Abstract
A series of 29 previously reported N4-substituted 5-nitroisatin-3-thiosemicarbazones 2–30 has been screened for leishmanicidal potential. Compounds 2–4, 7, 8, 10–13, 15–19, 21, 23, 24, 26, 28 and 30 exhibited good to excellent antileishmanial activities with IC50 values ranging from 0.44 ± 0.02 to 32.38 ± 0.66 µg/mL. Of these, 5, 7, 19 and 28 proved to be the most active antileishmanial agents, displaying activities with IC50 values 1.78 ± 0.35, 0.44 ± 0.02, 1.91 ± 0.04 and 4.28 ± 0.75 µg/mL, respectively, which were even better than the standard drug, pentamidine (IC50 = 5.09 ± 0.04 µg/mL). This study presents the first example of exhibition of leishmanicidal potential by isatin-thiosemicarbazones and as such furnishes a solid basis for further research on these compounds to develop more potent antileishmanial agents.
Introduction
Leishmaniasis is a tropical disease caused by protozoa of the genus Leishmania. There are four different forms of the disease with a broad range of chemical manifestations. These include cutaneous leishmaniasis (CL), diffused cutaneous leishmaniasis (DCL), mucocutaneous leishmaniasis (MCL) and visceral leishmaniasis (VL). Of these, the last, being the most severe, causes almost 100% mortality, if untreatedCitation1,Citation2.
The available chemotherapy for leishmaniasis relies upon pentavalent antimonial (Sbv) compounds, i.e. sodium stibogluconate (pentostam) or meglumine antimoniate (glucantime), which even though are still the first-line drugs, but are not totally safe and/or efficaciousCitation3. For the treatment of unresponsive cases, amphotericin B and pentamidine can be used, though they are not fully effective either and exhibit adverse side effectsCitation4. Miltefosine or hexadecylphosphocholine, an alkylphospholipid, originally developed as an anticancer agent, is the only oral antileishmanial drug found to be effective against both the CLCitation5 and VLCitation6, although causing serious gastrointestinal problemsCitation7. Furthermore, drug-resistant strains of Leishmania spp. have appeared, which necessitate the search for new, more effective and non- or least toxic chemotherapeutic agents.
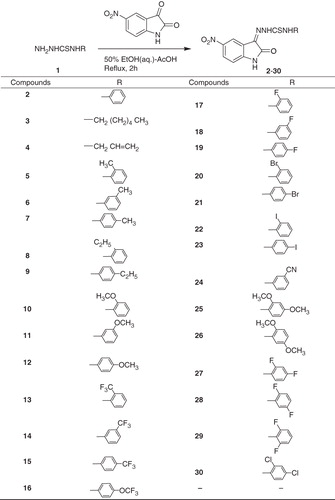
Thiosemicarbazones are an important class of organic compounds, which possesses a number of medicinal properties, including antibacterial, antifungal, antiprotozoal, antitumoral, antiviral and more particularly, the antileishmanial activitiesCitation8–21. Isatin-derived thiosemicarbazones have also been found to possess a variety of biological activities, such as antimicrobial, antineoplastic, antiplasmodial and antiviralCitation22–34. However, antileishmanial potential of this group of thiosemicarbazones has not been explored. Very recently, during random screening of the organic compounds prepared in our laboratory, we found that certain N4-substituted isatin-thiosemicarbazones bearing strong inductively electron-attracting substituents at position-5 of the isatin scaffold exhibited antileishmanial activity. This observation stimulated us to study the leishmanicidal properties of a series of 29 previously reported N4-alkyl/alkenyl/aryl substituted 5-nitroisatin-3-thiosemicarbazonesCitation35,Citation36, prepared by the condensation of 5-nitroisatin with appropriate thiosemicarbazides (). This paper, therefore, describes the in vitro leishmanicidal potential of these compounds. It also describes the effects of the nature of substituents attached to N4 of the thiosemicarbazone moiety on this very potential of the compounds. Further studies on such compounds with certain structural modifications are in progress.
Materials and methods
All reagents and solvents were used as obtained from the suppliers or recrystallized/redistilled as necessary. The 5-nitroisatin-3-thiosemicarbazones were prepared by treating 5-nitroisatin with appropriate thiosemicarbazides. The details of the reactions along with physical, analytical and spectral data of the compounds were reported elsewhereCitation35,Citation36. In vitro leishmanicidal screening of the prepared compounds was made at Dr. Panjwani Center for Molecular Medicine and Drug Research, H.E.J. Research Institute of Chemistry, University of Karachi, Karachi, Pakistan.
In vitro antileishmanial activity
Leishmania major were grown in bulk in modified Novy-MacNeal-Nicolle (NNN) biphasic medium by using normal physiological saline. Leishmania promastigotes were cultured with Rosewell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% heat inactivated foetal bovine serum. Parasites at log phase were centrifuged at 2000 rpm for 10 min and washed three times with saline at the same speed and time. Parasites were diluted with fresh culture medium to a final density of 106 cells/mL.
In a 96-well microtiter plate, 180 µL of medium was added in first row and 100 µL of medium was added in other wells. A 20 µL of the experimental compound was added in medium and serially diluted. A 100 µL of parasite culture was in all wells and two rows were left for negative and positive controls. Negative controls received only medium, whereas the positive control contained varying concentrations of standard leishmanicidal compound, pentamidine. The plate was incubated at 21–22 °C for 72 h. The culture was examined microscopically on an improved Neubauer counting chamber and IC50 values of compounds were calculated by Software Ezfit 5.03 (Perella Scientific, Amherst, NH). All assays were performed in triplicate.
Results and discussion
This study describes the in vitro determination of the leishmanicidal effects of 29 N4-substituted 5-nitroisatin-3-thiosemicarbazones 2–30, the synthesis of which has been reported elsewhereCitation35,Citation36.
In vitro antileishmanial activity
The synthetic thiosemicarbazones 2–30 were screened for their antileishmanial activity according to the literature protocolCitation37. Compound 2, i.e. 1-(5-nitro-2-oxindolin-3-ylidene)-4-phenylthiosemicarbazide severed as a reference point to evaluate the effects of different substituents (alkyl, alkenyl and aryl bearing one or two functions about the phenyl ring) attached to N4 of the thiosemicarbazone moiety on the leishmanicidal potential of these compounds. All the compounds except 29 were found to be active against Leishmania major, showing varied leishmanicidal activity (IC50 = 0.44 ± 0.02–82.12 ± 0.86 µg/mL) (). Compounds 3 and 4 having n-hexyl and allyl substituents at N4 of the thiosemicarbazone moiety were found to exhibit good antileishmanial activity (IC50 = 25.84 ± 0.205 and 27.14 ± 0.06 µg/mL, respectively), whereas 2 bearing phenyl group at the same position displayed weak activity (IC50 = 74.31 ± 0.69 µg/mL). Amongst the aryl-substituted compounds 5–7 possessing methyl substituents at different positions of the phenyl ring, the ortho-substituted compound 5 exhibited significant activity with an IC50 value of 1.78 ± 0.35 µg/mL, while the para-substituted compound 7 displayed excellent leishmanicidal effect (IC50 = 0.44 ± 0.02 µg/mL); however, the meta-substituted isomer 6 showed moderate activity (IC50 = 52.66 ± 0.28 µg/mL). To the contrary, amongst the ethyl-substituted compounds 8 and 9, the para-substituted compound 9 was found to demonstrate weak leishmanicidal activity (IC50 = 71.45 ± 0.61 µg/mL), when compared with the ortho-substituted compound 8, which displayed good activity (IC50 = 29.11 ± 0.70 µg/mL). However, all the three methoxy-substituted compounds 10–12, regardless of the positions of substituents about the phenyl ring, exhibited good activity with IC50 values 27.37 ± 1.05, 32.38 ± 0.66 and 31.43 ± 0.18 µg/mL, respectively. In the cases of trifluoromethyl-substituted compounds 13–15, the ortho- and para-substituted compounds 13 and 15 were found to show significant activity (IC50 = 12.98 ± 0.145 and 20.75 ± 0.01 µg/mL, respectively), when compared with the meta-substituted compound 14, which exhibited weak activity (IC50 = 65.53 ± 1.14 µg/mL). On the contrary, all the three monofluoro-substituted compounds 17–19 demonstrated significant activity with IC50 values ranging from 1.91 ± 0.04 to 13.00 ± 0.08 µg/mL. Of these, the para-substituted compound 19 showed much pronounced activity with an IC50 value of 1.91 ± 0.04 µg/mL. It is pertinent to mention that compound 16, the only trifluoromethoxy derivative (para-substituted) tested in the assay, showed leishmanicidal activity comparable with that of the standard drug, pentamidine (IC50 = 5.74 ± 0.01 versus 5.09 ± 0.04 µg/mL). Amongst the two bromo-substituted compounds 20 and 21, the latter having a para substituent displayed good antileishmanial activity with an IC50 value of 26.95 ± 0.08 µg/mL, whereas the former with an ortho substituent exhibited moderate activity (IC50 = 51.20 ± 0.27 µg/mL). In contrast, amongst the two iodo-substituted compounds 22 and 23, compound 23 possessing a para substituent showed significant leishmanicidal effect (IC50 = 25.93 ± 0.03 µg/mL), while the ortho-substituted compound 22 demonstrated weak activity (IC50 = 63.89 ± 1.39 µg/mL). Interestingly, compound 24, the only cyano derivative (meta-substituted) tested in the assay, was found to exhibit significant activity (IC50 = 25.86 ± 0.18 µg/mL), when compared with the corresponding trifluoromethyl derivative 14, which demonstrated weak leishmanicidal influence (IC50 = 65.53 ± 1.14 µg/mL). Of the remainder active N4-aryl- substituted compounds 25–28 and 30, compound 25 having methoxy substituents at ortho, para (2,4) positions of the phenyl ring displayed moderate antileishmanial activity (IC50 = 53.65 ± 0.51 µg/mL), whereas 26 with the same functions at ortho, meta (2,5) positions exhibited good activity (IC50 = 26.79 ± 0.90 µg/mL). This shows that in the case of 25, substitution of additional methoxy substituent at para position of the phenyl ring caused significant reduction in activity, as the respective ortho-substituted compound 10 gave an IC50 value of 27.37 ± 1.05 µg/mL in this assay. However, such an additional substitution at meta position of the phenyl ring was not found to cause any considerable change in the activity of compound 26 (IC50 = 27.37 ± 1.05 versus 26.79 ± 0.90 µg/mL). More interestingly, in compound 28, substitution of additional fluoro function at meta position of the phenyl ring caused pronounced enhancement in the leishmanicidal activity (IC50 = 4.28 ± 0.075 µg/mL), as the respective ortho-substituted compound 17 gave an IC50 value of 12.94 ± 0.02 µg/mL in the present assay. Furthermore, as in compound 25, such an additional substitution of fluoro group at para position of the phenyl ring of compound 27 resulted into a decrement in its activity but to a level of much higher degree (IC50 12.94 ± 0.02 µg/mL → 82.12 ± 0.86 µg/mL). Compound 27 thus proved to be the least active derivative in the present series. Compound 30, the only dichloro derivative (ortho, para-substituted) tested in this assay, demonstrated significant activity (IC50 = 15.58 ± 0.11 µg/mL). These structure–activity relationships (SARs) may serve as a basis for structural modifications aimed at the development of certain leishmanicidal agents of medicinal interest/importance.
Table 1. In vitro antileishmanial activity of compounds 2–30.
The exact mechanism of antileishmanial activity displayed by the test thiosemicarbazones 2–30 is not known. However, it is generally accepted that the mode of leishmanicidal action of this class of agents involves primarily the inhibition of cysteine proteases found in different Leishmania species. Such an enzymatic inhibition may be attributed to nucleophilic addition of the active site cysteine thiol to the thiocarbonyl function of the thiosemicarbazone moiety, as recently proposed for cruzain, the related major parasitic cysteine protease from Trypanosomal cruziCitation10,Citation12,Citation29. The antileishmanial activity shown by our test compounds 2–30 may also be attributed to inhibition of the ribonucleoside diphosphate reductase (RDR), an iron (Fe)-dependent enzyme catalyzing the rate-limiting step of DNA synthesis in the parasite. Notably, it is the ability of these compounds to act as tridentate metal chelators that leads to their leishmanicidal activity. The chelation of Fe from intracellular Fe pools results in the inhibition of RDR. The tridentate chelating thiosemicarbazones have been proposed to act by inhibiting RDRCitation13,Citation38–40. Such compounds have also been proposed to act by inhibiting dihydrofolate reductaseCitation41,Citation42.
It is only very recently that the thiosemicarbazone-metal complex, i.e. benzaldehyde thiosemicarbazone derived from limonene complexed with copper, termed as BenzCo, has been found to exhibit leishmanicidal activity against the promastigote, axenic amastigote and intracellular amastigote forms of Leishmania amazonensis. This leishmanicidal effect demonstrated by BenzCo was associated with the production of reactive oxygen species (ROS) leading to mitochondrial dysfunction, ultimately causing parasite deathCitation43.
ROS may lead to oxidative damage of virtually any biomolecule. Mitochondria are particularly susceptible to such a damage caused by ROS that are continuously generated by the mitochondrial respiratory chainCitation44. The generation of ROS can also be induced by certain drugs, affecting parasite mitochondrial functionsCitation45–48. The same principle worked for BenzCo in killing parasites. The treatment of parasites with BenzCo dose-dependently generated ROS and enhanced mitochondrial membrane lipid peroxidation, resulting in irreversible loss of mitochondrial functions such as mitochondrial respiration, oxidative phosphorylation and ion transportCitation44.
Conclusions
Conclusively, we have demonstrated the potential of N4-substituted 5-nitroisatin-3-thiosemicarbazones to exhibit antileishmanial activity. To the best of our knowledge, such a group of compounds has been scarcely studied previously for this activity. All the compounds of the present series except 29 were found to be active in the leishmanicidal assay. Of the active compounds, 20, i.e. 2–4, 7, 8, 10–13, 15–19, 21, 23, 24, 26, 28 and 30 displayed good to excellent antileishmanial activity. Compounds 5, 7, 19 and 28 proved to be potent antileishmanial agents, showing leishmanicidal activity even better than the standard drug, pentamidine. These compounds may represent valid leads for further studies aimed at the development of efficacious antileishmanial compounds of medicinal interest. The SAR studies revealed that the antileishmanial potential of the trial compounds depended mainly on the electronic effects of the substituents attached to N4 of the thiosemicarbazone moiety. Nevertheless, extensive studies are required to determine the mechanism by which these compounds exhibit the leishmanicidal activity.
Declaration of interest
We acknowledge partial funding of this research work and the award of Indigenous PhD scholarship to NM by Higher Education Commission, Pakistan.
References
- Tropical disease research. Progress 1999–2000. Geneva: World Health Organisation; 2001 . Available from: http://www.who.int/tdr/publications/documents/progress-99-00.pdf [last accessed 12 Sep 2013]
- Olliaro PL, Bryceson ADM. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today 1993;9:323–8
- Rath S, Trivelin LA, Imbrunito TR, et al. Antimoniais empregados no tratamento da leishmaniose: Estado da arte. Quim Nova 2003;26:550–5
- McGregor A. WHO warns of epidemic leishmania. The Lancet 1998;351:575
- Soto J, Arana BA, Toledo J, et al. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis 2004;38:1266–72
- Prasad R, Kumar R, Jaiswal BP, et al. Miltefosine: an oral drug for visceral leishmaniasis. Indian J Pediatr 2004;71:143–4
- Sangraula H, Sharma KK, Rijal S, et al. Orally effective drugs for Kala-azar (visceral leishmaniasis): focus on miltefosine and sitamaquine. J Assoc Physicians India 2003;51:686–90
- Dodd RH, Ouannès C, Robert-Gèro M, et al. Hybrid molecules: growth inhibition of Leishmania donovani promastigotes by thiosemicarbazones of 3-carboxy-β-carbolines. J Med Chem 1989;32:1272–6
- Bharti N, Husain K, Garza MTG, et al. Synthesis and in vitro antiprotozoal activity of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazone derivatives. Bioorg Med Chem Lett 2002;12:3475–8
- Du X, Guo C, Hansell E, et al. Synthesis and structure-activity relationship study of potent trypanocidal thiosemicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J Med Chem 2002;45:2695–707
- Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev Med Chem 2004;4:31–9 and references therein
- Aguirre G, Boiani L, Cerecetto H, et al. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg Med Chem 2004;12:4885–93
- Greenbaum DC, Mackey Z, Hansell E, et al. Synthesis and structure-activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei and Trypanosoma cruzi. J Med Chem 2004;47:3212–19
- Husain K, Bhat AR, Azam A. New Pd(II) complexes of the synthesized 1-N-substituted thiosemicarbazones of 3-indole carboxaldehyde: characterization and antiamoebic assessment against E. histolytica. Eur J Med Chem 2008;43:2016–28
- Kandemirli F, Saracoglu M, Cavusoglu I, et al. Structure-activity relationship study by ETM method on potent trypanocidal thiosemicarbazone inhibitors of the trypanosomal cysteine protease cruzain. Phil Nat 2009;1:179–93
- Sharma K, Sing R, Fahmi N, et al. Microwave assisted synthesis, characterization and biological evaluation of palladium and platinum complexes with azomethines. Spectrochim Acta A 2010;75:422–7
- Glinma B, Kpoviessi SDS, Fatondji RH, et al. Synthesis, characterization and anti-trypanosomal activity of R-(-)carvone and arylketones-thiosemicarbazones and toxicity against Artemia salina Leach. J App Pharm Sci 2011;8:65–70
- Sakirigui A, Kpoviessi SDS, Gbaguidi F, et al. Selective trypanocide activity of some substituted thiosemicarbazones of citral from benin Cymbopogon citratus essential oil and their toxicity against Artemia salina Leach. IJRRAS 2012;12:454–62
- Demoro B, Sarniguet C, Sanchez-Delgado R, et al. New organoruthenium complexes with bioactive thiosemicarbazones as co-ligands: potential anti-trypanosomal agents. Dalton Trans 2012;41:1534–43
- Navarro M, Gabbiani C, Messori L, et al. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: recent achievements and perspectives. Drug Discov Today 2010;15:1070–8
- Fatondji HR, Kpoviessi S, Gbaguidi F, et al. Structure-activity relationship study of thiosemicarbazones on an African trypanosome: Trypanosoma brucei brucei. Med Chem Res 2013;22:2151–62
- da Silva JFM, Garden SJ, Pinto AC. The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc 2001;12:273–324 and references therein
- Pandeya SN, Smitha S, Jyoti M, et al. Biological activities of isatin and its derivatives. Acta Pharm 2005;55:27–46 and references therein
- Vine KL, Matesic L, Locke JM, et al. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000–2008. Anti-Cancer Agents Med Chem 2009;9:397–414 and references therein
- Aboul-Fadl T, Bin-Jubair FAS. Anti-tubercular activity of isatin derivatives. Int J Res Pharm Sci 2010;1:113–26 and references therein
- Chiyanzu I, Hansell E, Gut J, et al. Synthesis and evaluation of isatins and thiosemicarbazone derivatives against cruzain, falcipain-2 and rhodesain. Bioorg Med Chem Lett 2003;13:3527–30
- Chiyanzu I, Clarkson C, Smith PJ, et al. Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorg Med Chem 2005;13:3249–61
- Bal TR, Anand B, Yogeeswari P, et al. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives. Bioorg Med Chem Lett 2005;15:4451–5
- Chibale K. Economic drug discovery and rational medicinal chemistry for tropical diseases. Pure Appl Chem 2005;77:1957–64
- Pirrung MC, Pansare SV, Sarma KD, et al. Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents. J Med Chem 2005;48:3045–50
- Terzioglu N, Karali N, Gursoy A, et al. Synthesis and primary antiviral activity evaluation of 3-hydrazono-5-nitro-2-indolinone derivatives. Arkivoc 2006(i):109–18
- Quenelle DC, Keith KA, Kern ER. In vitro and in vivo evaluation of isatin-β-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antivir Res 2006;71:24–30
- Hall MD, Salam NK, Hellawell JL, et al. Synthesis, activity, and pharmacophore development for isatin-β-thiosemicarbazones with selective activity toward multidrug-resistant cells. J Med Chem 2009;52:3191–204
- Ermut G, Karali N, Cetin I, et al. Synthesis and chemotherapeutic activities of 5-chloro-1H-indole-2,3-dione 3-thiosemicarbazones. Marmara Pharm J 2013;17:147–54
- Pervez H, Manzoor N, Yaqub M, et al. Synthesis and urease inhibitory properties of some new N4-substituted 5-nitroisatin-3-thiosemicarbazones. Lett Drug Des Discov 2010;7:102–8
- Pervez H, Manzoor N, Yaqub M, et al. Synthesis and biological evaluation of some N4-substituted 5-nitroisatin-3-thiosemicarbazones. Med Chem Res 2012;21:2251–62
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. The Netherlands: Harwood Academic Publishers; 2001:60–4
- Finch RA, Liu M-C, Grill SP, et al. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): a potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol 2000;59:983–91
- Li J, Zheng LM, King I, et al. Syntheses and antitumor activities of potent inhibitors of ribonucleotide reductase: 3-amino-4-methylpyridine-2-carboxaldehyde-thiosemicarbazone (3-AMP), 3-amino-pyridine-2-carboxaldehyde-thiosemicarbazone (3-AP) and its water-soluble prodrugs. Curr Med Chem 2001;8:121–33
- Danuta SK, Patricia Q, Richardson DR. Thiosemicarbazones: the new wave in cancer treatment. Future Med Chem 2009;1:1143–51 and references therein
- Lebrun E, Tu YX, van Rapenbusch R, et al. Inhibition of bovine dihydrofolate reductase and enhancement of methotrexate sensitivity by N4-(2-acetoxyethoxymethyl)-2-acetylpyridine thiosemicarbazone. Biochim Biophys Acta 1990;1034:81–5
- Choi I-H, Kim C. Flexible docking of an acetoxyethoxymethyl derivative of thiosemicarbazone into three different species of dihydrofolate reductase. Arch Pharm Res 2002;25:807–16
- Britta EA, Silva APB, Ueda-Nakamura T, et al. Benzaldehyde thiosemicarbazone derived from limonene complexed with copper induced mitochondrial dysfunction in Leishmania amazonensis. PLoS One 2012;7:e41440 . doi:10.1371/journal.pone.0041440
- Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radical Bio Med 1999;26:463–71 and references therein
- Das R, Roy A, Dutta N, Majumder HK. Reactive organic species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 2008;13:867–82
- Roy A, Ganguly A, BoseDasgupta S, et al. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of FOF1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol 2008;74:1292–307
- Pelizzaro-Rocha KJ, Veiga-Santos P, Lazarin-Bidoia D, et al. Trypanocidal action of eupomatenoid-5 is related to mitochondrion dysfunction and oxidative damage in Trypanosoma cruzi. Microbes Infect 2011;13:1018–24
- Fonseca-Silva F, Inacio JDF, Canto-Cavalheiro MM, et al. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One 2011;6:e14666 . doi:10.1371/journal.pone.0014666