal mol−1. On the other hand, the 2D ligand interaction () demonstrates that the compound binds with the amino acids of the active site (Thr199, Thr201 and His64) as well as the Zn atom through a network of hydrogen bonds (2.30–3.04 Å) some of them being mediated by water molecules present in the active site.
Regarding the docking results of compound 11, the 3D and 2D ligand interaction simulations ( and ) show that 11 binds in a same fashion to the co-crystallized ligand displaying a set of hydrogen bonds with the active pocket amino acids His64 and Thr199 as well as water mediated Zn interactions leading to an overall binding energy of −11.99 Kcal mol−1.
These observed favorable interactions between CA IX and the new sulfonamide derivatives might, at least in part, explain the observed biological activity of this series of compounds. Further investigations to explore more the plausible mechanism of action of these derivatives are underway.
Conclusion
The objective of the present study was to synthesize and investigate the antiproliferative activity of some novel sulfonamide/sulfone derivatives carrying the biologically active dihydropyridone, thiophene, thiazole, chromene and benzochromene moieties. Compounds 6, 9, 11, 16 and 17 showed promising anticancer activity higher than that of doxorubicin as a reference drug. Molecular modeling and docking of the synthesized sulfonamide derivatives in the active site of CA IX showed interaction modes comparable to that of the co-crystallized ligand.
Declaration of interest
This study was supported by Deanship of Scientific Research at King Saud University through the Research Group Project no. RGP-VPP-302. The authors declare no conflict of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University.
References
- Available from http://www.who.int/gho/ncd/mortality_morbidity/cancer_text/en/index.html [last accessed 12 June 2013]
- Matsuo H, Wakasugi M, Takanaga H, et al. Possibility of the reversal of multidrug resistance and the avoidance of side effects by liposomes modified with MRK-16, a monoclonal antibody to P-glycoprotein. Contr Rel 2001;77:77–86
- Fahad-Ullah M. Cancer multidrug resistance (MDR): a major impediment to effective chemotherapy. Asian Pac J Cancer Preven 2008;9:1–6
- (a) Marques SM, Enyedy EA, Supuran CT, et al. Pteridine--sulfonamide conjugates as dual inhibitors of carbonic anhydrases and dihydrofolate reductase with potential antitumor activity. Bioorg Med Chem 2010;18:5081–9. (b) Ghorab MM, Ragab FA, Heiba HI, et al. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Med Chem Res 2011; 20:388–400. (c) Ghorab MM, Ragab FA, Heiba HI, et al. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur J Med Chem 2010;45:3677–84. (d) Abbate F, Casini A, Owa T, et al. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:217–23. (e) Stephens CE, Felder TM, Sowell JW, et al. Synthesis and antiviral/antitumor evaluation of 2-amino- and 2-carboxamido-3-arylsulfonylthiophenes and related compounds as a new class of diarylsulfones. Bioorg Med Chem 2001;9:1123–32
- (a) Moorthy NS, Cerqueira NS, Ramos MJ, Fernandes PA. QSAR analysis of 2-benzoxazolyl hydrazone derivatives for anticancer activity and its possible target prediction. Med Chem Res 2012;21:133–44. (b) Patila BR, Machakanura SS, Hunoora RS, et al. Synthesis and anti-cancer evaluation of cyclotriphosphazene hydrazone derivatives. Der Pharma Chemica 2011;3:377–88
- (a) Greenfeld DL, Benhar I. Risks and untoward toxicities of antibody-based immunoconjugates. Adv Drug Delivery Rev 2012;64:1782–99. (b) Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retinal Eye Res 2013;32:102–80. (c) Renoir JM. Estradiol receptors in breast cancer cells: associated co-factors as targets for new therapeutic approaches. Steroids 2012;77:1249–61. (d) Lee EH, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Tox 2012;50:4136–43
- (a) Bassyouni FA, Tawfik HA, Soliman AM, Abdel Rehim M. Synthesis and anticancer activity of some new pyridine derivatives. Res Chem Intermed 2012;38:1291–310. (b) Amr AE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg Med Chem 2006;14:5481–8
- (a) Ahmed MM, Khan MA, Rainsford KD. Synthesis of thiophene and NO-curcuminoids for antiinflammatory and anti-cancer activities. Molecules 2013; 18:1483–501. (b) El-Gaby MSA, Ismail ZH, Abdel-Gawad SM, et al. Synthesis of thiazolidine and thiophene derivatives for evaluation as anticancer agents. Phosphorous, sulfur and silicon 2009;184:2645–54
- (a) Ghorab MM, Shaaban MA, Refaat HM, et al. Anticancer and radiosensitizing evaluation of some new pyranothiazole-Schiff bases bearing the biologically active sulfonamide moiety. Eur J Med Chem 2012;53:403–7. (b) Lu X, Liu X, Wan B, et al. Synthesis and evaluation of anti-tubercular and antibacterial activities of new 4-(2,6-dichlorobenzyloxy)phenyl thiazole, oxazole and imidazole derivatives. Part 2. Eur J Med Chem 2012;49:164–71
- (a) Fallah-Tafti A, Tiwari R, Shirazi AN, Akbarzadeh T, et al. 4-Aryl-4H-chromene-3-carbonitrile derivatives: evaluation of Src kinase inhibitory and anticancer activities. Med Chem 2011;7:466–72. (b) Ghorab MM, Al-Said MS, Nissan YM. Dapson in heterocyclic chemistry, Part V: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active dihydropyridine, dihydroisoquinoline, 1,3-dithiolan, 1,3-dithian, acrylamide, pyrazole, pyrazolopyrimidine and benzochromenemoieties. Chem Pharm Bull 2012;60:1019–28
- (a) Supuran CT. Inhibition of carbonic anhydrase IX as a novel anticancer mechanism. World J Clin Oncol 2012;3:98–103. (b) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (c) Said HM, Supuran CT, Hageman C, et al. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des 2010;16:3288–99. (d) Supuran CT. Carbonic anhydrases as drug targets. Curr Pharm Des 2008;14:601–2
- Skehan P, Storegng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Nat Cancer Inst 1990;82:1107–12
- Mckibben BP, Cartwright CH, Castelhano L. Practical synthesis of tetrasubstituted thiophenes for use in compound libraries. Tetrahedron Lett 1999;40:5471–4
- Scrowston RM. Recent advances in the chemistry of benzo[b]thiophenes. Adv Heterocyclic Chem 1981;29:171–249
- Tormos JV, Khodorkovsky YU, Neilands OY, Belyakov SV. A novel cyclocondensation of xanthates containing active methylene groups with isothiocyanates. Spectral data and X-ray structures of the products. Tetrahedron 1992;48:6863–74
- Zhou JF, Gong GX, Zhu FX, Zhi SJ. Microwave promoted one-pot synthesis of 3-(2′-amino-3′-cyano-4′-arylpyrid-6′-yl) coumarins.. Chin Chem Lett 2009;20:37–9
- Volmajer J, Toplak R, Leban I, Marechal AM. Synthesis of new iminocoumarins and their transformations into N-chloro and hydrazono compounds. Tetrahedron 2005;61:7012–21
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300